Abstract
Ebp1, an ErbB3 receptor-binding protein, inhibits the proliferation and induces the differentiation of human cancer cells. Ebp1 binds nuclear Akt and prevents DNA fragmentation by inhibiting caspase-activated DNase. Here, we show that Ebp1 possesses two different isoforms, p48 and p42, which differentially mediate PC12 cell survival and differentiation. The longer-form p48 localizes in both the cytoplasm and the nucleus and suppresses apoptosis, whereas the shorter-form p42 predominantly resides in the cytoplasm and promotes cell differentiation. EGF strongly stimulates p42 to bind ErbB3, and the association depends on PKC-mediated phosphorylation of Ebp1. By contrast, p48 does not bind to ErbB3 regardless of EGF treatment. Overexpression of p48 provokes cell proliferation, which is inhibited by p42. Moreover, nerve growth factor elicits extensive sprouting in p42 stably transfected PC12 cells, whereas p48 cells reveal modest neurite outgrowth. Although mitogen-activated protein kinase cascade remains similar in both cells, Akt is more active in p48 cells than in p42 cells. Thus, Ebp1 might regulate cell survival and differentiation through two distinctive p48 and p42 isoforms.
Keywords: Akt, cell death, cell proliferation
A member of the PA2G4 family, Ebp1, was initially identified as an ErbB-3-binding protein during yeast two-hybrid analysis. Ebp1 translocates from the cytoplasm to the nucleus of human breast cancer cells after treatment with the ErbB-3 ligand heregulin (1). Ebp1 is basally phosphorylated by PKC. Inhibition of Ebp1 phosphorylation by PKC inhibitor disrupts its association with ErbB3 (2). Ectopic expression of Ebp1 in ErbB-2, ErbB-3-expressing breast carcinoma cell lines resulted in inhibition of colony formation, a decreased proliferation rate, and suppression of growth in soft agar. Moreover, overexpression of Ebp1 led to a more differentiated phenotype in AU565 breast cancer cells (3). Epb1 has significant homology to the 38-kDa murine protein p38-2G4, which displays a cell-cycle-dependent expression pattern and binds to DNA (4). EBP1 is localized in the cytoplasm and the nucleolus, and its nucleolar localization requires amino acid sequences present at both the N and C termini of the molecule (5). Its nuclear distribution was consistent with previous findings in biochemical fractionation. In an effort to isolate factors involved in the repair of acid-depurinated DNA, Ebp1 was also purified as a nuclear protein from salt extract of permeable mouse ascite sarcoma cells. Interestingly, it was copurified with a major apurinic/apyrimidinic endonuclease (APEX nuclease) by sequential column chromatography. Northern blotting analysis reveals two transcripts with 1.7 kb being the major one and 2.2 kb the minor one (6). These observations are consistent with the report that two Ebp1 mRNAs occur in several normal human organs (1). However, Ebp1 appears as a single protein migrating at 48 kDa on SDS/PAGE, but it contains three in-frame ATG codons in the N terminus. It is possible that different transcripts might contain different translation initiation sites.
Recently, we showed that nuclei from nerve growth factor (NGF)-treated PC12 cells are resistant to DNA fragmentation initiated by the activated cell-free apoptosome, consisting of human embryonic kidney (HEK) 293 cell cytosol supplemented with purified active caspase-3. Using a cell-free apoptotic assay and NGF-treated PC12 nuclear extracts, we purified Ebp1 as a factor that contributes to inhibition of DNA fragmentation. Immunodepletion of Ebp1 from nuclear extracts or knockdown of Ebp1 in PC12 cells abolishes the protective effects of NGF, whereas overexpression of Ebp1 prevents apoptosis. Ebp1 is phosphorylated on Ser-360 by PKC in vitro and in vivo. A mutant form of Ebp1 (S360A), which cannot be phosphorylated, barely binds Akt or inhibits DNA fragmentation, whereas Ebp1 S360D, which mimics phosphorylation, strongly binds Akt and suppresses apoptosis (7). These findings are consistent with a recent report (8) that Ebp1 binds Bcl2 mRNA in human leukemia HL-60 cells, potentially contributing to overexpression of Bcl-2 protein, which is an essential protein-suppressing apoptosis.
In this article, we show that Ebp1 expresses two different proteins: p48 and p42. p48 resides in both the cytoplasm and the nucleus, whereas p42 occurs exclusively in the cytoplasm. Overexpression of p48 promotes cell growth, whereas p42 inhibits cell proliferation. Interestingly, NGF triggers extensive neurite sprout in p42 stably transfected PC12 cells; by contrast, neurite outgrowth is substantially diminished in p48 cells. p48 suppresses apoptosis, whereas p42 fails, which is consistent with their effects on Akt kinase activity. Therefore, Ebp1 isoforms distinctively regulate cell survival and differentiation.
Results
Ebp1 Encodes Two Isoforms: p48 and p42.
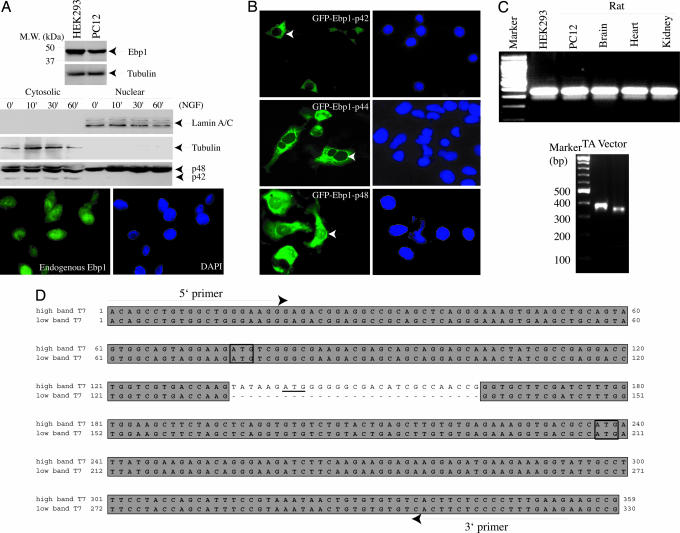
Ebp1 is ubiquitously expressed in all tissues and cells with two mRNA transcripts ≈2.2 and 1.7 kb in size (1, 6). However, it migrates as a single band at 48 kDa on SDS/PAGE (Fig. 1A Top). To determine whether the different-sized transcripts encode different proteins, we performed subcellular fractionation with PC12 cells. Immunoblotting analysis revealed two bands with apparent molecular masses of 48 and 42 kDa in the cytoplasmic fraction, whereas only a 48-kDa band selectively appeared in the nuclear fraction, consistent with previous findings that Ebp1 occurs in both the cytoplasm and the nucleus of HeLa cells and AU565 cells (9). NGF treatment did not alter Ebp1 subcellular distribution (Fig. 1A Bottom). To determine whether different ATG-translated proteins reside in different subcellular compartments, we transfected PC12 cells with GFP–Ebp1 constructs starting from the first, second, and third ATG. Ebp1 encoded by the second or third ATG exclusively resides in the cytoplasm regardless of growth factor stimulation. Similar subcellular distribution patterns occurred in HEK293 cells whether the proteins were tagged with GST or Flag (data not shown). By contrast, GFP–Ebp1 (first ATG) localized in both the cytoplasm and the nucleolus (Fig. 1B), consistent with previous reports that endogenous Ebp1 also localizes in the nucleolus (5). These findings suggest that Ebp1 encodes two different protein isoforms, correlating with its two mRNA transcripts. To examine whether different ATG starting codons are included in these two mRNAs, we conducted RT-PCR with a variety of cells and tissues with primers around the first and third ATG. Surprisingly, we observed two different sizes of PCR products with distinct abundance on agarose gel. These different bands were subcloned into TA vector (Fig. 1C). Sequence analysis revealed that the smaller band skips 29 nucleotides containing the second ATG (Fig. 1D), which presumably resulted from alternative splicing of pre-mRNA. Because of the frameshift, the smaller mRNA can only translate a protein from the third ATG with a calculated molecular mass of 37.97 kDa, and it might appear at 42 kDa on SDS/PAGE. Thus, p48 protein should result from the larger mRNA, which could initiate translation at the first or second ATG. However, endogenous Ebp1 resides in both the cytoplasm and the nucleolus, fitting with the subcellular localization of the first ATG-encoded Ebp1. Thus, p48 Ebp1 should stem from one ATG.
Fig. 1.
Ebp1 encodes two isoforms: p48 and p42. (A) Subcellular fractionation of PC12 cells. (Top) In whole-cell lysate, Ebp1 from rat PC12 and human HEK293 cells appeared as one band on SDS/PAGE. PC12 cells were treated with NGF for various time points. The cytosolic and nuclear fractions were prepared. p48 distributed in both the cytoplasmic and nuclear fractions, where p42 exclusively resided in the cytoplasmic fraction. (Middle) The identity of each fraction was verified with its specific marker. (Bottom) Immunofluorescent staining revealed that endogenous Ebp1 distributed in both the cytoplasm and the nucleus. (B) Ebp1 subcellular distribution. GFP-tagged Ebp1 with first (p48), second (p44), and third (p42) ATG constructs were transfected into PC12 cells and examined under a fluorescent microscope. p48 distributed in both the cytoplasm and the nucleolus, whereas both p44 and p42 occurred exclusively in the cytoplasm. (C) RT-PCR analysis of Ebp1. Total RNA was extracted from various cells and tissues. The first-strand cDNA was prepared with oligo(dT) primer. The two PCR products were ligated into pCR2.1 vector (Invitrogen) and sequenced. (D) ClustalW-formatted sequence alignment. The small band skipped 29 nucleotides containing the second ATG. The primers used in the assay are underlined.
PKC Phosphorylation of p48 Regulates Its Nucleolar Residency.
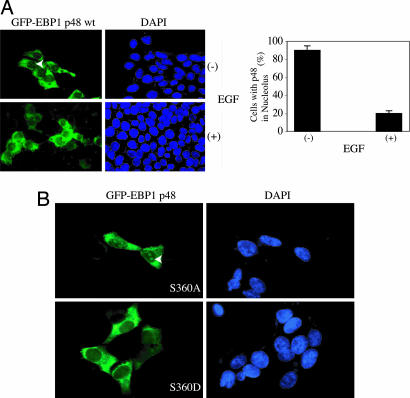
EGF and heregulin trigger Ebp1 phosphorylation (2). To examine whether phosphorylation on Ebp1 mediates its subcellular distribution, we transfected GFP–Ebp1 constructs into HEK293 cells and treated cells with or without EGF. Ebp1 (first ATG) revealed nucleolar distribution in ≈90% of transfected cells; however, after 45 min of EGF stimulation, distribution decreased to only ≈20% (Fig. 2A). NGF treatment also dispersed Ebp1 from the nucleolus in PC12 cells (data not shown). Subcellular fractionation demonstrated that Ebp1 still resided in the nucleus after NGF stimulation (Fig. 1A), indicating that growth factor might just relocate it from the nucleolus to the nucleoplasm. Ebp1 is potently phosphorylated by PKC-δ on Ser-360. To evaluate whether PKC-mediated phosphorylation is responsible for its translocation, we transfected cells with p48 Ebp1 S360A, a mutant unable to be phosphorylated, and S360D, a mutant mimicking PKC-mediated phosphorylation. Ebp1 S360A remained in the nucleolus regardless of growth factor stimulation. By contrast, Ebp1 S360D was absent from the nucleolus (Fig. 2B), underscoring that PKC phosphorylation on Ebp1 dictates its nucleolar localization.
Fig. 2.
PKC phosphorylation of p48 regulates its nucleolar residency. (A) EGF provokes p48 relocation from the nucleolus to the nucleoplasm. HEK293 cells were transfected with GFP–Ebp1 p48 and stimulated with EGF for 30 min. (Left) EGF treatment elicited p48 translocation from the nucleolus to the nucleoplasm. (Right) Quantitative analysis revealed that EGF stimulation substantially decreased p48 Ebp1’s nucleolar residency. (B) GFP–Ebp1 S360A (Upper) but not S360D (Lower) resided in the nucleolus.
p42 Suppresses Cell Growth and p48 Enhances Cell Growth.
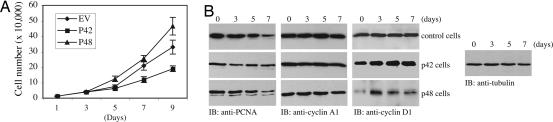
Ectopic expression of Ebp1 in human breast cancer cells resulted in cell growth arrest and induction of differentiation (3). To investigate the effect of different Ebp1 isoforms on cell proliferation, we prepared PC12 cell lines stably transfected with a tetracycline-inducible form of p48 and p42. Compared with control cells (empty vector-transfected cells), cell proliferation analysis demonstrated that induced p42 Ebp1 strongly inhibited cell growth, whereas p48 Ebp1 potently stimulated cell proliferation (Fig. 3A). To further evaluate the growth effect of Ebp1 isoforms, we determined the cell-cycle distribution profiles of p48 and p42 cells. Before NGF treatment, FACS analysis showed that 50% (±5.7) of control cells, 54.4% (±5.5) of p48 cells, and 64.1% (±5.2) of p42 cells were in G1 phase. NGF treatment slightly decreased G1-phase distribution in control cells. By contrast, NGF gradually increased G1 phase accumulation for both p42 and p48 cells. Notably, ≈20% (±3.1) of cells distributed in S phase for control and p42 cells, but ≈31.2% (±3.9) of p48 cells were in S phase, underscoring that p48 cells are more active in DNA synthesis and proliferation. NGF treatment decreased the S-phase profile in both p42 and p48 cells; however, it evidently did not alter control cell partition. It is noteworthy that ≈20.5% (±2.1) of control cells were in G2/M phase. In contrast, only ≈10% of p42 and p48 cells were in G2/M phase, which was not significantly changed upon NGF treatment (Table 1, which is published as supporting information on the PNAS web site).
Fig. 3.
p42 suppresses cell growth and p48 enhances cell growth. (A) Growth curve for Ebp1 isoforms’ stably transfected PC12 cell lines. The stably transfected PC12 cells were cultured in 10% FBS medium in the absence of tetracycline. The cell number was counted at different time points. The data shown are mean ± SD of triplicate experiments. (B) Immunoblotting analysis of cell-cycle proteins. p42 and p48 cell lines were induced and incubated with 50 ng/ml NGF for different times. At each time point, cell lysate was prepared and analyzed by immunoblotting with anti-cyclin A, PCNA, and cyclin D1 antibodies.
The antiproliferative effects of NGF in PC12 cells are coupled to alterations in disposition of a number of proteins associated with the cell cycle (10–14). Previous study showed that NGF does not block cell proliferation but slows it down. Cyclin A protein level remains constant and the proliferating cell nuclear antigen (PCNA), a molecular marker for cells in S phase, decreases after 6 days of NGF treatment (11). Conversely, cyclin D1 protein is greatly increased, and its nuclear translocation is also up-regulated (13). To evaluate whether Ebp1 isoforms affect cell-cycle-dependent protein expression, we monitored the protein levels in Ebp1 stable cell lines after NGF incubation. As expected, cyclin A remained stable in all cell lines regardless of NGF treatment. In addition, the PCNA level decreased in all cells after 7 days of incubation with NGF. By contrast, the cyclin D1 expression level substantially increased upon NGF treatment in both control and p42 cells. Surprisingly, it decreased upon NGF treatment in p48 cells, in alignment with its resistance to impede cell proliferation (Fig. 3B). Collectively, these data suggest that Ebp1 isoforms distinctively affect cell cycle and cell proliferation.
Ebp1 p42 but Not p48 Stimulates PC12 Cell Differentiation.
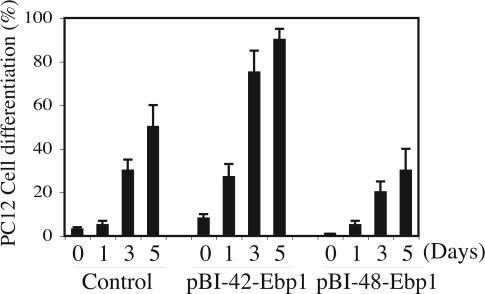
Stimulation of PC12 cells with NGF leads to cessation of the cell division and differentiation into sympathetic neuron-like cells with neurite outgrowth. To examine the physiological role of Ebp1 isoforms in this process, we induced Ebp1 p42 and p48 in PC12 cells and stimulated them with NGF for various days. Interestingly, some of the p42 cells displayed neurite sprouting even before NGF treatment, which was not observed in control or p48 cells. NGF elicited substantial neurite process in p42 cells compared with control cells. By contrast, p48 cells showed significantly attenuated neurite outgrowth (Fig. 8, which is published as supportinig information on the PNAS web site), indicating that p42 and p48 possess different effects on mediating PC12 cell differentiation. After 3–5 days of NGF treatment, quantitative analysis of differentiation ratios revealed that 70–90% of the p42 cells were differentiated, whereas only 30–50% of the control cells displayed neurite process. Strikingly, ≈15–25% of the p48 cells demonstrated neurite outgrowth (Fig. 4). This observation is consistent with the finding that ectopic expression of p42 but not p48 suppressed PC12 cell proliferation (Fig. 3). Taken together, these findings demonstrate that Ebp1 isoforms have different effects on PC12 cell differentiation.
Fig. 4.
p42 but not p48 promotes PC12 cell differentiation. Quantitative analysis of PC12 cell differentiation. A total of 100–200 cells were counted and scored for the expression of neurites longer than two cell bodies. Data are mean ± SD values of triplicate determinations taken from a single experiment. The experiments were replicated three times.
Ebp1 p48 but Not p42 Inhibits Apoptosis.
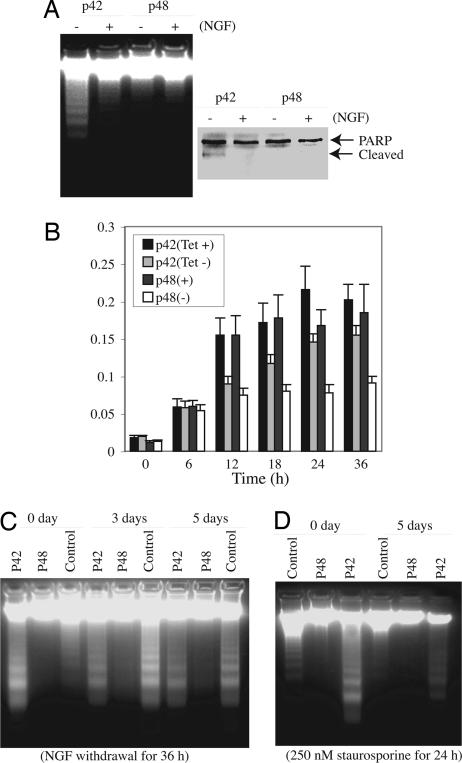
To investigate whether these two isoforms possess different activities in suppressing apoptosis, we induced p48 and p42 in stably transfected PC12 cells and treated the cells with staurosporine. In the absence of NGF, pronounced DNA fragmentation occurred in p42 cells but not in p48 cells (Fig. 5A Left, − lanes). In the presence of NGF, DNA fragmentation was substantially diminished in p42 cells and completely abolished in p48 cells (Fig. 5B Left, + lanes). The cleavage of nuclear poly(ADP-ribose) polymerase (PARP) correlated with DNA fragmentation activities (Fig. 5A Right). To further assess the antiapoptotic effect of Ebp1 isoforms, we treated the induced or uninduced cells with staurosporine for various time points. Caspase-3 activity assay showed that staurosporine increased caspase-3 activity in both uninduced p42 and p48 cells. Induction of Ebp1 diminished caspase-3 activation after 12 h of drug treatment. At 24 h, induction of p42 decreased caspase-3 activity by 20–30%. By contrast, p48 suppressed caspase-3 activity by 50–60% (Fig. 5B). To further assess the physiological role of Ebp1 in promoting cell survival, we differentiated PC12 cells with NGF, and then induced apoptosis by NGF and serum deprivation. NGF withdrawal triggered pronounced DNA fragmentation in both differentiated p42 and control PC12 cells but not in p48 cells (Fig. 5C, 3 days and 5 days). For undifferentiated naive cells, serum starvation elicited demonstrable DNA fragmentation in p42 cells and control PC12 cells but not in p48 cells (Fig. 5C, 0 day). Staurosporine also caused similar apoptotic effects in undifferentiated cells (Fig. 5D, 0 day). By contrast, DNA fragmentation was markedly diminished in the differentiated counterpart (Fig. 5D, 3 days). PARP cleavage tightly correlated with DNA fragmentation activities (data not shown). Therefore, these data further support that p48 reveals much stronger activity in suppressing apoptosis than p42 does. Collectively, these results demonstrate that Ebp1 p48 is necessary for promoting neuronal cell survival, and Ebp1 isoforms display different activities in preventing apoptosis.
Fig. 5.
p48 but not p42 inhibits apoptosis. (A) Ebp1 p48 but not p42 isoforms inhibit apoptosis. p48 and p42 stable cell lines were induced and treated with 250 nM staurosporine for 24 h. Induction of p48 but not p42 prevented DNA fragmentation even in the absence of NGF. (Left) In the presence of NGF, DNA fragmentation was completely blocked in both cells. (Right) PARP cleavage coupled to the DNA fragmentation pattern. (B) Caspase-3 activity assay. p42 and p48 cell lines were cultured in the presence or absence of tetracycline for 24 h, followed by treatment with 250 nM staurosporine for various times. The data shown are mean ± SD of triplicate samples. (C) p48 enhances cell survival. Induced p48 cells, p42 cells, and control PC12 cells were cultured in the medium containing NGF for 3 and 5 days. The differentiated cells were then cultured in NGF-free medium for 36 h. The soluble DNA was extracted and analyzed on a 2% agarose gel. (D) p48 but not p42 prevents DNA fragmentation. The NGF-differentiated cells (5 days) and undifferentiated cells were treated with 250 nM staurosporine for 24 h, and the degraded DNA was analyzed.
p42 and p48 Differentially Interact with ErbB3.
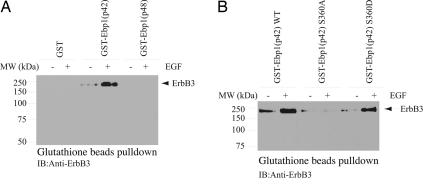
Ebp1 binds to ErbB3 receptor in human serum-starved breast cancer cell lines and dissociates from ErbB3 with treatment with the ErbB3 ligand heregulin (1). To explore whether Ebp1’s two isoforms differentially interact with the ErbB3 receptor, we performed coimmunoprecipitation assay with HEK293 cells cotransfected with ErbB-3 and GST-p42 and p48. Ebp1 was pulled down with glutathione beads, and the associated proteins were analyzed with anti-ErbB3 antibody. p42 but not p48 robustly bound to ErbB3; surprisingly, EGF stimulation strongly enhanced the interaction (Fig. 6A). The expression of GST-Ebp1 and ErbB3 was verified (Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 6.
p42 but not p48 associates with ErbB3 receptor. (A) p42 but not p48 binds ErbB3 receptor. HEK293 cells were cotransfected with ErbB3 and GST-p42 or p48, and then stimulated with EGF. Ebp1 was pulled down with glutathione beads, and the precipitated proteins were analyzed with anti-ErbB3 antibody. EGF stimulation increased the interaction between ErbB3 and p42 but not between ErbB3 and p48. (B) p42 S360D but not S360A binds ErbB3. HEK293 cells were cotransfected with ErbB3 and GST-WT p42, S360A, and S360D, then stimulated with EGF. Ebp1 was pulled down with glutathione beads, and the precipitated proteins were analyzed with anti-ErbB3 antibody.
To test whether Ebp1 phosphorylation by PKC is required for its binding to ErbB3, we performed a binding assay with Ebp1 p42-S360A, a mutant that cannot be phosphorylated by PKC, and S360D, a mutant mimicking phosphorylation. GST-p42 constructs were cotransfected with ErbB3 into HEK293 cells, followed by EGF stimulation for 10 min. WT p42 strongly associated with ErbB3, which was up-regulated upon EGF stimulation. S360D robustly bound to ErbB3; by contrast, S360A failed to interact with ErbB3 regardless of EGF treatment (Fig. 6B). These results demonstrate that Ebp1 phosphorylation by PKC plays an essential role in its association with ErbB3, consistent with a previous report (2) that Ebp1 association with ErbB3 was abrogated by a PKC inhibitor.
p48 Selectively Binds Nuclear Akt and Sustains Its Kinase Activity.
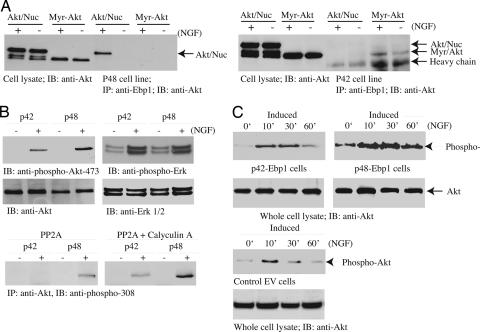
Previous study established that Ebp1 strongly binds to nuclear Akt in PC12 cells in response to NGF treatment. To explore whether Ebp1 isoforms differentially associate with nuclear or cytoplasmic Akt, we infected p48 and p42 cells with adenovirus expressing myristoylated-Akt and myc-NLS-Akt, and then stimulated them with NGF. Coimmunoprecipitation assay showed that p48 specifically interacted with nuclear Akt in response to NGF stimulation. However, p48 did not associate with myristoylated-Akt regardless of NGF treatment. By contrast, Ebp1 p42 only weakly bound to myristoylated-Akt but not to nuclear Akt, and NGF slightly up-regulated the interaction (Fig. 7A). To investigate whether Ebp1 association with Akt affects its kinase activity, we treated Ebp1 cell lines with NGF for various time points. Akt activation was monitored with its anti-phospho-473 antibody. Interestingly, NGF provoked much higher Akt activation in p48 cells than in p42 cells. In contrast, Erk phosphorylation remained the same in both cells (Fig. 7B), suggesting that Ebp1 p48 selectively up-regulates Akt activation. Purified active PP2A completely dephosphorylated Akt immunoprecipitated from p42 cells but not from p48 cells. By contrast, in the presence of calyculin A, a PP2A inhibitor, phospho-Akt 308 remained intact, suggesting that p48 Ebp1 might shield Akt from dephosphorylation by PP2A (Fig. 7B). Time-course experiments revealed that NGF elicited sustained and stronger Akt activity in p48 Ebp1-induced cells compared with p42 and control cells (Fig. 7C). Presumably, it provides a molecular mechanism for why p48 reveals much stronger activity than p42 in suppressing apoptosis.
Fig. 7.
p48 selectively binds nuclear Akt and sustains its kinase activity. (A) p48 but not p42 strongly interacts with nuclear Akt. p48 and p42 stable cell lines were induced in the medium without tetracycline for 24 h, infected with adenovirus expressing myristolated-Akt or nuclear Akt, and stimulated with NGF for 30 min. Ebp1 was immunoprecipitated and its associated proteins were analyzed with anti-Akt antibody. (Left) NGF stimulated nuclear but not cytoplasmic Akt to bind p48. (Right) By contrast, NGF enhanced cytoplasmic but not nuclear Akt to interact with p42. (B) Akt activation is stronger in p48 but not in p42 cells. p48 and p42 stable cell lines were induced and treated with NGF for 30 min. Cell lysate was monitored by immunoblotting with anti-phospho-Akt-473, anti-Akt, anti-phospho-Erk, and anti-Erk1/2 antibodies. Akt was immunoprecipitated from Ebp1 cells and incubated with purified active PP2A (1 unit, 0.5 μg) at 30°C for 1 h in the presence or absence of calyculin A. PP2A dephosphorylated Akt immunoprecipitated from p42 but not p48 cells, which can be inhibited by phosphatase inhibitor calyculin A (10 μM) pretreatment. (C) Akt activation was sustained and stronger in p48 cells compared with control and p42 cells.
Discussion
In the present study, we have discovered that Ebp1 possesses two isoforms: p42 and p48, which distinctively regulate cell proliferation, differentiation, and survival. Exogenously transfected p48 resides in the cytoplasm and nucleolus. p48’s nucleolar residency is negatively regulated by PKC phosphorylation, whereas p42 exclusively occurs in the cytoplasm. The molecular basis that selectively regulates Ebp1 isoforms’ subcellular distribution remains unknown. Presumably, the N terminus of p48 distinctively associates with some cellular targets mediating its nucleolar residency. p42 protein suppresses cell proliferation and enhances cell differentiation. On the other hand, p48 isoform blocks cell differentiation and promotes cell proliferation and survival. Moreover, these two isoforms differentially interact with the ErbB3 receptor. Growth factors trigger p48 to bind nuclear Akt, leading to escalation of its activation and repressing DNA fragmentation.
The interaction between ErbB3 and Ebp1 appears to be regulated by PKC, and heregulin stimulation triggers Ebp1 to dissociate from the ErbB3 receptor and translocate into the nucleus, where it binds to retinoblastoma (Rb) and affects Rb transcriptional regulation (1). However, we show that p42 and p48 display different binding affinities to the ErbB3 receptor. Surprisingly, p42 associates with the ErbB3 receptor and EGF further up-regulates it, whereas p48 does not interact with the ErbB3 receptor regardless of EGF stimulation (Fig. 6). Whether the interaction is related to the biological effect of p42 on cell growth inhibition and differentiation remains elusive. The different effects by heregulin and EGF might result from distinct phosphorylation sites on ErbB3 and Ebp1 (1, 15). Moreover, p42 S360A does not bind to ErbB3, whereas p42 S360D robustly binds to ErbB3, indicating that PKC-mediated Ebp1 phosphorylation is indispensable for its association with ErbB3. This observation is consistent with a previous report that PKC inhibitor antagonizes Ebp1 to interact with ErbB3 (2). The N-terminal 54-residue extension in p48 blocks Ebp1 from binding ErbB3, suggesting that the N terminus might mask the ErbB3 binding motif on Ebp1.
Compared with control PC12 cells, overexpression of p42 accumulates cells in the G1 phase, whereas overexpression of p48 enriches cells in the S phase (Table 1). However, both isoforms decrease G2/M-phase cells. It has been shown that ectopic expression of Ebp1 in ErbB-2, ErbB-3-expressing breast carcinoma cell lines results in inhibition of colony formation, a decreased proliferation rate, an accumulation of cells in the G2/M phase of the cell cycle, and suppression of growth in soft agar (3). It is unclear why Ebp1 triggers different cell-cycle profiles in different cell lines. It remains elusive whether both p42 and p48 also bind to the transcription machinery, repressing their transcriptional activity. However, p48 promotes cell proliferation and prevents cell differentiation. Conceivably, p48 might not interfere with transcription factors, even though it evidently resides in the nucleus. Both p42 (third ATG) and p44 (second ATG) suppress cell growth and initiate cell differentiation. Nevertheless, p48 stimulates cell growth and prevents neurite outgrowth (Figs. 3 and 4). PC12 differentiation requires cessation of cell division and proliferation. From this point of view, p42 and p48 isoforms act harmoniously in the physiological events.
One of the most remarkable aspects of the Ebp1 isoforms is that p48 robustly prevents DNA fragmentation, for which p42 weakly suppresses, indicating that the N terminus of p48 Ebp1 is essential for inhibiting the DNA cleavage activity of caspase-activated DNase during apoptosis. Interestingly, caspase-3 activity and PARP cleavage are more substantially attenuated in p48 cells than in p42 cells, suggesting that p48 antagonizes apoptosis at multiple levels. p48 strongly binds to nuclear Akt, whereas p42 faintly associates with plasma membrane Akt (Fig. 7). Accordingly, Akt activation is much stronger in p48 cells than in p42 cells. Presumably, it accounts for the robust antiapoptotic activity of p48. We shown that p48 protects Akt T308 dephosphorylation from PP2A phosphatase (Fig. 7B). Whether it also shields Akt from PHLPP, an Akt S473 phosphatase, remains unknown (16). Taken together, our findings demonstrate that Ebp1 isoforms distinctively regulate cell proliferation, differentiation, and survival through different subcellular distribution, binding partners, and downstream effectors.
Materials and Methods
Cells and Reagents.
PC12 cells were maintained in medium A (DMEM with 10% FBS, 5% horse serum, and 100 units penicillin-streptomycin) at 37°C with 5% CO2 atmosphere in a humidified incubator. Ebp1 stably transfected PC12 cells (Tet-off cell line) were cultured in medium with 100 μg/ml G418, 100 μg/ml hygromycin B, and 2 μg/ml tetracycline. The transfected genes were induced by culturing in medium without tetracycline for 24 h. Phospho-Akt-473 or 308, Akt, and lamin A/C antibodies were from Cell Signaling Technology, Beverly, MA. Anti-Ebp1 and anti-caspase-3 antibodies were from Upstate Biotechnology, Lake Placid, NY. Anti-ErbB3, PCNA, cyclin D1, and cyclin A antibodies were from Santa Cruz Biotechnology. All other chemicals were from Sigma.
DNA Fragmentation in PC12 Cells.
Oligonucleosomal fragmentation of genomic DNA was determined as described (17). In brief, 3 × 106 induced or uninduced PC12 cells were incubated with 250 nm staurosporine for 16 h. At the end of the incubation, cells were pelleted, washed twice with ice-cold PBS, and lysed on ice for 60 min in 250 μl of 1% Nonidet P-40/proteinase K (0.5 mg/ml) in PBS. Samples were centrifuged, the supernatants were removed and incubated with 5 μl Rnase A (10 μg/ml) at 37°C for 40 min, and 1 ml of anhydrous ethanol was added. Tubes were placed at 20°C for 20 min and then centrifuged to pellet the DNA. After the samples were dry, the same amount of DNA (10 μg) was electrophoresed at 80 V for 3 h in a 2% agarose gel containing ethidium bromide in buffer containing 40 mm Tris acetate and 1 mm EDTA (pH 8.0). DNA bands were visualized under UV light.
Subcellular Fractionation of PC12 Cells.
PC12 cells were treated with NGF for various time points. Cells were washed with PBS and lysed as described (18). Briefly, cells (1 × 106 to 5 × 106/ml) were washed once with 1 ml of PBS and once with 1 ml of lysis buffer (10 mM Hepes/10 mM KCl/1.5 mM MgCl2/0.5 mM PMSF/10 μg/ml leupeptin, pH 7.9). Cells were lysed by suspending the cell pellet in 20 μl of lysis buffer containing 0.1% Nonidet P-40 for 10 min on ice. To isolate nuclei, the lysates were microcentrifuged for 5 min at 12,000 × g, and the nuclear pellet was washed with lysis buffer without Nonidet P-40. Nuclear proteins were obtained by resuspending the nuclear pellet in 20 μl of extraction buffer (420 mM NaCl/20 mM Hepes/1.5 mM MgCl2/0.2 mM EDTA/25% glycerol, pH 7.9) for 10 min at 4°C. The nuclear suspension was microcentrifuged, the pellet was discarded, and the supernatant was diluted in dilution buffer (50 mM KCl/20 mM Hepes/0.2 mM EDTA/20% glycerol, pH 7.9).
RT-PCR of Ebp1 from Total RNA of HEK293 and PC12 Cells.
The total RNA was extracted from HEK293 cells and PC12 cells with Trizol (Invitrogen). The first-strand cDNA was prepared with oligo(dT) primer. PCR was performed with two primers: 5′ primer, 5′-ACA GCC TGT GGC TGG GAA GGG-3′ in 5′ UTR and 3′ primer, 5′-CTT CAA AGG GGA GAA GTG-3′ in coding region from 261 to 279 nucleotides starting from one ATG. The two PCR products were ligated into pCR2.1 vector (Invitrogen) and sequenced.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 NS045627 and American Cancer Society Grant RSG-04-077-01-TBE (to K.Y.).
Abbreviations
- NGF
nerve growth factor
- HEK
human embryonic kidney
- PCNA
proliferating cell nuclear antigen
- PARP
poly(ADP-ribose) polymerase.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Yoo J. Y., Wang X. W., Rishi A. K., Lessor T., Xia X. M., Gustafson T. A., Hamburger A. W. Br. J. Cancer. 2000;82:683–690. doi: 10.1054/bjoc.1999.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessor T. J., Hamburger A. W. Mol. Cell. Endocrinol. 2001;175:185–191. doi: 10.1016/s0303-7207(01)00387-2. [DOI] [PubMed] [Google Scholar]
- 3.Lessor T. J., Yoo J. Y., Xia X., Woodford N., Hamburger A. W. J. Cell. Physiol. 2000;183:321–329. doi: 10.1002/(SICI)1097-4652(200006)183:3<321::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Radomski N., Jost E. Exp. Cell Res. 1995;220:434–445. doi: 10.1006/excr.1995.1335. [DOI] [PubMed] [Google Scholar]
- 5.Squatrito M., Mancino M., Donzelli M., Areces L. B., Draetta G. F. Oncogene. 2004;23:4454–4465. doi: 10.1038/sj.onc.1207579. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa Y., Watanabe S., Akiyama K., Sarker A. H., Tsutsui K., Inoue H., Seki S. Acta Med. Okayama. 1997;51:195–206. doi: 10.18926/AMO/30763. [DOI] [PubMed] [Google Scholar]
- 7.Ahn J. Y., Liu X., Liu Z., Pereira L., Cheng D., Peng J., Wade P. A., Hamburger A. W., Ye K. EMBO J. 2006;25:2083–2095. doi: 10.1038/sj.emboj.7601111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bose S. K., Sengupta T. K., Bandyopadhyay S., Spicer E. K. Biochem J. 2006;396:99–107. doi: 10.1042/BJ20051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia X., Cheng A., Lessor T., Zhang Y., Hamburger A. W. J. Cell. Physiol. 2001;187:209–217. doi: 10.1002/jcp.1075. [DOI] [PubMed] [Google Scholar]
- 10.Buchkovich K. J., Ziff E. B. Mol. Biol. Cell. 1994;5:1225–1241. doi: 10.1091/mbc.5.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan G. Z., Ziff E. B. J. Neurosci. 1995;15:6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Movsesyan V., Whalin M., Shibutani M., Katagiri Y., Broude E., Guroff G. Exp. Cell Res. 1996;227:203–207. doi: 10.1006/excr.1996.0268. [DOI] [PubMed] [Google Scholar]
- 13.van Grunsven L. A., Thomas A., Urdiales J. L., Machenaud S., Choler P., Durand I., Rudkin B. B. Oncogene. 1996;12:855–862. [PubMed] [Google Scholar]
- 14.van Grunsven L. A., Billon N., Savatier P., Thomas A., Urdiales J. L., Rudkin B. B. Oncogene. 1996;12:1347–1356. [PubMed] [Google Scholar]
- 15.Olayioye M. A., Graus-Porta D., Beerli R. R., Rohrer J., Gay B., Hynes N. E. Mol. Cell Biol. 1998;18:5042–5051. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao T., Furnari F., Newton A. C. Mol. Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Walton M. I., Whysong D., O’Connor P. M., Hockenbery D., Korsmeyer S. J., Kohn K. W. Cancer Res. 1993;53:1853–1861. [PubMed] [Google Scholar]
- 18.Blass M., Kronfeld I., Kazimirsky G., Blumberg P. M., Brodie C. Mol. Cell Biol. 2002;22:182–195. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.