Abstract
The sigma factor RpoH (σ32) is the key regulator of the heat shock response in Escherichia coli. Many structural and functional properties of the sigma factor are poorly understood. To gain further insight into RpoH regions that are either important or dispensable for its cellular activity, we generated a collection of tetrapeptide insertion variants by a recently established in vitro linker insertion mutagenesis technique. Thirty-one distinct insertions were obtained, and their sigma factor activity was analyzed by using a groE-lacZ reporter fusion in an rpoH-negative background. Our study provides a map of permissive sites which tolerate linker insertions and of functionally important regions at which a linker insertion impairs sigma factor activity. Selected linker insertion mutants will be discussed in the light of known sigma factor properties and in relation to a modeled structure of an RpoH fragment containing region 2.
The induction of heat shock proteins (Hsps) at elevated temperatures is a universal cellular response. The majority of Hsps are either chaperones or proteases involved in protein quality control (7, 42). The evolutionary conservation of Hsps is contrasted by a multitude of regulatory mechanisms established for temperature perception and heat shock gene expression. Numerous positive- and negative-control mechanisms acting at the transcriptional or posttranscriptional level have been described previously (7, 13, 25, 26, 31, 33, 42).
In Escherichia coli, expression of more than 30 heat shock genes is under the control of the alternative sigma factor RpoH (σ32) (7, 42). Its intracellular level is very low during growth at 30°C and increases transiently after temperature upshift (35). The cellular concentration of σ32 is tightly controlled at four different levels: transcription and translation of the rpoH gene and activity and stability of the RpoH protein (42). Heat induction of σ32 mainly occurs at the posttranscriptional level. An extended secondary structure in the rpoH transcript blocks translation at low temperatures (20, 22). Thermal melting of that structure permits ribosome entry followed by translation initiation. Once produced, the fate of σ32 is determined by its interaction with a number of other proteins. Under nonstress conditions, the sigma factor is neutralized through an interaction with DnaK and DnaJ (6, 36). Sequestration by the chaperones serves two regulatory functions. It inactivates σ32 by preventing it from interaction with the RNA polymerase core enzyme and renders it susceptible to FtsH-mediated degradation (36, 38). Accumulation of unfolded proteins under heat stress conditions titrates away the DnaK system, leaving behind free RpoH, which associates with RNA polymerase and in turn initiates transcription of heat shock genes. Accumulation of σ32 only occurs in the initial phase of the heat shock response (35). Elevated temperatures introduce a conformational change in σ32 which specifically abolishes interaction with DnaK (5). The structurally altered sigma factor is rapidly turned over by cellular proteases, presumably in a DnaK-independent manner (16, 21). As a consequence, the cellular level of σ32 decreases and the heat shock response is shut off.
Its physiological function requires σ32 to be a remarkably flexible protein. An interaction with any of the factors described above as well as DNA binding, promoter melting, transcription initiation, and promoter escape is likely to be accompanied by conformational changes in the transcription factor. The plasticity of full-length sigma factors is thought to be the reason that they have evaded crystallization trials. A first glimpse of the enzyme was provided by investigation of the crystal structure of a proteolytic subfragment of σ70, the primary sigma factor of E. coli (17). Recently, the structure of three stably folded domains of the housekeeping sigma factor from Thermus aquaticus was solved (4). Combined with RNA polymerase holoenzyme structures from T. aquaticus (23, 24) and Thermus thermophilus (41), we now have a fairly good picture of the bacterial transcription machinery containing the principal sigma factor.
To gain some insight into structural and functional properties of the alternative sigma factor σ32 of E. coli, we undertook a linker insertion mutagenesis (LIM) study. The generation of short in-frame insertions has been widely used to analyze the structure-function relationship of proteins (11, 18). LIM is a powerful strategy to distinguish functionally important regions from regions that are tolerant of insertions, regardless of whether the structure of the target protein is known. Several methods based on the random incorporation of transposable elements have been reported. Following imperfect excision of the inserted element, only a short linker composed of the transposon ends and the duplicated target nucleotides is left behind. Depending on the system used, the linker consists of 12 to 279 bp resulting in corresponding insertions of 4 to 93 amino acids (11). Linker insertion libraries of various proteins have been successfully used to map permissive sites that tolerate insertions without significant loss of activity. Other reported applications are membrane topology mapping and modulation of enzymatic activities (11, 18).
In our study, we carried out IS21-based LIM using the E. coli rpoH gene as a target. IS21 is a transposable element from the broad-host-range plasmid R68 (28). IS21 transposes infrequently when present in a single copy. Spontaneously occurring tandem duplications of IS21, however, form cointegrates with target replicons very efficiently (10). Cointegrate formation requires two IS21-encoded proteins, the cointegrase IstA (P45) and the helper protein IstB (P30) (30). Since both proteins function efficiently when supplied in trans, LIM can be carried out in vivo or in a crude extract containing both proteins. To exploit this system, IS21-IS21 junction regions containing unique restriction sites have been engineered and cloned into an R6K suicide replicon (32). Cointegration of this vector into a target replicon and subsequent elimination of the bulk of the suicide plasmid by restriction digest with SalI creates small in-frame linkers consisting of 12 nucleotides coding for four amino acids. Eight nucleotides (including the SalI restriction site) derive from IS21, while four nucleotides derive from target site duplication during the cointegration event. Depending on the reading frame, three classes of tetrapeptide linkers, each coding for two invariant and two variable amino acids, are produced (see Table 2). The total number of possible tetrapeptide sequences is 205 (32). By making use of a recently developed in vitro mutagenesis protocol, we identified a number of permissive sites in σ32. Other linker insertions that abolished sigma factor activity pointed out structurally and functionally important sites in the heat shock transcription factor.
TABLE 2.
Summary of RpoH-linker insertion mutants and β-galactosidase activity of a groES-lacZ fusion in E. coli ΔrpoH carrying plasmids encoding these derivatives
| Protein | Frequency of insertion | Duplicated nucleotides | Inserted amino acids | Sigma factor region | β-Galactosidase activity (MU) | Comments |
|---|---|---|---|---|---|---|
| RpoH-WT | 1,700 | |||||
| No RpoH | 66 | |||||
| RpoH-33 | 2× | GAGG | VSTE | 1.2 | 1,572 | |
| RpoH-57 | 5× | TCAC | CRHH | 2.1 | 107 | |
| RpoH-63 | 1× | ATAT | IVDN | 2.1 | 433 | |
| RpoH-64 | 1× | ATTG | VSTI | 2.1 | 914 | |
| RpoH-77 | 1× | GATT | LSTD | 2.2 | 953 | |
| RpoH-83 | 17× | ACAT | IVDN | 2.2 | 46 | |
| RpoH-84 | 1× | CATC | CRHI | 2.2 | 29 | |
| RpoH-101 | 1× | CCTG | CRHL | 2.3 | 826 | |
| RpoH-105 | 14× | CCGT | VVDT | 2.3 | 57 | |
| RpoH-106 | 2× | CGTT | CRHV | 2.3 | 47 | |
| RpoH-125 | 1× | AAGT | VVDK | 2.4 | 51 | |
| RpoH-130.1 | 1× | AAGC | AVDK | Between 2.4 and RpoH box | 63 | |
| RpoH-130.2 | 1× | AAGCGC | Between 2.4 and RpoH box | 35 | Frame shift | |
| RpoH-132 | 1× | CAGC | LSTQ | RpoH box | 27 | |
| RpoH-143 | 1× | AACCAAG | CRQTK | 3.1 | 1,779 | Pentapeptide insertion |
| RpoH-146 | 6× | TCTG | CRHL | 3.1 | 727 | |
| RpoH-155 | 1× | AAAT | IVDK | 3.1 | 282 | |
| RpoH-156.1 | 1× | ATGG | VSTM | 3.1 | 107 | |
| RpoH-156.2 | 1× | AAATGG | 3.1 | 154 | Frame shift | |
| RpoH-159 | 3× | CCGT | CRHR | 3.1 | 548 | |
| RpoH-163 | 1× | TAAC | TVDI | 3.1 | 1,157 | |
| RpoH-169 | 3× | ACGT | CRQR | 3.1 | 67 | |
| RpoH-173 | 1× | ATCA | CRQC | 3.1 | 43 | |
| RpoH-184 | 1× | CTGT | LSTL | 3.1 | 799 | |
| RpoH-186 | 1× | TTCC | CRHS | 3.1 | 684 | |
| RpoH-195 | 1× | ATGG | VSTM | 3.2 | 547 | |
| RpoH-200.1 | 1× | CTAT | CRHY | 3.2 | 1,359 | |
| RpoH-200.2 | 1× | TATC | LSTY | 3.2 | 696 | |
| RpoH-243 | 2× | CGCT | LSTR | 4.1 | 1,742 | |
| RpoH-252 | 1× | ACGT | LSTT | 4.2 | 1,612 | |
| RpoH-282 | 1× | ATTG | VSTI | 4.2 | 1,569 |
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains and plasmids used in this study are listed in Table 1. All strains were grown in Luria-Bertani (LB) medium supplemented with ampicillin (Ap) (200 μg/ml) and chloramphenicol (Cm) (30 μg/ml) where applicable. The cloning host E. coli DH5α and the expression strain ED8767 were propagated at 37°C. Unless otherwise described, the ΔrpoH strain was grown at room temperature or 30°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| E. coli strains | ||
| ED8767 | supE44 supF58 hsdS3(rB− mB−) recA56 galK2 galT22 metB1 | 29 |
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 29 |
| KY1612 | MC4100 ΔrpoH30::kan zhf-50::Tn10 (λimm21pF13-PgroE-lacZ) | 43 |
| Plasmids | ||
| pEC5007 | pUC18 carrying E. coli rpoH, Ap | 27 |
| pEC5217 | pEC5007 with eliminated SalI site, Ap | This study |
| pME6 | R6K suicide replicon carrying IS21-IS21 junction with SalI site, Cm | 32 |
| pME3913 | ColE1 replicon carrying istA(P45)istB(P30), Ap | 30 |
DNA manipulations.
Recombinant DNA techniques were performed by standard protocols (29). Target plasmids for pME6-based LIM must be devoid of SalI restriction sites. To destroy the SalI site in the pUC18-derived polylinker of pEC5007 (Table 1), the plasmid was digested with SalI, filled in with Klenow enzyme, and religated. Sequencing revealed that not only the SalI site but also the PstI and HindIII sites had been eliminated in the resulting plasmid pEC5217.
For construction of plasmids encoding RpoH-33/143, RpoH-64/143, and RpoH-77/143, the parental plasmids were digested with XmnI, which cuts in the bla gene of pUC18 and in rpoH. Appropriate fragments were isolated and ligated. Replacement of a 0.6-kb PstI/EcoRI fragment from the RpoH-243 plasmid by the equivalent fragment from the RpoH-143 plasmid resulted in a plasmid coding for RpoH-143/243.
LIM.
The in vitro linker insertion approach is based on an in vivo method described by Seitz et al. (32). The IS21 transposition proteins IstA (P45) and IstB (P30) were expressed in E. coli ED8767 from plasmid pME3913 and supplied in a crude extract that was prepared as follows. A 100-ml culture grown at 37°C in LB-Ap medium was induced with 1 mM IPTG (isopropyl-α-d-thiogalactopyranoside) at an optical density at 600 nm (OD600) of 0.6. Following further growth for 2 h, the cells were harvested at 4°C and resuspended in 333 μl of chilled buffer A (25 mM HEPES [pH 7.5], 1 mM Na2EDTA) per OD600 of 1. All subsequent steps were carried out in the cold room at 4°C. A one-ninth volume of chilled solution B (1 M KCl, 20 mM dithiothreitol) was added, and cells were incubated under agitation in the presence of 0.25 mg of lysozyme/ml for 20 min. After the addition of a 1/50 volume of 0.5 M MgCl2 and further incubation for 30 min, the cell suspension was frozen in liquid nitrogen. Cell lysis occurred during thawing on ice for 3 to 4 h. Cell debris was removed by centrifugation at 12,000 × g for 30 min at 4°C, and aliquots of 10 μl from the supernatant were frozen in liquid nitrogen and stored at −70°C. A 1-μl crude extract prepared by this procedure should contain 20 to 30 μg of protein.
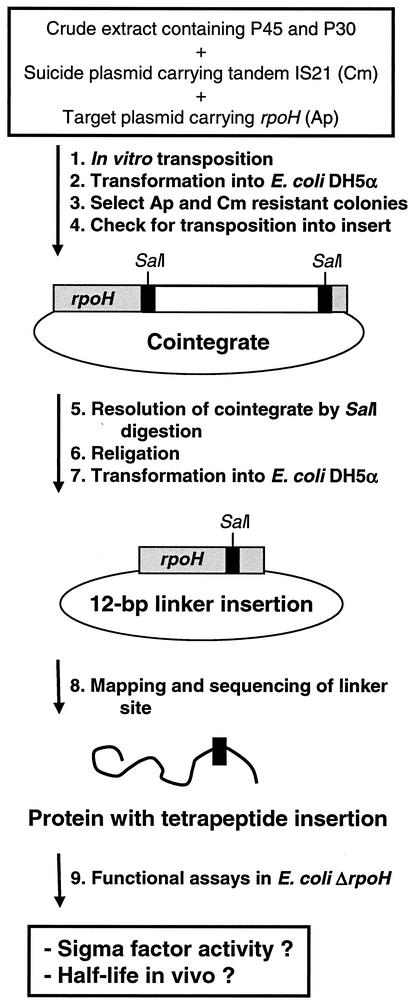
In vitro transposition was initiated by adding 0.27 μg of target plasmid (pEC5217) and 0.37 μg of suicide plasmid (pME6) to 5 μl of crude extract and 15 μl of solution C (25 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 50 mM KCl, 1.8 mM ATP, 5% polyvinylpyrrolidone K90). After incubation for 30 min at 30°C, plasmids were isolated by cetyltrimethylammonium bromide precipitation. Aliquots were transformed into E. coli DH5α, and colonies growing on LB-Ap-Cm plates at 37°C were further investigated as illustrated in Fig. 1. Briefly, isolated plasmids were digested with EcoRI and BamHI to distinguish whether cointegration had occurred in the vector or the insert. Plasmids carrying pME6 in their insert were digested with SalI to release the bulk of the integrated suicide plasmid. E. coli DH5α was transformed with religated plasmids and grown at 30°C. Linker insertion sites in isolated resolved plasmids were roughly mapped by restriction digests and then sequenced with an appropriate primer.
FIG. 1.
In vitro LIM strategy. For details, see text.
Determination of sigma factor activity.
Plasmids carrying linker insertions in rpoH were transformed into E. coli KY1612, which carries a groE-lacZ fusion (Table 1). β-Galactosidase of cells grown at 30°C was measured according to standard procedures (19).
RpoH stability test.
Degradation of RpoH in E. coli cultures grown at 30°C was examined by Western blot analysis as described previously (2, 40). Decay of active RpoH variants was monitored in the ΔrpoH KY1612 strain to facilitate immunodetection of plasmid-encoded RpoH proteins. Protein synthesis was stopped by the addition of Cm (200 μg/ml). Rabbit anti-E. coli σ32 serum was used at a 3,000-fold dilution. Bound peroxidase-coupled secondary anti-rabbit immunoglobulin G was detected with a Pierce SuperSignal kit.
Structural modeling of σ32.
The homology-based RpoH structure was modeled with Swiss-Model Server software, version 36.0002 (8, 9). The entire protein sequence of RpoH was submitted. A three-dimensional model for a RpoH fragment was returned on the basis of a σ70 fragment of E. coli RNA polymerase (PDB entry 1SIG) and a T. aquaticus RNA polymerase sigma subunit fragment (PDB entry 1KU2A). The structure was visualized and edited with the Swiss PDB viewer.
RESULTS
In vitro LIM of RpoH.
The in vitro linker insertion technology used in this study has been developed in the laboratory of Dieter Haas (University of Lausanne) (unpublished data) and is based on a previously described in vivo method (32). An outline of the in vitro approach is depicted in Fig. 1. More than 1,000 cointegrates were generated, and 364 randomly picked Ap- and Cm-resistant colonies were further investigated. Restriction analysis revealed that cointegration had occurred in the rpoH gene in at least 76 of these clones. Upon cointegrate resolution by SalI restriction, which retains only eight nucleotides from the integrated suicide plasmid, the exact linker insertion site was determined by automated sequencing. In total, 31 different linker insertion mutants were obtained (Table 2 and Fig. 2).
FIG. 2.
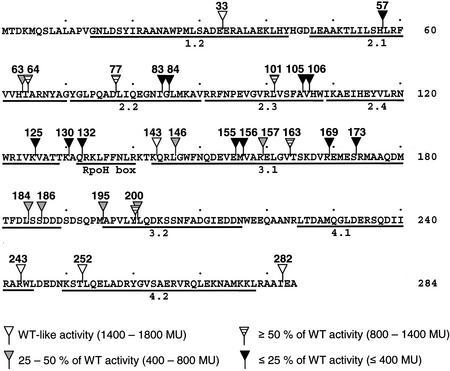
Overview of linker insertion mutants obtained in this study. The amino acid position after which the linker has been inserted is indicated above each corresponding triangle. RpoH mutants were categorized into four groups according to their capacity to initiate transcription from a groE-lacZ promoter in vivo. The groups are defined at the bottom of the figure. Conserved sigma factor regions are indicated below the RpoH sequence.
The insertions were situated throughout the gene. Unexpectedly, IS21 exhibited a strong preference for certain positions in rpoH. In 31 of the 76 sequenced plasmids, the linker was found to be located at either of two positions. A total of 17 and 14 identical linker insertions leading to RpoH-83 and RpoH-105, respectively, were isolated (Table 2). Cointegration of IS21 usually results in the duplication of four nucleotides at the target site. Occasionally, a duplication of five or more nucleotides can occur (32). An atypical duplication of six nucleotides in RpoH-130.2 and RpoH-156.2 adding to the eight IS21-derived residues resulted in out-of-frame products. A duplication of seven nucleotides in RpoH-143 produced a pentapeptide insertion (Table 2).
Permissive and nonpermissive sites in RpoH.
The potential of plasmid-encoded mutated RpoH proteins to initiate transcription from an RpoH-dependent groE-lacZ fusion was assayed in the KY1612 ΔrpoH E. coli strain (43). The β-galactosidase activity derived from all 29 in-frame insertions was measured (Table 2). Four tetrapeptide insertions and the only pentapeptide insertion retained wild-type-like sigma factor activity. With the exception of RpoH-143, which includes a mutation immediately downstream of the RpoH box, these permissive insertions were located within the 50 N- or C-terminal amino residues (Fig. 2). The activity in 12 RpoH mutants was either completely abolished or reduced to less than 25% of wild-type activity. The remaining 12 proteins generated β-galactosidase activity at a level of between 25 and 80% of that produced by wild-type RpoH (Table 2).
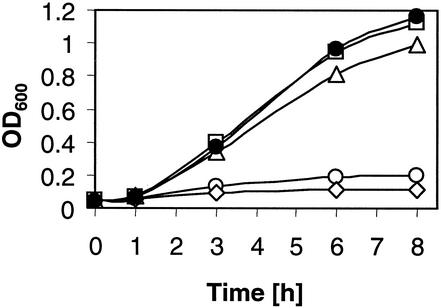
During the creation of the linker insertion mutants, it became apparent that ΔrpoH strains producing inactive RpoH derivatives grew slower on LB plates than strains bearing fully or partially complementing RpoH variants. It is known that strains lacking RpoH are temperature sensitive and only grow at low temperatures (43). A growth experiment in liquid culture at 37°C demonstrated that RpoH proteins with full and partial activity (such as RpoH-33) complemented the growth defect of E. coli KY1612 (Fig. 3). Partially active variants, such as RpoH-77, fully complemented the growth defect, indicating that reduced amounts of RpoH-dependent gene products are sufficient to support cell growth at elevated temperatures. An inactive variant, i.e., RpoH-83, however, did not restore growth to the rpoH mutant strain.
FIG. 3.
Growth complementation of a ΔrpoH strain by mutated RpoH proteins. E. coli strain KY1612 was transformed with the vector pUC18 (open circles) or plasmids expressing wild-type RpoH (filled circles), RpoH-33 (open triangles), RpoH-77 (open squares), or RpoH-83 (open diamonds). LB-Ap medium was inoculated with overnight cultures at an OD600 of 0.05, and growth at 37°C was recorded over time.
Combination of permissive sites.
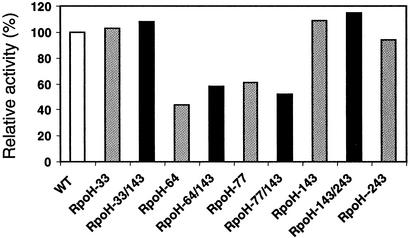
A protein with restriction site-carrying linkers at two different permissive sites might become useful for various applications, e.g., for construction of internal deletions, for error-prone mutagenesis of subfragments, for replacement of internal fragments by heterologous fragments leading to hybrid proteins, or for overproduction of specific subfragments of the protein of interest. Four such RpoH mutants were constructed. Dual-linker mutants retained full activity when they derived from two individual proteins with wild-type-like activity (RpoH-33/143 and RpoH-143/243 in Fig. 4). When a fully permissive linker was combined with a linker generating partially active sigma factor, the resulting proteins (RpoH-64/143 and RpoH-77/143) also showed partial activity. These results indicate that the simultaneous presence of two linkers in RpoH can be tolerated and that the linker originating from the mutant with lowest activity determines the overall activity.
FIG. 4.
Transcriptional activity of RpoH proteins carrying linkers in two permissive sites. Relative β-galactosidase activities derived from the groE-lacZ promoter by RpoH variants carrying a single linker (grey columns) or two linkers (black columns) were compared with the activity of wild-type RpoH (white column).
Stability of linker insertion mutants.
Under nonstress conditions, E. coli RpoH is rapidly turned over by FtsH and other cellular proteases. Shown in Fig. 5 is the degradation of WT-RpoH and six active linker insertion variants. Plasmid-encoded RpoH proteins were produced in the KY1612 ΔrpoH strain to avoid signals derived from chromosomally encoded RpoH. Active RpoH variants were chosen to ensure the presence of the FtsH protease whose synthesis is at least in part RpoH dependent (12). RpoH decay over time was assayed by Western blot analysis after protein synthesis was blocked by Cm addition. Consistent with previous reports, the wild-type sigma factor was degraded, with an estimated half-life of around 1 min (Fig. 5, top panel). Turnover of all linker insertion mutants tested was similar to the wild-type protein, indicating that recognition and degradation sites in the mutated RpoH proteins had not been impeded.
FIG. 5.
Stability of RpoH derivatives. The decay of RpoH proteins after Cm addition (t = 0) was monitored by immunoblot analysis.
DISCUSSION
LIM is a powerful random approach for investigations of structural and functional aspects of any protein of interest. It is particularly attractive for proteins whose high-resolution structure is unknown. Insertions of between 4 and 93 amino acids can be generated depending on the system used (11, 18). We reasoned that large insertions would be more disruptive and deleterious to protein activity than linker peptides consisting of only a few amino acids. Hence, we chose a novel in vitro LIM technology that introduces short linkers (usually tetrapeptides). The E. coli heat shock sigma factor RpoH, whose structure has not yet been solved, was studied. Although random integration throughout the rpoH sequence was achieved, several sites were largely overrepresented. Some clustering of insertions had also been observed after IS21-governed in vivo transposition into arcB of Pseudomonas aeruginosa (32). Specific target sequences could not be detected. Linker insertion into the most frequent hot spots of rpoH rendered the resulting sigma factors inactive. The reason for repeated insertions into these hot spots remains unknown. No obvious sequence motif within or around the duplicated target site was discernible, suggesting that not the DNA sequence itself but some other feature is targeted by the cointegrase IstA and its helper protein IstB.
Our study has revealed a number of interesting permissive and nonpermissive locations in the RpoH protein. Linker insertions that strongly reduced or abolished sigma factor activity were found at various positions throughout the protein, implying that individual mutants are defective at different steps in transcription initiation. Insertion mutants with substantial sigma factor activity and normal degradation properties pointed out positions that are not essential for transcription initiation and RpoH turnover. Tolerant regions were identified immediately downstream of the RpoH box and in the N- and C-terminal regions of RpoH. The wild-type-like features of RpoH-282 located at the very end of the sigma factor are in good agreement with previous data, showing that a C-terminal truncation of five residues retains activity (37). It also has been reported that the C-terminal end of RpoH is not essential for its degradation (2, 37). However, extended C-terminal truncations into region 4.2 impaired RpoH activity, presumably because binding to the −35 promoter region is impossible (2, 3, 37). The intervening region between regions 4.1 and 4.2 appears to be rather flexible, because it tolerated two different tetrapeptide insertions, namely, RpoH-243 and RpoH-252. Apparently, both mutants were able to correctly orient region 4.2 to the −35 promoter sequence, allowing for full groE-lacZ expression. According to investigations of the crystal structure of a C-terminal fragment of T. aquaticus σA (4), RpoH-252 would be at the very beginning of the helix-turn-helix motif important for DNA binding. Residues R243, W244, and L245 have previously been implicated in RpoH-core interaction (15). Moderate core binding defects of corresponding RpoH mutants during glycerol gradient sedimentation became more pronounced in the presence of σ70 competitor. The equivalent region in σ70 also contributes to the extensive core binding surface of the sigma factor (34). However, full activity of RpoH-243 indicates that this region is not critical for core binding under in vivo conditions.
RpoH-143 is the only fully active mutant bearing a linker in the central region of the sigma factor. The insertion site is located between the RpoH box and region 3.1. The RpoH box resides in the more extended region C (Y116 to R145) and is diagnostic of heat shock sigma factors. Residues I123, F136, and F137 have been shown to be involved in core contacts (1, 15). RpoH-125, -130, and -132 presumably are inactive, because interaction with core RNA polymerase is disturbed.
Core binding and in vitro transcription activity of RpoH mutated in L161 was shown to be only slightly reduced (15). Accordingly, flanking linker insertion mutants RpoH-157 and RpoH-163 both exhibited considerable sigma factor activity. The carboxy terminus of region 3.1 and the amino-terminal part of region 3.2 of σ70-type factors have an extended unfolded conformation (24, 41). Since RpoH mutants in this region (RpoH-184, -186, -195, -200.1, and -200.2) showed substantial activity, the corresponding region in RpoH-type sigma factors might also be flexible and disordered. RpoH-200.1 (CHRY insertion) and RpoH-200.2 (LSTY insertion) were both active sigma factors. However, they clearly deviated in their activity levels (1,359 and 696 MU, respectively), indicating that different linker sequences at the same position can alter functionality to various extents.
The observed phenotypes of RpoH mutants in region 2 can be reconciled in light of the modeled structure of this segment shown in Fig. 6. E. coli RpoH (red in Fig. 6A) was modeled against known partial structures from E. coli σ70 and T. aquaticus σA, which are shown in yellow and blue, respectively (4, 17). The almost perfect superimposition of all three structures reflects the high degree of sequence conservation in this sigma factor region. Region 2 carries out many important sigma factor activities, including core binding, −10 recognition, and DNA strand opening. Overall it is a tightly packed region consisting of a three-helix domain (from blue to yellow in Fig. 6B). The primary interface between the core RNA polymerase and sigma factor has been assigned to region 2.2 (14, 24, 34, 41). RpoH variants mutated in Q80 were largely deficient in core binding and in vitro transcription (14). As one would expect, RpoH-83 and -84, which have a linker inserted between the most highly conserved sigma factor residues and interrupt an extended helix including region 2.2 (green in Fig. 6B), are completely inactive. Further downstream, the same helix contains two residues, K87 and R91, whose equivalents in σ70 have been shown to be particularly important for open complex formation (39). Additional amino acids important for strand nucleation by σ70 lie at the beginning of the next helix flanking RpoH residues 105 and 106, which represent two additional nonpermissive sites in the heat shock sigma factor. The linker between both helical parts of region 2.3 tolerated an insertion without much loss of activity (RpoH-101), suggesting that the connecting loop is quite flexible.
FIG. 6.
Predicted structure of E. coli RpoH from residues H44 to F137. (A) Superimposed structures of E. coli σ70 (yellow) from A370 to Y446 (17), T. aquaticus σA (blue) from R193 to L286 (4) and the corresponding RpoH fragment (red). (B) Backbone ribbon presentation of the RpoH fragment showing all linker insertions in this region. The corresponding sigma factor activity is symbolized by triangles whose definitions are as described for Fig. 2.
Region 2.1 of E. coli σ70 forms two α helices that are connected by a 45° kink (17). The suggestion that this kink might be important for core RNA polymerase binding is supported by the inactivity of RpoH-57, which carries a tetrapeptide linker in the predicted kink region of RpoH. Interestingly, a stretch from the C terminus of region 2.1 to the N terminus of region 2.2 was found to be quite tolerant of linker insertions. RpoH-63, -64, and -77 retained considerable sigma factor activity. In agreement with this observation, a P74R version of RpoH interacted with RNA polymerase efficiently but displayed reduced transcriptional activity (14). Moreover, σ70 variants mutated in residues equivalent to RpoH positions 61, 62, 66, 75, and 79 were slightly reduced in core binding affinity (34).
The random mutational approach used in the present study has confirmed and expanded our knowledge about functionally important regions of the heat shock sigma factor RpoH. As one would expect, insertions into regions known to be involved in key steps of transcription initiation were deleterious to sigma factor activity. The finding that many positions throughout the protein tolerated short peptide insertions supports the concept that sigma factors have a modular structure comprised of several stably folded subdomains. Its overall plasticity most likely primes this important bacterial transcription factor perfectly for the large conformational changes that it goes through during core interaction, promoter binding, and transcription initiation.
Acknowledgments
We thank Hauke Hennecke for generous support and continuous interest in this project. Cornelia Reimmann and Dieter Haas are gratefully acknowledged for providing components and advice concerning the linker insertion mutagenesis system. We are indebted to Bernd Bukau for the generous gift of E. coli σ32 antiserum.
This study was supported by the Swiss Federal Institute of Technology, Zürich.
REFERENCES
- 1.Arsène, F., T. Tomoyasu, A. Mogk, C. Schirra, A. Schulze-Specking, and B. Bukau. 1999. Role of region C in regulation of the heat shock gene-specific sigma factor of Escherichia coli, σ32. J. Bacteriol. 181:3552-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertani, D., A. B. Oppenheim, and F. Narberhaus. 2001. An internal region of the RpoH heat shock transcription factor is critical for rapid degradation by the FtsH protease. FEBS Lett. 493:17-20. [DOI] [PubMed] [Google Scholar]
- 3.Blaszczak, A., C. Georgopoulos, and K. Liberek. 1999. On the mechanism of FtsH-dependent degradation of the σ32 transcriptional regulator of Escherichia coli and the role of the DnaK chaperone machine. Mol. Microbiol. 31:157-166. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, E. A., O. Muzzin, M. Chlenov, J. L. Sun, C. A. Olson, O. Weinman, M. L. Trester-Zedlitz, and S. A. Darst. 2002. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol. Cell 9:527-539. [DOI] [PubMed] [Google Scholar]
- 5.Chattopadhyay, R., and S. Roy. 2002. DnaK-Sigma 32 interaction is temperature-dependent. Implication for the mechanism of heat shock response. J. Biol. Chem. 277:33641-33647. [DOI] [PubMed] [Google Scholar]
- 6.Gamer, J., G. Multhaup, T. Tomoyasu, J. S. McCarty, S. Rüdiger, H. J. Schönfeld, C. Schirra, H. Bujard, and B. Bukau. 1996. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor σ32. EMBO J. 15:607-617. [PMC free article] [PubMed] [Google Scholar]
- 7.Gross, C. A. 1996. Function and regulation of the heat shock proteins, p. 1382-1399. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Resnikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
- 8.Guex, N., A. Diemand, and M. C. Peitsch. 1999. Protein modelling for all. Trends Biochem. Sci. 24:364-367. [DOI] [PubMed] [Google Scholar]
- 9.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 10.Haas, D., B. Berger, S. Schmid, T. Seitz, and C. Reimmann. 1996. Insertion sequence IS21: related insertion sequence elements, transpositional mechanisms, and application to linker insertion mutagenesis, p. 238-249. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. ASM Press, Washington, D.C.
- 11.Hayes, F., and B. Hallet. 2000. Pentapeptide scanning mutagenesis: encouraging old proteins to execute unusual tricks. Trends Microbiol. 8:571-577. [DOI] [PubMed] [Google Scholar]
- 12.Herman, C., D. Thévenet, R. D'Ari, and P. Bouloc. 1995. Degradation of σ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. USA 92:3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurme, R., and M. Rhen. 1998. Temperature sensing in bacterial gene regulation—what it all boils down to. Mol. Microbiol. 30:1-6. [DOI] [PubMed] [Google Scholar]
- 14.Joo, D. M., N. Ng, and R. Calendar. 1997. A σ32 mutant with a single amino acid change in the highly conserved region 2.2 exhibits reduced core RNA polymerase affinity. Proc. Natl. Acad. Sci. USA 94:4907-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joo, D. M., A. Nolte, R. Calendar, Y. N. Zhou, and D. J. Jin. 1998. Multiple regions on the Escherichia coli heat shock transcription factor σ32 determine core RNA polymerase binding specificity. J. Bacteriol. 180:1095-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanemori, M., H. Yanagi, and T. Yura. 1999. Marked instability of the σ32 heat shock transcription factor at high temperature. Implications for heat shock regulation. J. Biol. Chem. 274:22002-22007. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra, A., E. Severinova, and S. A. Darst. 1996. Crystal structure of a σ70 subunit fragment from E. coli RNA polymerase. Cell 87:127-136. [DOI] [PubMed] [Google Scholar]
- 18.Manoil, C., and B. Traxler. 2000. Insertion of in-frame sequence tags into proteins using transposons. Methods 20:55-61. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Morita, M., M. Kanemori, H. Yanagi, and T. Yura. 1999. Heat-induced synthesis of σ32 in Escherichia coli: structural and functional dissection of rpoH mRNA secondary structure. J. Bacteriol. 181:401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita, M. T., M. Kanemori, H. Yanagi, and T. Yura. 2000. Dynamic interplay between antagonistic pathways controlling the σ32 level in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5860-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita, M. T., Y. Tanaka, T. S. Kodama, Y. Kyogoku, H. Yanagi, and T. Yura. 1999. Translational induction of heat shock transcription factor σ32: evidence for a built-in RNA thermosensor. Genes Dev. 13:655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami, K. S., S. Masuda, E. A. Campbell, O. Muzzin, and S. A. Darst. 2002. Structural basis of transcription initiation: an RNA polymerase holoenzyme-DNA complex. Science 296:1285-1290. [DOI] [PubMed] [Google Scholar]
- 24.Murakami, K. S., S. Masuda, and S. A. Darst. 2002. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science 296:1280-1284. [DOI] [PubMed] [Google Scholar]
- 25.Narberhaus, F. 2002. mRNA-mediated detection of environmental conditions. Arch. Microbiol. 178:404-410. [DOI] [PubMed] [Google Scholar]
- 26.Narberhaus, F. 1999. Negative regulation of bacterial heat shock genes. Mol. Microbiol. 31:1-8. [DOI] [PubMed] [Google Scholar]
- 27.Narberhaus, F., P. Krummenacher, H. M. Fischer, and H. Hennecke. 1997. Three disparately regulated genes for σ32-like transcription factors in Bradyrhizobium japonicum. Mol. Microbiol. 24:93-104. [DOI] [PubMed] [Google Scholar]
- 28.Reimmann, C., R. Moore, S. Little, A. Savio, N. S. Willetts, and D. Haas. 1989. Genetic structure, function and regulation of the transposable element IS21. Mol. Gen. Genet. 215:416-424. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Schmid, S., T. Seitz, and D. Haas. 1998. Cointegrase, a naturally occurring, truncated form of IS21 transposase, catalyzes replicon fusion rather than simple insertion of IS21. J. Mol. Biol. 282:571-583. [DOI] [PubMed] [Google Scholar]
- 31.Schumann, W. 2000. Function and regulation of temperature-inducible bacterial proteins on the cellular metabolism. Adv. Biochem. Eng. Biotechnol. 67:1-33. [DOI] [PubMed] [Google Scholar]
- 32.Seitz, T., B. Berger, V. T. Nguyen, C. Tricot, V. Villeret, S. Schmid, V. Stalon, and D. Haas. 2000. Linker insertion mutagenesis based on IS21 transposition: isolation of an AMP-insensitive variant of catabolic ornithine carbamoyltransferase from Pseudomonas aeruginosa. Protein Eng. 13:329-337. [DOI] [PubMed] [Google Scholar]
- 33.Servant, P., and P. Mazodier. 2001. Negative regulation of the heat shock response in Streptomyces. Arch. Microbiol. 176:237-242. [DOI] [PubMed] [Google Scholar]
- 34.Sharp, M. M., C. L. Chan, C. Z. Lu, M. T. Marr, S. Nechaev, E. W. Merritt, K. Severinov, J. W. Roberts, and C. A. Gross. 1999. The interface of sigma with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev. 13:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straus, D. B., W. A. Walter, and C. A. Gross. 1987. The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature 329:348-351. [DOI] [PubMed] [Google Scholar]
- 36.Tatsuta, T., T. Tomoyasu, B. Bukau, M. Kitagawa, H. Mori, K. Karata, and T. Ogura. 1998. Heat shock regulation in the ftsH null mutant of Escherichia coli: dissection of stability and activity control mechanisms of σ32 in vivo. Mol. Microbiol. 30:583-593. [DOI] [PubMed] [Google Scholar]
- 37.Tomoyasu, T., F. Arsène, T. Ogura, and B. Bukau. 2001. The C terminus of σ32 is not essential for degradation by FtsH. J. Bacteriol. 183:5911-5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomoyasu, T., T. Ogura, T. Tatsuta, and B. Bukau. 1998. Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in Escherichia coli. Mol. Microbiol. 30:567-581. [DOI] [PubMed] [Google Scholar]
- 39.Tomsic, M., L. Tsujikawa, G. Panaghie, Y. Wang, J. Azok, and P. L. deHaseth. 2001. Different roles for basic and aromatic amino acids in conserved region 2 of Escherichia coli σ70 in the nucleation and maintenance of the single-stranded DNA bubble in open RNA polymerase-promoter complexes. J. Biol. Chem. 276:31891-31896. [DOI] [PubMed] [Google Scholar]
- 40.Urech, C., S. Koby, A. B. Oppenheim, M. Münchbach, H. Hennecke, and F. Narberhaus. 2000. Differential degradation of Escherichia coli σ32 and Bradyrhizobium japonicum RpoH factors by the FtsH protease. Eur. J. Biochem. 267:4831-4839. [DOI] [PubMed] [Google Scholar]
- 41.Vassylyev, D. G., S. Sekine, O. Laptenko, J. Lee, M. N. Vassylyeva, S. Borukhov, and S. Yokoyama. 2002. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature 417:712-719. [DOI] [PubMed] [Google Scholar]
- 42.Yura, T., M. Kanemori, and M. T. Morita. 2000. The heat shock response: regulation and function, p. 3-18. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 43.Zhou, Y. N., N. Kusukawa, J. W. Erickson, C. A. Gross, and T. Yura. 1988. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor σ32. J. Bacteriol. 170:3640-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]