Abstract
The identification of multipotential mesenchymal stem cells (MSCs) derived from adult human tissues, including bone marrow stroma and a number of connective tissues, has provided exciting prospects for cell-based tissue engineering and regeneration. This review focuses on the biology of MSCs, including their differentiation potentials in vitro and in vivo, and the application of MSCs in tissue engineering. Our current understanding of MSCs lags behind that of other stem cell types, such as hematopoietic stem cells. Future research should aim to define the cellular and molecular fingerprints of MSCs and elucidate their endogenous role(s) in normal and abnormal tissue functions.
Keywords: cell differentiation, cell signaling, mesenchymal stem cells, stem cells, tissue engineering
Introduction
Despite the pluripotency of embryonic stem (ES) cells, legal and moral controversies concerning their use for therapeutic and clinical application have prompted active examination of the reservoirs of progenitor cells harbored within the adult organism. In principle, such unspecialized cells are considered to be quiescent, but capable of self-renewing; their asymmetric division produces one identical daughter stem cell and a second progenitor cell that becomes committed to a lineage-specific differentiation program [1]. These cells remain in their 'undifferentiated' state from suppression by some intrinsic or extrinsic factor, until stimulated. Such adult stem cells have been discovered and characterized in a multitude of tissues, suggesting the potential for therapeutic application in their host tissue [2-4]. As these cells are capable of differentiation along specific lineages and of being recruited to tissues in need, the promise for autologous clinical implantation or genetically engineered stem cells for protein or drug delivery without the risk of immunorejection looms on the horizon. However, the success of future clinical applications depends critically upon a thorough understanding of the biology of these cells, and new findings are continuously being reported. For example, there is recent evidence suggesting that the pluripotent stem cell, once thought to be restricted to the fates of a lineage hierarchy, is capable of transdifferentiation. Some recent examples include the observations that the hematopoietic stem cells of bone marrow have been shown to become hepatic oval cells [5-7]; that muscle satellite cells exhibit hematopoietic potential [8]; that neural stem cells have been shown to produce lineage-committed hematopoietic progenitors [9]; and that mesenchymal stem cells from bone marrow have traveled to skeletal muscle [10], differentiated into neuronal tissue [11,12], supplied mesangial cells during repair processes [13], and given rise to cardiomyocytes in vitro [14,15]. These observations strongly imply a critical influence of microenvironmental cues on cell fate.
Sources of mesenchymal stem cells
This review focuses on the adult mesenchymal stem cell (MSC), which has the potential to differentiate into chondrocytes, osteoblasts, adipocytes, fibroblasts, marrow stroma, and other tissues of mesenchymal origin. Interestingly, these MSCs reside in a diverse host of tissues throughout the adult organism and possess the ability to 'regenerate' cell types specific for these tissues (Table 1). Examples of these tissues include adipose tissue [16], periosteum [17,18], synovial membrane [19], muscle [20], dermis [21], pericytes [22-24], blood [25], bone marrow [26], and most recently trabecular bone [27,28]. Currently, bone marrow aspirate is considered to be the most accessible and enriched source of MSCs, although trabecular bone may also be considered an alternative source, in view of recent efficient isolation of multipotential cells from this tissue [29]. Given the wide distribution of the sources of MSCs, the bone marrow stroma may be considered to be the source of a common pool of multipotent cells that gain access to various tissues via the circulation, subsequently adopting characteristics that meet the requirements of maintenance and repair of a specific tissue type. In fact, the presence of MSCs in tissues other than the marrow stroma strongly suggests the existence of cell populations with more limited capacity for differentiation; specifically, monopotent or bipotent cells may have differentiation potentials developmentally adapted to (and perhaps restricted to) the tissues in which they reside.
Table 1.
Sources of multipotential adult mesenchymal stem cells
| Source tissue | Multilineage differentiation potential | Representative references |
| Bone marrow | Adipocyte | [26,143] |
| Astrocyte, neuron | [12,137] | |
| Cardiomyocyte | [14,15,131-133] | |
| Chondrocyte | [26,46,76,79] | |
| Hepatocyte | [6,7,144] | |
| Mesangial cell | [13] | |
| Muscle | [10,35] | |
| Neuron | [11,12] | |
| Osteoblast | [26,33,43,145-147] | |
| Stromal cell | [148] | |
| Various embryonic tissue lineages | [139] | |
| Muscle | Adipocyte, myotubes, osteocyte | [138] |
| Endothelial cell, neuron | [20,149] | |
| Chondrocyte | [112] | |
| Osteocyte | [20] | |
| Trabecular bone | Adipocyte, chondrocyte, osteoblast | [27-29] |
| Dermis | Adipocyte, chondrocyte, muscle, osteoblast | [21] |
| Adipose tissue | Chondrocyte, muscle, osteoblast | [16] |
| Stromal cell | [150] | |
| Periosteum | Chondrocyte, osteoblast | [17,18] |
| Pericyte | Chondrocyte | [22] |
| Osteoblast | [23,24] | |
| Blood | Adipocyte, fibroblast, osteoblast, osteoclast | [25] |
| Synovial membrane | Adipocyte, chondrocyte, muscle, osteoblast | [18] |
Bone marrow contains three main cell types: endothelial cells, hematopoietic stem cells, and stromal cells. In a ground-breaking study, Friedenstein et al. [30] isolated cells, clonogenic fibroblast precursor cells (CFU-F), from whole bone marrow and showed that they were capable of forming bone- and cartilage-like colonies. Long-term bone marrow cultures also revealed the presence of adherent stromal cells that supported and maintained the hematopoietic component as a feeder layer [31]. After the endothelial cells, monocytes, and lymphocytes were removed using negative immunoselection, these long-term cultures revealed stromal cells that coexpressed phenotypic characteristics of the osteoblastic and adipocytic lineages, thereby indicating their progenitor status [32]. Many subsequent studies have substantiated the multipotent mesenchymal progenitor nature of cells isolated according to Friedenstein's method [e.g. [33-35]]. These studies have prompted interest not only in the differentiation potential of MSCs, but also in the mechanisms governing their lineage-specific differentiation, particularly to bone and cartilage. For example, Pittenger et al. [26] showed that cells isolated from human marrow aspirates were capable of remaining in a stable undifferentiated state when cultured long-term in vitro, and that colonies derived from single isolated cells could be induced to differentiate along osteogenic, adipogenic, and chondrogenic lineages when provided the appropriate cues. Concurrent with such discoveries, varying methods of isolation or preparation of more homogeneous, potentially clonally derived MSC populations have emerged. Kuznetsov et al. [36] found the stromal-cell population to be capable of forming colonies in response to the following growth factors: platelet-derived growth factor, transforming growth factor beta (TGF-β), basic fibroblast growth factor, and epidermal growth factor when cultured in serum-containing medium. More recently, Digirolamo et al. [37] have shown that cells with the highest colony-forming efficiency exhibited the greatest replicative potential, and also readily differentiated into osteoblasts and adipocytes. As such, techniques for the isolation and in vitro culture expansion of bone-marrow-derived MSCs range from aspiration and density-gradient centrifugation to simple, direct plating methods to size sieving [38,39]. Although preliminary studies suggest that cells isolated using different methodologies are, in fact, the same and appear to retain similar potentials for differentiation, there is as yet no clear-cut definition of the human MSC, in view of the multitude of methods and procedures used in their isolation and characterization.
Characteristics of mesenchymal stem cells
MSCs are described as multipotent because of their ability, even as clonally isolated cells, to exhibit the potential for differentiation into a variety of different cells/tissue lineages (Fig. 1). However, in most studies, it remains to be determined whether true stem cells are present, or whether the population is instead a diverse mixture of lineage-specific progenitors. Inconsistency in published reports of the growth characteristics and differentiation potential of MSCs underscores the need for a functional definition of these cells. At present, there is lack of a unifying definition as well as information on specific markers that define the cell types characterized as MSCs, with the sole definition being their ability to differentiate along specific mesenchymal lineages when induced to do so, to remain in a quiescent undifferentiated state until provided the signal to divide asymmetrically, and finally, to undergo many more replicative cycles than normal, fully differentiated cells.
Figure 1.
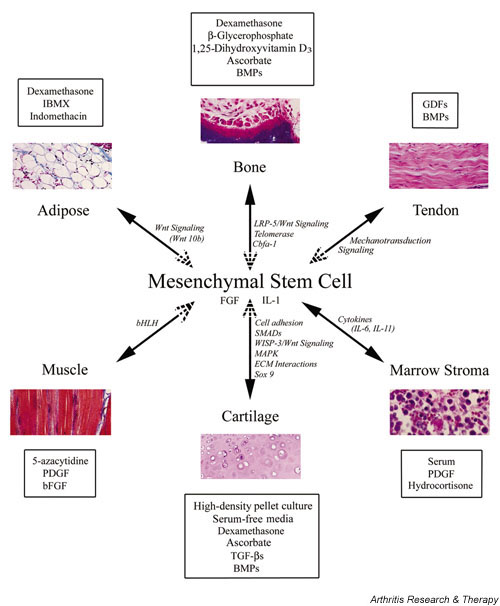
Lineage potential of adult human MSCs. MSCs are characterized by their multilineage differentiation potentials, including bone, cartilage, adipose tissue, muscle, tendon, and stroma. This figure depicts some of the in vitro culture conditions (boxed) that promote the respective differentation process into a specific lineage. Signaling pathways and/or components or events shown to be involved in lineage-specific differentiation are in italics. See text for details. Dotted arrowheads denote potential 'reverse' differentiation events. bFGF, basic fibroblast growth factor; bHLH, basic helix–loop–helix; BMP, bone morphogenetic protein; Cbfa1, core binding factor alpha 1; ECM, extracellular matrix; FGF, fibroblast growth factor; GDF, growth/differentiation factor; IBMX, 3-isobutyl-1-methylxanthine; LRP, low-density lipoprotein receptor-related peptide; MAPK, mitogen-activated protein kinase; PDGF, platelet-derived growth factor; SMAD, vertebrate homologue of Drosophila Mothers Against Decapentaplegic (MAD); TGF-β, transforming growth factor beta; WISP, Wnt-1-inducible protein.
Some groups have used the term 'marrow stromal cell' interchangeably with 'mesenchymal stem cell' [40]. While these two types of cell are likely to have a common ancestor, the stromal characteristic can be thought of as a committed lineage with limited potential for differentiation. Studies examining and comparing the morphology, phenotype, and in vitro function of MSCs and marrow-derived stromal cells have shown the MSCs to be more homogeneous and fibroblastoid, while the marrow-derived stromal cells were less homogeneous, with both fibroblastic and hematopoietic characteristics present to varying degrees. Although both cell types were able to support hematopoiesis, the undifferentiated MSCs were not as efficient, and while the cells displayed similar mRNA and cytokine profiles, their individual responses to IL-1 treatment differed [41]. Therefore, we propose that the stromal compartment of the bone marrow itself contains MSCs and that the stromal cell is actually an early differentiated progeny of the MSC.
Despite improvements in long-term culture expansion, MSCs display finite life spans, uncharacteristic of immortalized 'stem' cells. Although MSCs are present throughout life, their total number is inversely correlated to the age of the patient and depends upon the site of extraction and the systemic disease state [42]. Bruder et al. [43] characterized the long-term growth kinetics and osteogenic differentiation potential of MSCs aspirated from bone marrow of the iliac crest; the cells averaged 38 ± 4 population doublings following extensive subcultivation and cryopreservation before they reached senescence. Retroviral transduction of human MSCs with the human telomerase gene has successfully extended the life span to more than 260 population doublings, while allowing the cells to remain stably undifferentiated with full multilineage differentiation potential [44,45]. For the purpose of further elucidating the mechanisms regulating the lineage-specific differentiation pathways of MSCs, immortalized clonal sublines have been established using the human papilloma virus E6/E7 genes with and without transduced telomerase reverse transcriptase [28,46,47]. Okamoto et al. have shown the immortalized parental population to be composed of a heterogenous combination of uni-, bi-, and tripotential progenitor cells [47]. These findings again point to the intrinsic heterogeneity as well as the need for thorough characterization of the MSC population.
At present, the characterization of human MSCs lags significantly behind that of bone marrow hematopoietic cells. MSCs isolated directly from bone marrow are positive for CD34, the hallmark antigen for positive immunoselection of the hematopoietic stem cell, but lose this antigen upon in vitro culture. While these results suggest a common precursor for these two cell populations, they can be distinguished based upon the CD50 surface antigen, which is common only to the hematopoietic stem cell [48]. Isolation and enrichment of the MSC population has been greatly facilitated by the Stro-1 monoclonal antibody [49]. Stro-1 immunoselection of cells derived from human bone marrow revealed all fibroblast-colony-forming units to be positive for Stro-1 but to lack the CD34 antigen, indicating CD34 to be a nonspecific marker of human MSCs [50]. The Stro-1-positive population of bone-marrow-derived cells has been shown to be capable of differentiating into multiple mesenchymal lineages, including hematopoiesis-supportive stromal cells with a vascular-smooth-muscle-like phenotype, adipocytes, osteoblasts, and chondrocytes [51]. In addition, the cell-surface antigen activated leukocyte-cell adhesion molecule, which reacts with the monoclonal antibody SB-10, has been shown to be expressed in undifferentiated cells but is lost during mesenchymal differentiation. This surface antigen has been suggested to act as a cell adhesion molecule involved in osteogenesis during bone morphogenesis [52]. The presence of specific, distinct antigens that are identified by the monoclonal antibodies SH2, SH3, and SH4 on the cell surface of marrow-derived MSCs and that are absent from osteocytes and osteoblasts suggests that these recognized epitopes are developmentally regulated [53]. More recently, the antigen binding the SH2 antibody was identified as endoglin (CD105), the receptor for TGF-β3, which potentially plays a role in mediating the chondrogenic differentiation of MSCs as well as their interactions with hematopoietic cells [54]. The SH3 and SH4 antibodies have been shown to react with CD73 (ecto-5'-nucleotidase), which plays a role in the activation of B lymphocytes in lymphoid tissue but whose role has yet to be elucidated in human MSCs [55]. As progress in phenotyping the MSC and its progeny continues, the use of selective markers has resulted in the enhanced propagation and enrichment of the MSC population, while maintaining them in an undifferentiated state without diminishing the differentiation potential. Walsh et al. [56] found that fibroblast growth factor-2 increases the proliferative potential of human-bone-marrow-derived MSCs ex vivo. This increase in colony size and overall cell number in response to treatment with fibroblast growth factor-2 was accompanied by an increase in the expression of Stro-1 and in the abundance of alkaline phosphatase-positive cells, suggesting that osteoblast progenitor cells are preferentially targeted by the growth factor [56]. Other studies, however, have shown the differential potential of human MSCs to be unaffected by fibroblast growth factor-2 treatment, notwithstanding the proliferative effects [57]. The phenotypic characterization of MSCs from human bone marrow has been further realized through the identification of the cytokine expression profile of undifferentiated cells. Constitutive expression of cytokines, such as granulocyte-colony stimulating factor, stem cell factor, leukemia inhibitory factor, macrophage-colony stimulating factor, and IL-6 and IL-11 is consistent with the ability of MSCs to support hematopoiesis and provide factors that regulate the marrow milieu itself [58].
Applications of mesenchymal stem cells in tissue engineering and regenerative medicine
Bone
The challenges of engineering a tissue with numerous cell types, each expressing individual differentiation patterns, are significant for bone. The regeneration of bone is a key issue at the forefront of current tissue engineering applications, owing to the ease of use and accessibility of osteoprogenitor cells. The molecular mechanisms of human MSC regulation and the importance of specific growth factors during the different stages of osteogenic differentiation are subjects of intensive investigation.
Molecular regulation of osteogenic differentiation
The induction of MSC osteogenesis is a highly programmed process, best illustrated in vitro. Treatment with the synthetic glucocorticoid dexamethasone stimulates MSC proliferation and supports osteogenic lineage differentiation [59,60]. Organic phosphates, such as β-glycerophosphate, also support osteogenesis by playing a role in the mineralization and modulation of osteoblast activities [61,62]. Free phosphates can induce the mRNA and protein expression of osteogenic markers such as osteopontin, and these phosphates have known effects on the production and nuclear export of a key osteogenesis regulatory gene, Cbfa1 (core binding factor alpha1) [63-65]. Other supplements, such as ascorbic acid phosphate and 1,25-dihydroxyvitamin D3, are commonly used for osteogenic induction, with the latter involved in increasing alkaline phosphatase activity in osteogenic cultures and promoting the production of osteocalcin [66]. In addition to established supplements, members of the bone morphogenetic protein (BMP) family of growth factors are also routinely used for osteoinduction. BMP-2 alone appears to increase bone nodule formation and the calcium content of osteogenic cultures in vitro, while concomitant application of BMP-2 and basic fibroblast growth factor increases MSC osteogenesis both in vivo and in vitro [67].
A number of signaling pathways have been shown to participate in MSC osteogenesis. The secreted signaling proteins known as Wnts have been implicated in various differentiation programs, including osteogenesis. An established Wnt coreceptor, the low-density lipoprotein receptor-related peptide5 (LRP-5) has been linked to osteoporosis–pseudoglioma syndrome in humans [68]. Patients with this syndrome have very low bone mass, are prone to fracture and bone deformation, and have an overall decrease in trabecular bone volume. Our laboratory has shown that trabecular bone harbors a population of MSCs [27], which may be the affected cell population in this disease, thereby leading to alterations in bone formation and remodeling. In mice, LRP-5 mediates Wnt signaling via the canonical pathway (i.e. through intracellular β-catenin) [69,70]. In these in vitro mouse cultures, the application of Wnt-3a can induce the activity of alkaline phosphatase without altering the levels of Cbfa1. It has also been shown that mice with targeted disruptions of LRP-5 expression have a decreased level of osteoblast proliferation and display a phenotype similar to humans with osteoporosis–pseudoglioma syndrome [69].
Interestingly, the misexpression of telomerase was recently found not only to extend the life of MSCs in vitro, but also to increase their osteogenic differentiation potential [44,45].
Bone tissue engineering
The use of natural and synthetic biomaterials as carriers for MSC delivery has shown increasing promise for orthopaedic therapeutic applications, especially bone formation. Recent advances in the field of biomaterials have led to a transition from nonporous, biologically inert materials to more porous, osteoconductive biomaterials, and, in particular, the use of cell-matrix composites [71]. The parameters that need to be considered in the selection of a suitable delivery vehicle include physicochemical properties, such as surface area, porosity, local acidification, material chemistry, dimensional architecture, mechanical integrity, degradation characteristics, natural versus synthetic, and potential for drug delivery; and biological properties, such as the ability of the scaffold to support cellular attachment, proliferation, differentiation, matrix deposition, angiogenesis, prevention of dedifferentiation, and enrichment with a suitable quantity of cells. A number of delivery vehicles have been successfully used in cell-matrix composites in vivo, such as porous ceramics of hydroxyapatite and β-tricalcium phosphate loaded with autologous MSCs [72]. These constructs were capable of healing critical-sized segmental bone defects not capable of being healed by resident cells or by the addition of the osteoconductive device alone. A recent in vitro study comparing the biodegradable polymers poly-L-lactide (PLA) and poly-L-lactide-co-glycolide (PLGA) on the basis of adherence and proliferation of seeded trabecular-bone-derived osteoprogenitor cells showed that PLGA was the better substrate for the attachment and subsequent osteogenic differentiation of these progenitor cells [73].
Cartilage
Joint pain is a major cause of disability, which most often results from damage to the articular cartilage by trauma or degenerative joint diseases such as primary osteoarthritis. Articular cartilage functions to provide uncompromised movement by minimizing friction between joints and allows load bearing through distribution of and resistance to compressive forces, but possesses very limited potential for healing. Current treatment methods for restoration of function due to articular cartilage damage, other than total joint arthroplasty, include autografting, allografting, periosteal and perichondrial grafting, stimulation of intrinsic regeneration by intentionally drilling full-thickness defects, pharmacological intervention, and, finally, autologous cell transplantation such as the periosteal flap technique [74] marketed by Genzyme Corp. (Cambridge, MA, USA). Despite such advances, cartilage damage often cannot be repaired to a fully functional normal state, or the procedures have higher failure rates in younger patients [75]. A potential resolution of this disease state is the regeneration of cartilage tissue using autologous MSCs, thereby obviating any donor-site morbidity as is seen with current repair methods, but requiring an understanding of the mechanisms responsible for the generation, maintenance, and particularly the regeneration of cartilage tissues.
Molecular regulation of chondrogenic differentiation
The induction of chondrogenesis in MSCs depends on the coordinated activities of many factors, including parameters such as cell density, cell adhesion, and growth factors. For example, culture conditions conducive for chondrogenic induction of MSCs require high-density pelleting and growth in serum-free medium containing specific growth factors and supplements. The TGF-β superfamily of proteins and their members, such as the bone morphogenetic proteins (BMPs), are well-established regulatory factors in chondrogenesis. TGF-β1 was initially used for in vitro culture and can induce chondrogenesis under these conditions [76,77], although TGF-β3 has recently been shown to induce a more rapid and thorough expression of chondrogenic markers [78,79]. Another TGF-β family member, BMP-6, appears to increase the size and weight of pellet cultures and to increase the amount of matrix proteoglycan produced [80]. BMP-2 and BMP-9 have also been used in three-dimensional MSC culture systems, such as those seeded in the hydrogel alginate, and under these conditions can induce markers of chondrogenesis [81].
Similar to their role in chondrogenesis during development, the Wnt and Wnt-related family of signaling proteins are also involved in adult cartilage homeostasis. While a number of Wnts have been shown to inhibit chondrogenesis in vitro and in vivo [82,83], we have recently identified Wnt-3a to be chondrostimulatory in mouse C3H10T1/2 cells [84]. In humans, mutations in the Wnt-1-inducible signaling pathway protein3 (WISP-3) are associated with the autosomal recessive disorder progressive pseudorheumatoid dysplasia. Patients with this disorder present primarily with a continual loss of cartilage as they age, which is accompanied by destructive bone changes [85]. WISP-3 is closely related to WISP-1 and WISP-2, both of which are highly expressed in Wnt-1-transformed cells [86]. These WISP proteins are of the same family of proteins as connective tissue growth factor, which is regulated by TGF-β [87]. Interestingly, WISP-3 is expressed in adult human synoviocytes and articular cartilage, and other Wnts, such as Wnt-11, are expressed in developing cartilage [88] and are upregulated during MSC chondrogenesis [89], suggesting the involvement of the Wnt signaling cascade in MSC chondrogenic differentiation. Consistent with this hypothesis, Wnt family members are present in vivo in the joint and in vitro in chondrogenic pellet cultures. Wnt-5a is expressed constitutively in pellet cultures in vitro (GBoland et al., unpublished observation), whereas in rheumatoid arthritis, there is an established connection between elevated expression of Wnt-5a by activated synovium and established disease markers [90]. It is postulated that the presence of activated synoviocytes in the rheumatoid arthritic joint may be due to the migration of MSCs into the tissue, accompanied by high expression of Wnt-5a. In this activated synovium, blockade of Wnt signaling has been shown to lead to a decrease in the level of active cytokines such as IL-6 and IL-15 [90,91].
Other signaling cascades involved in crosstalk with TGF-β include the mitogen-activated protein kinase (MAPK) pathways. Recent reports have implicated p38 MAPK as a downstream target of TGF-β1, BMP-2, and growth/differentiation factor5 (GDF-5) in the chondrogenic differentiation of the mouse cell line ATDC5 [92]. Moreover, in the mouse osteoblastic cell line MC3T3-E1, TGF-β was shown by genetic screening in yeast to activate two novel proteins, TAK1-binding protein (TAB1) and TGF-β-activated kinase (TAK1) [93,94]. Potential downstream targets of activated TAK1 include MKK4/JNKK and MKK3/MAPKK6, which directly activate c-Jun N-terminal kinase (JNK) and p38 MAP kinase, respectively [95,96]. Another MAPK, extracellular signal-regulated kinase (ERK), has also been shown to increase in protein level and activity after TGF-β treatment, thereby contributing to gene expression and regulation [97,98]. Intracellular signals initiated by TGF-β ligand binding are principally mediated by the Smad family of proteins, particularly the receptor-activated Smads (2 and 3), the common-mediator Smad (4), and the inhibitory Smads (6 and 7) [99,100]. Mutations of the TGF-β superfamily genes and their specific receptors in mice have led to multiple skeletal defects [101,102]. More recent studies involving homozygous Smad-3-deficient mice have revealed abnormal hypertrophic differentiation of articular chondrocytes, leading to the progressive loss of articular cartilage resembling the pathology of osteoarthritic degenerative joint disease [103]. In addition, the ERK MAP kinases also phosphorylate the Smad2 proteins via receptor tyrosine kinases, thereby suggesting some crosstalk between the MAP kinase and Smad signaling proteins [104,105]. Indeed, our recent studies have shown that activation of the p38, ERK, and JNK MAP kinases is required for the chondrogenic induction and maintenance of TGF-β1 treated trabecular-bone-derived MSC cultures (Tuli et al., unpublished observation). Inhibition of the individual MAP kinase pathways with specific chemical inhibitors either completely abolished or significantly reduced expression levels of cartilage-specific genes in a pattern distinct to each pathway, thus indicating that p38, ERK, and JNK are independently essential for the TGF-β1-mediated induction of chondrogenesis.
A potential mechanism by which the MAP kinases mediate the effects of TGF-β1 is through the cell–cell adhesion molecule N-cadherin, previously shown to mediate embryonic mesenchymal condensation, a requisite cell–cell interaction in developmental chondrogenesis [106-108]. Treatment of cell pellets with TGF-β1 led to a transient increase in N-cadherin levels, followed by rapid decrease below basal levels (R Tuli et al., unpublished observation). The addition of MAP kinase inhibitors to these TGF-β1-treated cultures led to alterations in N-cadherin protein levels, suggesting regulation of in vitro chondrogenic differentiation of MSCs by cellular signaling as well as mechanisms of interaction similar to those previously identified in embryonic developmental model systems (R Tuli et al., unpublished observation). While the mechanisms of TGF-β-mediated stimulation of chondrogenesis remain incompletely understood, Wnt signaling via the MAP kinases is probably involved. Activation of the Frizzled receptor by Wnt-7a, and the subsequent activation of adenomatous polyposis coli (APC) and β-catenin have been shown to interfere with the progression from precartilage condensation to nodule formation by prolonging the expression of cell adhesion molecules [[109]; Tuli et al., unpublished observations].
At the level of transcriptional regulation, changes in the levels of cellular binding of the transcription factors Sp-1 and AP-2 to their cognate response DNA sequences contained within the proximal promoter region of the gene of a cartilage matrix component, aggrecan, are indeed the targets of TGF-β1-induced MSC chondrogenesis, and alterations of AP-2 binding, but not Sp-1, are mediated by the activity of p38 MAP kinase [110]. These results suggest a possible signal transduction cascade whereby TGF-β1 activation of p38 MAP kinase results in the inhibition of AP-2 DNA binding, resulting in increased expression of the aggrecan gene. Another key factor known to play a role in chondrogenic lineage commitment and differentiation, and in the activation of cartilage-specific genes, is the transcription factor Sox9 [89], whose mRNA levels are increased during chondrogenesis, particularly at early time points (GBoland et al., unpublished observation).
Cartilage tissue engineering
MSC-based repair of full-thickness articular cartilage defects has been attempted in animal models, using various carrier matrices [111-115]. Natural polymers such as collagen have shown promise in early applications. Using autologous MSCs dispersed in a collagen-type-I gel, Wakitani et al. [111] succeeded in repairing full-thickness defects on the weight-bearing surface of medial femoral condyles. The regenerating cartilage was subsequently replaced by bone in a proximal-to-distal fashion until the underlying subchondral bone was completely repaired without disruption of the overlying cartilage.
Use of synthetic polymers in such applications have also been promising, in particular the α-hydroxyesters PLA and PGA and their copolymer, PLGA. Recent work in our laboratory has also tested the efficacy of using such biomaterials, with modifications, in MSC-based cartilage tissue engineering. Caterson et al. recently evaluated the use of an amalgam consisting of PLA and the hydrogel alginate as a three-dimensional carrier for MSC-based cartilage formation in vitro [116]. Alginate significantly improved cell loading and retention within the construct and maintained a round cell shape to enhance the chondrogenic differentiation of MSCs, while PLA provided appropriate mechanical support and stability to the composite culture, suggesting the amalgam as a potential candidate bioactive scaffold. We have also successfully fabricated 'plug-like' cartilage constructs by press-coating PLA polymer blocks onto high-density cell pellets of human MSCs treated with TGF-β1 in a chondrogenic environment. Scanning electron microscopy and histological analysis revealed spatially distinct cellular zones, with the superficial layer resembling hyaline cartilage, and immunohistochemically detectable collagen typeII and cartilage proteoglycan link protein within the extracellular matrix, suggesting the potential utility of this construct for tissue-engineered therapy of articular cartilage defects [117]. Our recent attempts to fabricate a single-unit osteochondral plug on the PLA block using press-coated cartilage followed by seeded osteoblasts, all derived from the same MSC source, have been promising (R Tuli et al., unpublished observation). Recently, Li et al. have developed a novel nanofibrous biomaterial, based on PLGA and poly-ε-caprolactone, by using an electrospinning process to fabricate a unique three-dimensional scaffold with structural similarity to a natural collagen network, as well as the ability to support MSC attachment, proliferation, and differentiation [[118]; Li et al., unpublished observation]. In particular, the slower degradation rate of poly-ε-caprolactone compared with other polyesters may make it a highly suitable candidate biomaterial for the delivery of growth factors such as TGF-β1, and the properties can be further modified by copolymerizing with other polyesters. Such constructs may be applicable for the clinical reconstruction of articular cartilage defects.
Soft tissues
Tendon
In addition to the well-established bone, cartilage, and adipose lineages, the induction of MSC differentiation into other connective tissues, such as muscle, tendons, and ligaments is also being investigated. For tenogenesis, key factors include culture conditions, growth factors, and physical stimulation, such as mechanical loading.
Compared to the osteoblastic and chondrocytic lineages, little is known about the signaling pathways involved in tenogenesis of MSCs. Members of the TGF-β superfamily, specifically the growth/differentiation factors (GDFs), have been implicated in tendon formation. In some animal systems, GDFs5,6, and7 are seen to induce formation of tendon-like tissue upon implantation in vivo [119]. Similar effects have been seen upon adenoviral gene expression of BMP-13 (GDF6) in rats. The aforementioned GDF effects occur ectopically but are similar to the reparative effects seen in GDF treatment of damaged tendons [120,121].
For a tissue-engineering approach, marrow-derived MSCs have been used for Achilles tendon repair. MSCs seeded onto a collagen-type-I construct incorporated into healing tendons that subsequently exhibited greater load-related structural and material properties than unseeded constructs. These MSC-loaded scaffolds had better alignment of cells and collagen fibers and were more similar to the native tendon than unloaded controls [122]. Much of the improvement seen with MSC-loaded constructs was seen at a biochemical level and in maximum stress, modulus, and strain energy density, rather than a histological level, and without much improvement in the microstructure of the tissue itself [123]. Another factor in this process is the initial seeding density of the cells, showing a plateau of density-dependent effect at approximately 4 million cells per milliliter [124].
One important issue concerning cell-based tendon tissue engineering is the mechanical loading and subsequent activation of the forming tissue. While no specific studies addressing this in MSCs are available, information gathered from tendon/ligament fibroblasts strongly suggests that tensile strength and stretch loading are essential for the proper formation and alignment of the tendon or ligament structure [125].
Adipose tissue
In vitro adipogenic induction requires specific medium supplementations, including dexamethasone and 3-isobutyl-1-methylxanthine. Indomethacin, a nonsteroidal anti-inflammatory drug, binds to and activates the transcription factor peroxisome proliferator-activated receptor gamma (PPAR-γ), which is crucial for adipogenesis [126]. Known regulators of adipogenesis include several other transcription factors besides PPAR-γ, such as C/EBP-α and C/EBP-β. Also, during the adipogenic process, Wnt signaling, presumably through Wnt-10b expression by pre-adipocytes, is known to decrease adipogenesis in vitro and to play a role in the cell fate determination of mesenchyme [127]. It is believed that endogenous, canonical Wnt signaling maintains preadipocytes in an undifferentiated state by inhibiting C/EBP-α and PPAR-γ. When Wnt signaling is suppressed in pre-adipocytes and myoblasts, they proceed down the adipogenic lineage [127].
Several groups have also shown the ability of MSCs to interconvert between the adipogenic and osteogenic lineages [128,129]. The concept of interconvertibility is appealing because in vivo the bone marrow progressively adopts a more 'fatty' or adipose-like, versus hematopoietic, structure as a function of age. It has been proposed that the stromal elements of the marrow, perhaps containing MSCs, can differentiate into either the osteogenic or the adipogenic lineage, depending upon microenvironmental cues [128,129].
Muscle
Marrow MSCs have been induced into the myogenic lineage both in vivo and in vitro. While skeletal muscle itself contains stem cells known to be active in regeneration, these cells are distinct from MSCs and the subject is reviewed elsewhere [130]. Examination of the myogenic differentiation of MSCs is currently being applied to cardiac muscle as well as skeletal muscle. In particular, regeneration of cardiomyocytes is the goal of many groups, on the basis of previous experiments showing the induction of murine marrow stem cells into the cardiomyocyte phenotype [14,131]. Some groups have examined the treatment of myocardial infarction by application of autologous MSCs in the pig model, and these studies show engraftment, differentiation, and improved function in animals treated with autologous marrow MSCs [132]. In a recent human study, the intracoronary application of autologous bone-marrow cells after myocardial infarction led to significant improvements of function in comparison with a group given standard therapy. Not only was the infarct region itself much smaller in these patients, but also the level of function of the heart was vastly improved over those receiving only the standard therapeutic interventions [133]. While the exact mechanisms responsible for such phenotypic conversion remain unknown, these findings hold much promise for the future of tissue engineering and regeneration [134].
Mesenchymal stem cells versus embryonic stem cells
Embryonic stem (ES) cells are derived from the inner cell mass of the embryonic blastocyst. These cells can be maintained indefinitely in vitro without loss of differentiation potential, and when reimplanted into a host embryo, they give rise to progenies that differentiate into all tissues. However, much of what is known of EScells is derived from studies performed on the mouse, since human cell lines have only recently become available. Although instructive, such information may not necessarily apply to the capabilities of human EScells, further complicated by the current complexities of ethical issues. Controversies surrounding the legal and moral status of human embryos and the use of EScells encompass fundamental issues such as contraception, abortion, the definition of human life, and the rights and legal status of an embryo. A case in point is the position held by the administration of US president George W Bush, as articulated on August 9, 2001, which limits federal funding to research that uses EScell cultures in existence before that date. Despite such challenging considerations, it is instructive to explore the fundamental biological differences between MSCs and EScells, especially for applications of regenerative medicine.
The transient life span of EScells in vivo is in sharp contrast to that of MSCs, which reside much later into adult life. The seemingly unlimited potential of human EScells to self-renew and differentiate into a large variety of tissues was first characterized by Thomson et al. [135]. Although such cells can be propagated for more than two years with approximately 400 population doubling cycles while maintaining a normal karyotype and full differentiation potential, several key issues remain to be addressed. For example, use of allogeneic cells could involve the potential risks of immunorejection and heterotopic tissue formation (teratomagenesis). These problems could be circumvented using autologous cells created by 'somatic-cell nuclear transfer', but will eventually evoke ethical and legal issues similar to those surrounding reproductive cloning [136]. Adult-derived MSCs, initially thought to be limited in potential to mesenchymal tissues, have been shown to be capable of greater plasticity and transdifferentiation than previously expected [6,11-15,19,20,137,138]. Although MSCs display a finite life span in in vitro culture and approach senescence much more rapidly than ESCs, current techniques for the long-term culture expansion and maintenance of the undifferentiated phenotype of MSCs already allow them to be grown in sufficient number for clinical application [29,43]. Interestingly, another multipotent adult progenitor cell, capable of differentiating at the single-cell level into cells of visceral mesoderm, neuroectoderm, and endoderm in vitro (specifically, cells of the hematopoietic lineage), as well as epithelium of the liver, lung, and gut, was recently copurified along with the MSC from rodent bone marrow [139]. Although the existence of such multipotent adult progenitor cells needs to be confirmed in humans, adult MSCs are likely to offer the same therapeutic potential without evoking the ethical, moral, and legal issues associated with the use of ES cells.
Future of mesenchymal stem cells
To seriously consider the applications of MSCs for regeneration and tissue engineering, two key fundamental questions regarding these cells must be addressed: what exactly are these cells? and what is their endogenous function in their native tissue?
Addressing the question of stem-cell identity requires a focus on the cellular and genetic signature of MSCs. This question needs to be addressed in a similar manner to current analyses of other populations of stem cells. In the case of the hematopoietic stem cell, techniques such as flow cytometry to analyze specific cell-surface markers [140] and methods such as microarray analysis are being applied to establish a phenotypic and genotypic fingerprint of this cell population [141,142]. Moreover, not only MSCs need to be examined, but studies should also include the cells that make up the niche or microenvironment that supports the survival and differentiation of stem cells. These complementary approaches have been used to compare different groups of stem cells in order to identify core 'stem' genes and to examine supportive tissue to understand what genes and pathways are involved not only in stem-cell differentiation, but also in stem-cell support and maintenance.
The second important question addresses the native function of stem cells. These cells must exist in vivo to serve a specific purpose. One of their functions may be to serve as a repository of 'differentiation potentials' – a storehouse of cells waiting to differentiate into the needed lineage depending upon environmental needs and cues. Another possibility is that these cells function as 'director cells', remaining undifferentiated themselves but, once stimulated, actively direct the differentiation of cells around them. Answers to these questions should provide important clues to the basic biology and potential of MSCs. That is, if these cells are intended for regeneration, the undifferentiated state is thus a dormant state until they are called upon to differentiate and replace old or damaged tissue. If, instead, MSCs are director cells, their maintenance in the undifferentiated state is a controlled process and represents the preferred cellular phenotype rather than a waiting state. In this capacity, these cells would have specific and active roles, rather than simply serving as a repository of potential.
Another critical issue is the potential of MSCs. Are they part of a pyramid or a pancake (i.e. do they exist as part of a lineage hierarchy or a lineage web)? Do they undergo the traditional hierarchical differentiation process, or are they, as recent evidence suggests, capable of transdifferentiating from one lineage to another? What is the stage past which these cells lose their plasticity? And where along this path are we catching them? These questions apply not only to MSCs, but also to the larger field of stem-cell research, since there is no current consensus as to whether all these pools of stem cells are separate entities or whether they are all descendants of one common stem cell. Are the true 'stem' cells circulating and homing to tissues as they are needed? Is the same cell being called by many different names – the circulating fibrocyte, the 'bone marrow stem cell', which is often used for either hematopoietic or mesenchymal stem cells, the central nervous system stem cell, the hepatic stem cell, etc.? If many stem cells are found circulating, not only do the specific differentiation cues become important, but the homing mechanisms of the cells to the correct tissue become crucial also. As discussed here, the microenvironment plays a very critical role in MSC development. Growth factors, physical and mechanical stimuli, cell density, and cell–cell interactions all contribute to the end product of differentiation – the cellular phenotype and behavior. An important question to address now is whether these cell fate decisions are due to inductive pathways that become activated, or instead are due to the inactivation of repressive pathways, or both. What is the differentiation baseline of these cells? Are they normally suppressed or normally dormant?
A recent paper describes the ES-cell-like property of a subgroup of marrow-derived stem cells [139]. This raises some intriguing questions about the origins and functions of MSCs. Are these cells a developmental remnant of early embryonic stem cells? If so, what mechanisms operate to allow this particular group of cells to 'escape' developmental cues and remain undifferentiated in the adult organism? It is also known that the regenerative capacity of humans is very different from that of other metazoans and even different from that of other mammals. Are these differences in tissue-regenerative capacity related to the number of MSCs? For example, do axolotls, which are among the most efficient tissue regenerators, have MSCs, and, if so, are they more abundant than in humans? In addition, what is the developmental or evolutionary advantage to the decrease in MSC number? Were these cells slowly recruited from the stem-cell pool to contribute to the increasing complexity and tissue organization of the human system? If so, how can we utilize the potential of our remaining stem cells for tissue regeneration and repair?
In conclusion, MSCs derived from adult tissue present an exciting progenitor cell source for applications of tissue engineering and regenerative medicine. Modalities may include direct implantation and/or ex vivo tissue engineering, in combination with biocompatible/biomimetic biomaterials and/or natural or recombinantly derived biologics. MSCs may also be considered for gene therapy applications for the delivery of genes or gene products. Another intriguing prospect for the future is the use of MSCs to create 'off-the-shelf' tissue banks. To fully harness the potential of these cells, future studies should be directed to ascertain their cellular and molecular characteristics for optimal identification, isolation, and expansion, and to understand the natural, endogenous role(s) of MSCs in normal and abnormal tissue functions.
Abbreviations
APC = adenomatous polyposis coli; bHLH = basic helix–loop–helix; BMP = bone morphogenetic protein; Cbfa1 = core binding factor alpha 1; ECM = extracellular matrix; ERK = extracellular signal-regulated kinase; ES = embryonic stem; GDF = growth/differentiation factor; IBMX = 3-isobutyl-1-methylxanthine; IL = interleukin; JNK = c-Jun N-terminal kinase; LRP = low-density lipoprotein receptor-related peptide; MAPK = mitogen-activated protein kinase; MSC = mesenchymal stem cell; PLA = poly-L-lactide; PLGA = poly-L-lactide-co-glycolide; PPAR-γ = peroxisome proliferator-activated receptor-γ; SMAD = vertebrate homologue of Drosophila Mothers Against Decapentaplegic (MAD); TGF-β = transforming growth factor beta; WISP = Wnt-1-inducible protein.
References
- Lin H. The tao of stem cells in the germline. Annu Rev Genet. 1997;31:455–491. doi: 10.1146/annurev.genet.31.1.455. [DOI] [PubMed] [Google Scholar]
- de Wynter EA, Emmerson AJ, Testa NG. Properties of peripheral blood and cord blood stem cells. Baillière's Best Pract Res Clin Haematol. 1999;12:1–17. doi: 10.1053/beha.1999.0003. [DOI] [PubMed] [Google Scholar]
- Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- Rao MS. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257:137–148. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats – similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9:444–450. doi: 10.1159/000052644. [DOI] [PubMed] [Google Scholar]
- Fukuda K. Molecular characterization of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom Kyoto. 2002;42:1–9. doi: 10.1111/j.1741-4520.2002.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res. 1991;9:465–476. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bosch P, Musgrave DS, Lee JY, Cummins J, Shuler T, Ghivizzani TC, Evans T, Robbins TD, Huard J. Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18:933–944. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, Duenzl M, Lucas PA, Black AC. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- Diefenderfer DL, Brighton CT. Microvascular pericytes express aggrecan message which is regulated by BMP-2. Biochem Biophys Res Commun. 2000;269:172–178. doi: 10.1006/bbrc.2000.2148. [DOI] [PubMed] [Google Scholar]
- Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA., 2nd The pericyte as a possible osteoblast progenitor cell. Clin Orthop. 1992;275:287–299. [PubMed] [Google Scholar]
- Reilly TM, Seldes R, Luchetti W, Brighton CT. Similarities in the phenotypic expression of pericytes and bone cells. Clin Orthop. 1998;346:95–103. [PubMed] [Google Scholar]
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–1069. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Osyczka AM, Noth U, Danielson KG, Tuan RS. Different osteochondral potential of clonal cell lines derived from adult human trabecular bone. Ann N Y Acad Sci. 2002;961:73–77. doi: 10.1111/j.1749-6632.2002.tb03054.x. [DOI] [PubMed] [Google Scholar]
- Tuli R, Seghatoleslami MR, Tuli S, Wang ML, Hozack WJ, Manner PA, Danielson KG, Tuan RS. A simple, high-yield method for obtaining multipotential mesenchymal progenitor cells from trabecular bone. Mol Biotechnol. 2002. [DOI] [PubMed]
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- Dexter TM. Stromal cell associated haemopoiesis. J Cell Physiol. 1982;1(suppl):87–94. doi: 10.1002/jcp.1041130414. [DOI] [PubMed] [Google Scholar]
- Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Keating A, Horsfall W, Hawley RG, Toneguzzo F. Effect of different promoters on expression of genes introduced into hematopoietic and marrow stromal cells by electroporation. Exp Hematol. 1990;18:99–102. [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Friedenstein AJ, Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561–570. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]
- Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546–557. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG, Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nature Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- Osyczka AM, Noth U, O'Connor J, Caterson EJ, Yoon K, Danielson KG, Tuan RS. Multilineage differentiation of adult human bone marrow progenitor cells transduced with human papilloma virus type 16 E6/E7 genes. Calcif Tissue Int. 2002. [DOI] [PubMed]
- Okamoto T, Aoyama T, Nakayama T, Nakamata T, Hosaka T, Nishijo K, Nakamura T, Kiyono T, Toguchida J. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun. 2002;295:354–361. doi: 10.1016/s0006-291x(02)00661-7. [DOI] [PubMed] [Google Scholar]
- Waller EK, Olweus J, Lund-Johansen F, Huang S, Nguyen M, Guo GR, Terstappen L. The "common stem cell" hypothesis reevaluated: human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood. 1995;85:2422–2435. [PubMed] [Google Scholar]
- Simmons PJ, Gronthos S, Zannettino A, Ohta S, Graves S. Isolation, characterization and functional activity of human marrow stromal progenitors in hemopoiesis. Prog Clin Biol Res. 1994;389:271–280. [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002;170:73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Ricalton NS, Boynton RE, Connolly TJ, Jaiswal N, Zaia J, Barry FP. Mesenchymal stem cell surface antigen SB-10 corresponds to activated leukocyte cell adhesion molecule and is involved in osteogenic differentiation. J Bone Miner Res. 1998;13:655–663. doi: 10.1359/jbmr.1998.13.4.655. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton R, Murphy M, Haynesworth S, Zaia J. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun. 2001;289:519–524. doi: 10.1006/bbrc.2001.6013. [DOI] [PubMed] [Google Scholar]
- Walsh S, Jefferiss C, Stewart K, Jordan GR, Screen J, Beresford JN. Expression of the developmental markers STRO-1 and alkaline phosphatase in cultures of human marrow stromal cells: regulation by fibroblast growth factor (FGF)-2 and relationship to the expression of FGF receptors 1–4. Bone. 2000;27:185–195. doi: 10.1016/s8756-3282(00)00319-7. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1α. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bellows CG, Heersche JN, Aubin JE. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev Biol. 1990;140:132–138. doi: 10.1016/0012-1606(90)90060-v. [DOI] [PubMed] [Google Scholar]
- Liu F, Aubin JE, Malaval L. Expression of leukemia inhibitory factor (LIF)/interleukin-6 family cytokines and receptors during in vitro osteogenesis: differential regulation by dexamethasone and LIF. Bone. 2002;31:212–219. doi: 10.1016/s8756-3282(02)00806-2. [DOI] [PubMed] [Google Scholar]
- Chung CH, Golub EE, Forbes E, Tokuoka T, Shapiro IM. Mechanism of action of beta-glycerophosphate on bone cell mineralization. Calcif Tissue Int. 1992;51:305–311. doi: 10.1007/BF00334492. [DOI] [PubMed] [Google Scholar]
- Tenenbaum HC, Limeback H, McCulloch CA, Mamujee H, Sukhu B, Torontali M. Osteogenic phase-specific co-regulation of collagen synthesis and mineralization by beta-glycerophosphate in chick periosteal cultures. Bone. 1992;13:129–138. doi: 10.1016/8756-3282(92)90002-e. [DOI] [PubMed] [Google Scholar]
- Beck GR, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Izumo N, Fukuyama R, Meguro T, Nakamuta H, Kohno T, Koida M. Phosphate provides an extracellular signal that drives nuclear export of Runx2/Cbfa1 in bone cells. Biochem Biophys Res Commun. 2001;280:348–352. doi: 10.1006/bbrc.2000.4108. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) in vitro. Calcif Tissue Int. 1999;65:173–180. doi: 10.1007/s002239900678. [DOI] [PubMed] [Google Scholar]
- Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res. 1997;12:1606–1614. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Rose FR, Oreffo RO. Bone tissue engineering: hope vs hype. Biochem Biophys Res Commun. 2002;292:1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- El-Amin SF, Attawia M, Lu HH, Shah AK, Chang R, Hickok NJ, Tuan RS, Laurencin CT. Integrin expression by human osteoblasts cultured on degradable polymeric materials applicable for tissue engineered bone. J Orthop Res. 2002;20:20–28. doi: 10.1016/S0736-0266(01)00062-6. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- O'Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Cassiede P, Dennis JE, Ma F, Caplan AI. Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-β1 or PDGF-BB as assayed in vivo and in vitro. J Bone Miner Res. 1996;11:1264–1273. doi: 10.1002/jbmr.5650110911. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Colter DC, Prockop DJ. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001;284:411–418. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- Tufan AC, Tuan RS. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J. 2001;15:1436–1438. doi: 10.1096/fj.00-0784fje. [DOI] [PubMed] [Google Scholar]
- Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277:30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- Hurvitz JR, Suwairi WM, Van Hul W, El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM, Herd JK, Van Hul EV, Rezai-Delui H, Legius E, Le Merrer M, Al-Alami J, Bahabri SA, Warman ML. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nature Genet. 1999;23:94–98. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Lako M, Strachan T, Bullen P, Wilson DI, Robson SC, Lindsay S. Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene. 1998;219:101–110. doi: 10.1016/s0378-1119(98)00393-x. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001;44:772–781. doi: 10.1002/1529-0131(200104)44:4<772::AID-ANR133>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F. p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp Cell Res. 1999;250:351–363. doi: 10.1006/excr.1999.4535. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1997;272:8141–8144. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- Mucsi I, Skorecki KL, Goldberg HJ. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-β1 on gene expression. J Biol Chem. 1996;271:16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- Hartsough MT, Mulder KM. Transforming growth factor-β signaling in epithelial cells. Pharmacol Ther. 1997;75:21–41. doi: 10.1016/s0163-7258(97)00020-x. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signaling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- de Caestecker MP, Parks WT, Frank CJ, Castagnino P, Bottaro DP, Roberts AB, Lechleider RJ. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998;12:1587–1592. doi: 10.1101/gad.12.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120:177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- DeLise AM, Tuan RS. Alterations in the spatiotemporal expression pattern and function of N-cadherin inhibit cellular condensation and chondrogenesis of limb mesenchymal cells in vitro. J Cell Biochem. 2002;87:342–359. doi: 10.1002/jcb.10308. [DOI] [PubMed] [Google Scholar]
- DeLise AM, Tuan RS. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Develop Dyn. 2002;225:195–204. doi: 10.1002/dvdy.10151. [DOI] [PubMed] [Google Scholar]
- Stott NS, Jiang TX, Chuong CM. Successive formative stages of precartilaginous mesenchymal condensations in vitro: modulation of cell adhesion by Wnt-7A and BMP-2. J Cell Physiol. 1999;180:314–324. doi: 10.1002/(SICI)1097-4652(199909)180:3<314::AID-JCP2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tuli R, Seghatoleslami MR, Tuli S, Howard MS, Danielson KG, Tuan RS. p38 MAP kinase regulation of AP-2 binding in TGF-beta1-stimulated chondrogenesis of human trabecular bone-derived cells. Ann N Y Acad Sci. 2002;961:172–177. doi: 10.1111/j.1749-6632.2002.tb03077.x. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- Adachi N, Sato K, Usas A, Fu FH, Ochi M, Han CW, Niyibizi C, Huard J. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29:1920–1930. [PubMed] [Google Scholar]
- Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin Orthop. 1997;342:254–269. [PubMed] [Google Scholar]
- Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, Singh HN, Kraus KH, O'Byrne E, Pellas TC. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002;23:109–119. doi: 10.1016/s0142-9612(01)00086-2. [DOI] [PubMed] [Google Scholar]
- Radice M, Brun P, Cortivo R, Scapinelli R, Battaliard C, Abatangelo G. Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J Biomed Mater Res. 2000;50:101–109. doi: 10.1002/(sici)1097-4636(200005)50:2<101::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, Vaccaro AR, Tuan RS. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394–403. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Noth U, Tuli R, Osyczka AM, Danielson KG, Tuan RS. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng. 2002;8:131–144. doi: 10.1089/107632702753503126. [DOI] [PubMed] [Google Scholar]
- Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm GA, Li JZ, Alden TD, Hudson SB, Beres EJ, Cunningham M, Mikkelsen MM, Pittman DD, Kerns KM, Kallmes DF. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg. 2001;95:298–307. doi: 10.3171/jns.2001.95.2.0298. [DOI] [PubMed] [Google Scholar]
- Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–54. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- Awad HA, Butler DL, Harris MT, Ibrahim RE, Wu Y, Young RG, Kadiyala S, Boivin GP. In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res. 2000;51:233–240. doi: 10.1002/(sici)1097-4636(200008)51:2<233::aid-jbm12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hsieh AH, Tsai CM, Ma QJ, Lin T, Banes AJ, Villarreal FJ, Akeson WH, Sung KL. Time-dependent increases in type-III collagen gene expression in medical collateral ligament fibroblasts under cyclic strains. J Orthop Res. 2000;18:220–227. doi: 10.1002/jor.1100180209. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Park SR, Oreffo RO, Triffitt JT. Interconversion potential of cloned human marrow adipocytes in vitro. Bone. 1999;24:549–554. doi: 10.1016/s8756-3282(99)00084-8. [DOI] [PubMed] [Google Scholar]
- Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- Goldring K, Partridge T, Watt D. Muscle stem cells. J Pathol. 2002;197:457–467. doi: 10.1002/path.1157. [DOI] [PubMed] [Google Scholar]
- Fukuda K. Development of regenerative cardiomyocytes from mesenchymal stem cells for cardiovascular tissue engineering. Artif Organs. 2001;25:187–193. doi: 10.1046/j.1525-1594.2001.025003187.x. [DOI] [PubMed] [Google Scholar]
- Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1926. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- Akins RE. Can tissue engineering mend broken hearts? Circ Res. 2002;90:120–122. [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Nippert I. The pros and cons of human therapeutic cloning in the public debate. J Biotechnol. 2002;98:53–60. doi: 10.1016/s0168-1656(02)00085-8. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development. 2002;129:2987–2995. doi: 10.1242/dev.129.12.2987. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Rosu-Myles M, Khandaker M, Wu DM, Keeney M, Foley SR, Howson-Jan K, Yee IC, Fellows F, Kelvin D, Bhatia M. Characterization of chemokine receptors expressed in primitive blood cells during human hematopoietic ontogeny. Stem Cells. 2000;18:374–381. doi: 10.1634/stemcells.18-5-374. [DOI] [PubMed] [Google Scholar]
- Hackney JA, Charbord P, Brunk BP, Stoeckert CJ, Lemischka IR, Moore KA. A molecular profile of a hematopoietic stem cell niche. Proc Natl Acad Sci U S A. 2002;99:13061–13066. doi: 10.1073/pnas.192124499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Bianco P, Costantini M, Dearden LC, Bonucci E. Alkaline phosphatase positive precursors of adipocytes in the human bone marrow. Br J Haematol. 1988;68:401–403. doi: 10.1111/j.1365-2141.1988.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]


