Abstract
The crystal structure of a collagen-binding domain (CBD) with an N-terminal domain linker from Clostridium histolyticum class I collagenase was determined at 1.00 Å resolution in the absence of calcium (1NQJ) and at 1.65 Å resolution in the presence of calcium (1NQD). The mature enzyme is composed of four domains: a metalloprotease domain, a spacing domain and two CBDs. A 12-residue-long linker is found at the N-terminus of each CBD. In the absence of calcium, the CBD reveals a β-sheet sandwich fold with the linker adopting an α-helix. The addition of calcium unwinds the linker and anchors it to the distal side of the sandwich as a new β-strand. The conformational change of the linker upon calcium binding is confirmed by changes in the Stokes and hydrodynamic radii as measured by size exclusion chromatography and by dynamic light scattering with and without calcium. Furthermore, extensive mutagenesis of conserved surface residues and collagen-binding studies allow us to identify the collagen-binding surface of the protein and propose likely collagen–protein binding models.
Keywords: calcium-induced conformational change/domain reorientation/extracellular calcium-binding protein/molecular switch/square antiprismatic
Introduction
Gas gangrene is an infectious disease caused by a group of anaerobic spore-forming bacteria, histotoxic clostridia. They produce collagenases responsible for extensive tissue destruction. Enzymes produced by Clostridium histolyticum have been widely studied (Mookhtiar and van Wart, 1992). They are grouped into class I and class II, and are encoded by distinct chromosomal genes, colG and colH (Yoshihara et al., 1994; Matsushita et al., 1999). The full-length class I enzyme (ColG) possesses tandem collagen-binding domains (CBDs) at its C-terminus, while the class II enzyme (ColH) contains only one. These three domains are homologous to each other, consist of ∼110 amino acid residues and bind to various types of insoluble collagens (Matsushita et al., 1998; Toyoshima et al., 2001) by recognizing the triple-helical conformation of collagen (Matsushita et al., 2001). Calcium is known to be necessary for collagenolysis (Worthington Biochemical Co., 1988) and we have previously shown that the ion enhances the binding of CBD to collagen and collagenous peptides (Matsushita et al., 2001).
A plethora of collagen-binding protein structures are now available and reveal distinct morphologies and pathologies. Mammalian collagen-binding proteins including matrix metalloproteases (MMPs) aid in tissue remodeling and wound healing (Pilcher et al., 1999; Vogel, 2001). When unchecked, they can cause arthritis and promote tumor invasion. Bacterial CBDs are much smaller than eukaryotic CBDs (e.g. ∼200 amino acid residues for hemopexin-like domains in MMPs compared with ∼100 amino acids for clostridial CBDs). The mechanism of how bacteria evolved to recognize a eukaryote-specific molecule is unclear despite ample sequence information generated by genomics efforts. Thus far two families of bacterial CBDs have been characterized. Clostridial CBDs are all found as components of collagenases, and staphylococcal CBDs mediate bacterial adherence to collagen.
The clostridial CBD is currently being tested as a novel drug-delivering vehicle. There are diverse clinical applications for autocrine/paracrine peptide signaling molecules such as growth factors. However, they are easily washed out by the circulation, and hence their limited target specificity and short half-lives in vivo make their therapeutic effects unpredictable. As collagen is the primary component of the mammalian extracellular matrix, it is possible to anchor signaling molecules to the extracellular matrix, by linking them to a CBD (Nishi et al., 1998). A CBD-fusion protein carrying the basic fibroblast growth factor strongly stimulated fibroblast growth in mice at an injection site for up to 10 days (Nishi et al., 1998). To develop a drug delivery system by rational drug design, it is essential to understand how CBD interacts with collagen. Since clostridal CBD is the smallest protein to recognize insoluble collagen, it is suitable both for basic and application studies.
Here we report crystal structures of CBD with its N-terminal 12-residue-long domain linker still attached, both in the absence of calcium (apo-CBD) and in the presence of calcium (holo-CBD). We also propose its mode of binding and interaction with the collagen substrate and the role of calcium based on crystallographic, mutagenesis, gel filtration, fluorescence and collagen-binding assay results.
Results and discussion
Overall structure of the collagen-binding domain
The high-resolution apo-CBD structure (without calcium) forms a β-sandwich ‘jelly-roll’ composed of ten β-strands (Figure 1). Strands B, D, F′, G and I make up a predominantly hydrophilic backside of the protein while strands A, C, E, F and H make up a tyrosine-rich face of the protein structure (Figure 2A). The CBD with N-terminal linker crystallizes as a dimer with the tyrosine-rich surfaces oriented toward one another. One molecule of the asymmetric unit (ASU) reveals the N-terminal linker (residues 895–908) as an α-helix while the linker of the other molecule cannot be resolved prior to Lys908 (numbering is that for the mature ColG collagenase sequence). Unlike the typical jelly-roll structure, CBD contains two parallel β-strands (A and C) at one edge of the sandwich. The loop formed by residues 961–967 in one molecule is not clearly resolved and suggests inherent flexibility. In apo-CBD, residues Lys898, Glu899 and Asn902 of the helix interact with residues Glu961 and Asn963 of a loop region through three hydrogen bonds and may provide stability for both regions. The structure was refined to 1.00 Å, with Rfree = 16.6% and R = 14.2% with excellent geometry (Figure 3; Table I). The N-terminal helix was well ordered with an average B-factor of 14.0 Å2 for residues 895–908 compared with 8.1 Å2 for the rest of the molecule.
Fig. 1. Schematic representation of CBD. Apo-CBD (without calcium) has an N-terminal α-helix (α1) while holo-CBD (with calcium bound) has a β-strand (A′) and a 310 helix (η1). In both structures, G971 forces strand F to flip to the opposite face of the sandwich.
Fig. 2. CBD in the presence and absence of calcium. (A) In the high resolution apo-CBD structure, no calcium was observed, and the N-terminus residues 895–908 adopted an α-helix conformation (colored red) in one molecule of the ASU. (B) In the holo-CBD structure, two Ca2+ ions were found (colored orange), and the N-terminus residues 890–908 continued the β-sheet on the back face of the protein. Residues N963–N966 were not resolved. The tyrosine-rich surface is at the left and the back face is at the right of each molecule.
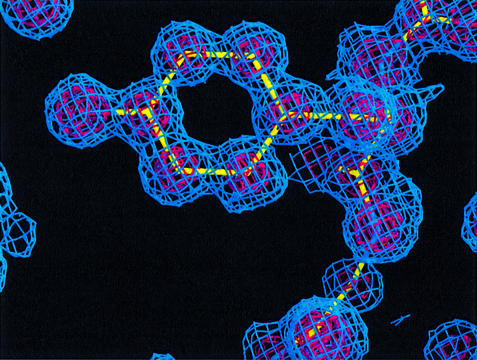
Fig. 3. 2Fo–Fc map of apo-CBD centered on Tyr994 and contoured at 1.25 (blue) and 5 (red) sigma. Tyr994 is the most critical residue in collagen binding.
Table I. Data collection and refinement statistics.
| Se-MET | Se-MET | Se-MET | Apo-CBD | Holo-CBD | Apo-Y990A | Holo-Y990A | |
|---|---|---|---|---|---|---|---|
| Wavelength (Å) | 0.97981 | 0.98013 | 0.94979 | 1.00000 | 0.97950 | 0.94800 | 1.54178 |
| dmin (Å) | 2.00 | 2.00 | 2.00 | 1.0 | 1.65 | 2.3 | 2.85 |
| Completeness (%)a | 99.9 (99.9) | 99.9 (99.9) | 99.9 (99.9) | 91.7 (72.64) | 98.6 (90.9) | 99.5 (99.5) | 99.7 (99.8) |
| I/σIa | 13.51 (3.45) | 11.40 (2.54) | 14.06 (3.34) | 30.70 (2.94) | 12.64 (4.36) | 4.1 (0.9) | 6.93 (1.88) |
|
Rsym (%)b |
15.8 |
12.6 |
17.2 |
7.4 |
5.8 |
10.7 |
15.3 |
| Refinement |
|
|
|
|
|||
| Resolution (Å) | 28.9–1.00 | 8–1.65 | 20–2.3 | 20–2.85 | |||
| R-factor (%) | 14.2 | 19.1 | 27.0 | 25.5 | |||
| Rfree (%) 10% of data | 16.6 | 25.4 | 35.5 | 36.9 | |||
| Ramachandran statistics | |||||||
| Most favorable (%) | 94.4 | 91.1 | 87.2 | 81.8 | |||
| Additionally allowed (%) | 5.6 | 8.9 | 12.3 | 18.2 | |||
| Disallowed (%) | 0.0 | 0.0 | 0.0 | 0.0 |
aData for the highest resolution shell are given in parentheses.
bRsym = Σ|I – <I>|/Σ|I|
The holo-CBD structure, crystallized from similar conditions to apo-CBD, reveals the coordination of two Ca2+ ions per molecule. The linker region that appeared as an α-helix in the apo-CBD structure has uncoiled and the peptide chain loops to the backside of the molecule as a new parallel β-strand (Figure 2B). The binding of Ca2+ forces two uncommon structural elements on holo-CBD. Residues 906–909 form a short 310 helix and the newly formed calcium-binding loop forces the bond between Glu901 and Asn902 into a cis-peptide conformation. The two molecules in the ASU are virtually identical; however, one molecule in the ASU has loop residues 963–966 unresolved, while 966 is resolved in the other with only 963–965 unresolved. A side-chain oxygen of Asp966 interacts with the amino nitrogen of Gly975 in the neighboring molecule, allowing the additional residue to be resolved. The two molecules in the ASU have a main-chain root mean square deviation (r.m.s.d.) of 0.36 Å.
The structural differences between apo-CBD and holo-CBD are both dramatic and subtle. The most significant structural change occurs in the N-terminal linker. For the holo-CBD structure, residues Lys908 and Asp907 start into a 310 helix, but the chain quickly loops back and coordinates the Ca2+ ions. It has been suggested that the 310 helix is a precursor to the formation of an α-helix (Wu and Zhao, 2001), which we observe in the apo-CBD structure. Less obvious is the change in the 961–968 loop region due to it being poorly resolved. Similar to apo-CBD, in holo-CBD, this loop is better resolved in one molecule of the ASU than the other. In apo-CBD, the loop is stabilized by the α-helix while an intermolecular hydrogen bond stabilizes Asp966 in holo-CBD. Finally, the side-chain χ1 angle of residue Tyr931 has rotated 64° toward the center of the tyrosine-rich face. This tyrosine orientation in holo-CBD is energetically unfavorable but necessary to accommodate the new structural region created by 904–908. Apo-CBD and holo-CBD structures are nearly identical excluding the loop region 961–968 with r.m.s.d. of 0.45 Å.
The role of calcium binding at the N-terminal linker
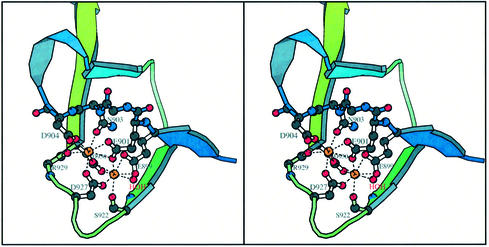
The structures of CBD reveal great variability in the N-terminus. That the N-terminal linker in apo-CBD is not resolvable up to Lys908 in one molecule of the ASU suggests that the linker is mobile in the absence of Ca2+; it adopts a β-strand in the presence of Ca2+. During the course of the Y990A structure refinement, two strong peaks were observed in each independent protein molecule. Based on their coordination and known role in protein activation, these peaks were modeled as Ca2+. Subsequently, wild-type crystals were grown in the presence of Ca2+ to ensure incorporation. The Ca2+ ions are well resolved with temperature factors <15 Å2. The cations bind between two loops and are mostly solvent inaccessible. Oxygen atoms from the side chains of residues Glu901 (both Oε1 and Oε2), Asn903, Asp904, Asp927, Asp930 and main-chain carbonyl of Arg929 form a pentagonal bipyramidal coordination around one Ca2+ ion (Figure 4). The geometric arrangement of the ligand set agrees with typical pentagonal bipyramidals, as observed in other calcium-binding proteins (McPhalen et al., 1991; Busch et al., 2000). The oxygen atoms of residues Glu899, Glu901, Asp927 and Asp930 side chains, main-chain carbonyl of Ser922 and one water form a coordination, best described as square antiprismatic. The water, Glu901 and Glu899 (providing two bonds) form one face while Ser922, Asp927 and Asp930 (providing two bonds) form the other face (Figure 4). Since Glu899 and Asp930 provide multiple bonds, the faces deviate from square geometry. The peptide bond between residues Glu901 and Asn902 adopts an uncommon and energetically unfavorable cis-peptide conformation that must be stabilized by calcium chelation. We note that in multiple homologous proteins, the residue at position 902 is Pro (Figure 5), and the peptide bond prior to Pro can adopt a cis conformation with relative ease. Our dual ion structure is dissimilar from that in all other calcium-binding proteins and represents a new motif.
Fig. 4. Stereo diagram of the dual-calcium-binding site. Holo-CBD is shown with coordinated Ca2+ ions (orange spheres). Calcium-binding residues are in ‘ball-and-stick’ rendering. The orientation is similar to that of Figure 2. The calcium on the right is coordinated to ligands with near square antiprismatic geometry while the one on the left is coordinated to them with near pentagonal bipyramidal geometry. This figure was prepared using Molscript (Kraulis, 1991).
Fig. 5. Multiple sequence alignment of homologous proteins using MULTALIN scoring. Red triangles indicate residues whose side chain binds calcium, and orange triangles show those with main-chain carbonyl-binding calcium. Residues when mutated to alanine that show >5-fold reduction in collagen-binding activity are indicated with blue stars. Secondary structure features are for (top) apo-CBD and (bottom) holo-CBD. The sequence numbers are for CBD S3b in the mature ColG. The first six sequences were found using BLAST (Altschul et al., 1997). The top five are known collagen-binding proteins. The Bacillus licheniformis protein is a cocaine esterase and the other three Bacillus and Deinococcus proteins are uncharacterized. The figure was prepared using ESPript (Gouet et al., 1999).
Structural changes upon calcium binding were tracked via measurements of the apparent molecular mass by size exclusion chromatography to provide information on the Stokes radius of CBD. After dialysis in HEPES-buffered saline (HBS) containing 1 mM EGTA, the apparent molecular mass of wild-type CBD increased by ∼38% (Table II). When this calcium-depleted CBD was back-dialyzed versus HBS containing 1 mM Ca2+, the apparent mass reduced back to essentially the same value as that without EGTA treatment (11.95 ± 0.01 kDa). Thus, the N-terminal conformational change seems to be reversible. This observation coincides with a previous result from dynamic laser light scattering that the hydrodynamic radius is increased by ∼12% when 1 mM EGTA was added instead of calcium, which corresponds to an ∼32% increase in the estimated molecular mass (Matsushita et al., 2001). Alanine mutations of Ca2+ chelating residues showed significant increases to apparent mass in the presence of 1 mM calcium. As a control, mutations in the non-calcium-binding residues examined did not alter the value significantly. The apparent mass of all proteins converged towards that of wild-type apo-CBD when calcium was removed with EGTA.
Table II. Apparent molecular mass of wild-type and mutant CBDs in HBS supplemented with 1 mM Ca2+ or EGTA measured by size exclusion chromatography.
| Apparent molecular mass (kDa) |
||
|---|---|---|
| Mutation | 1 mM Ca2+ | 1 mM EGTA |
| Wild-type | 11.92 ± 0.07 | 16.43 ± 0.02 |
| E899Aa | 14.39 ± 0.02 | 15.89 ± 0.01 |
| E901Aa | 13.27 ± 0.04 | 15.96 ± 0.01 |
| N903Aa | 15.73 ± 0.03 | 16.53 ± 0.01 |
| D904Aa | 13.75 ± 0.07 | 16.16 ± 0.02 |
| S906A | 11.75 ± 0.01 | 16.41 ± 0.03 |
| S906F | 11.47 ± 0.05 | 16.65 ± 0.01 |
| D927Aa | 15.79 ± 0.03 | 16.19 ± 0.01 |
| D930Aa | 15.37 ± 0.01 | 15.42 ± 0.01 |
| Q972A | 11.81 ± 0.01 | 16.47 ± 0.02 |
| V973A | 11.91 ± 0.02 | 16.51 ± 0.02 |
The values represent the average of triplicate trials plus deviations.
aResidue determined via crystallography to bind Ca2+.
Since the calcium-binding site places two Ca2+ ions unusually close to each other, mutations which would destabilize binding between either one or both of the Ca2+ ions were made in order to study thoroughly the impact of calcium on collagen binding (Table III). Asp927 and Asp930 individually interact with both Ca2+ ions, and their individual mutation to Ala only caused a two-fold reduction in binding activity, while the Stokes radius determined in the presence of Ca2+ was approximately the same as that of wild-type apo-CBD. Mutations of residues interacting with only one Ca2+ had little effect on collagen binding, yet they too show large increases in Stokes radius over that of holo-CBD. The binding to insoluble type I collagen coincided with the binding to a collagenous peptide (data not shown). The N-terminal truncated CBD, Δ905, lacks four residues—Glu899, Glu901, Asn903 and Asp904—which all help form one side of the calcium-binding cage, yet collagen-binding only decreased two-fold. Therefore we conclude that the N-terminal region of CBD is not an essential structural component for collagen binding or recognition but rather a cation-controlled multi-conformational domain-linker.
Table III. Effect of various mutations on binding to a mini-collagen peptide.
| Mutation | KD (× 10–5 M) | Ratio |
|---|---|---|
| Wild type | 5.72 ± 0.473 | 1 |
| E899Aa | 6.57 ± 0.533 | 1.15 |
| E901Aa | 5.85 ± 0.548 | 1.02 |
| D904Aa | 5.98 ± 0.528 | 1.05 |
| Δ905 | 12.6 ± 0.657 | 2.20 |
| Δ905-EGTAb | 12.7 ± 0.719 | 2.22 |
| S906A | 6.10 ± 0.573 | 1.07 |
| D927Aa | 14.9 ± 0.595 | 2.60 |
| R929A | 2.52 ± 0.295 | 0.441 |
| D930Aa | 14.3 ± 0.578 | 2.50 |
| Y931A | 10.0 ± 0.488 | 1.75 |
| E935A | 6.07 ± 0.505 | 1.06 |
| E941A | 5.82 ± 0.481 | 1.02 |
| F952Ac | 2.37 ± 0.346 | 0.414 |
| T955A | 6.11 ± 0.690 | 1.07 |
| T957A | 57.0 ± 2.40 | 9.97 |
| H959A | 11.7 ± 0.680 | 2.05 |
| N963A | 5.23 ± 0.452 | 0.914 |
| D966A | 5.07 ± 0.423 | 0.886 |
| I968A | 9.53 ± 0.579 | 1.67 |
| Y970A | 70.8 ± 2.59 | 12.4 |
| Q972A | 7.01 ± 0.581 | 1.23 |
| V973A | 3.99 ± 0.546 | 0.698 |
| D974A | 4.88 ± 0.503 | 0.853 |
| V978Ac | 2.56 ± 0.472 | 0.448 |
| K983A | 6.90 ± 0.579 | 1.21 |
| K988A | 7.75 ± 0.560 | 1.35 |
| Y990A | 6.46 ± 0.518 | 1.13 |
| L992A | 28.9 ± 1.12 | 5.05 |
| Y994A | d | |
| Y994F | 66.2 ± 1.15 | 11.6 |
| Y994S | d | |
| Y996A | 233 ± 271 | 40.7 |
CBD proteins were dissolved in HBS-Ca at varying concentrations from 1 × 10–7 M to 3 × 10–4 M. Binding to the mini-collagen peptide, Gly-(Pro-Hyp-Gly)8, was measured by surface plasmon resonance. Data were directly fit by least squares method to calculate values for apparent dissociation constant (KD) and the uncertainty.
aMutation of an amino acid residue crystallographically shown to bind Ca2+.
bMeasurement conducted in the presence of 1 mM EGTA.
cA core-oriented residue.
dValues of dissociation constant could not be determined due to low binding.
To estimate the dissociation constant for Ca2+, the apparent molecular mass of wild-type CBD was determined by size-exclusion chromatography at varying con centrations of Ca2+ (Figure 6A). At all concentrations examined, CBD eluted as a single peak, suggesting a rapid equilibrium between the Ca2+-bound form and the Ca2+-free form. Thus, respective observed values are the weighted averages of values contributed by the two forms in equilibrium. Since the two Ca2+ ions are located so close together, only 3.7Å from one another, obtaining the dissociation constant for each is quite difficult. However, we can calculate an ‘apparent’ KD value. Assuming that there is only one Ca2+ ion present (thus ‘apparent’), we calculated the KD value of this virtual Ca2+ ion to be 4.99 ± 1.23 × 10–6 M, with an R2 value of 0.984. From natural fluorescence measurements (Figure 6B), the value was calculated to be 3.67 ± 0.54 × 10–6 M, with an R2 value of 0.994. Although the experimental methods are quite different in their nature (apparent molecular mass and fluorescence intensity), the estimated KD values agree closely. The binding of CBD to a collagenous peptide and insoluble collagen at varying concentrations of Ca2+ paralleled well with these parameters (Figure 6C and D).
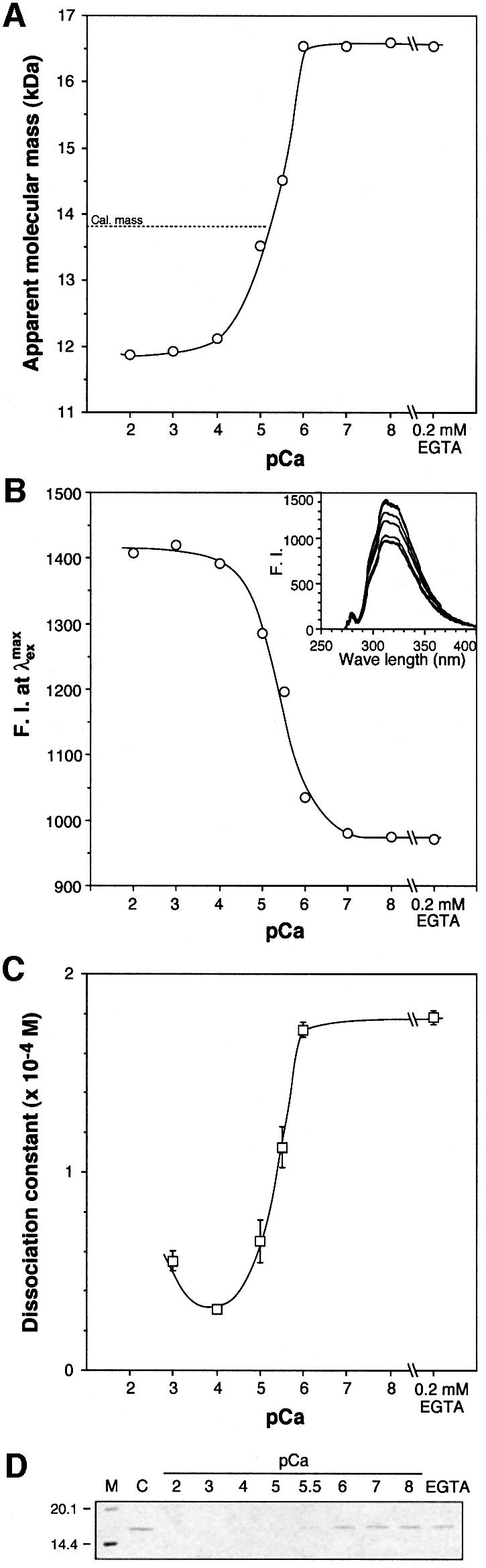
Fig. 6. Binding of CBD to Ca2+ ion and the substrates. All the experiments were carried out in HBS-based calcium buffers with varying pCa. (A) Apparent molecular mass of CBD measured by size exclusion chromatography. (B) Internal fluorescence of CBD. (C) Binding constant of CBD to a collagenous peptide measured by surface plasmon resonance. (D) Binding of CBD to insoluble collagen. After allowing CBD to bind to insoluble type I collagen, the protein left in the water phase was analyzed by SDS–PAGE.
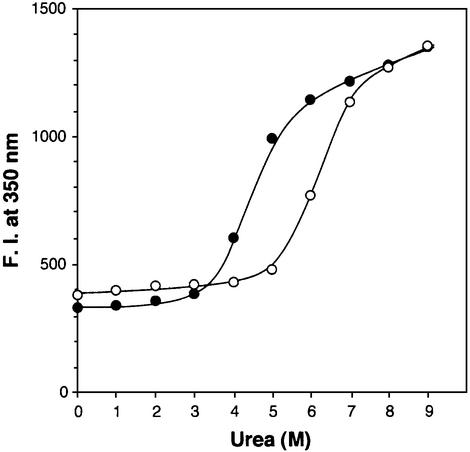
Collagenase is an extracellular protein. The intracellular Ca2+ concentration of Clostridium is likely to be similar to that of Escherichia coli (0.2–0.3 × 10–6 M) (Holland et al., 1999), which is well below CBD’s estimated KD value for Ca2+. However, extracellular mammalian tissue fluids contain Ca2+ concentrations at ∼1.2 mM (Maurer and Hohenester, 1997) with local concentrations down to 0.1 mM (Nicholson, 1980; Brown et al., 1995). Apo-CBD is significantly less stable than holo-CBD. When apo-CBD was incubated in varying concentrations of urea, it denatured at a 1.5 M less concentration of urea than holo-CBD (Figure 7). Thus, we speculate that the relative flexibility within a domain and/or between domains in the absence of calcium allows the enzyme to be efficiently secreted from the cell. At the extracellular matrix, calcium binds to the linker region to help CBD molecules to form a more rigid structure. In contrast, for the collagen-binding protein BM-40, which is also secreted, calcium binding is apparently crucial for its biosynthesis and processing in the endoplasmic reticulum or Golgi apparatus prior to secretion (Busch et al., 2000).
Fig. 7. Urea denaturation. CBD protein was dissolved in HBS supplemented with 0.2 mM EGTA (closed circles) or 1.0 mM excess CaCl2 (open circles) containing varying concentrations of urea. Internal fluorescence was measured by using an excitation wavelength of 290 nm with emission at 350 nm. Standard deviations of the triplicate measurement were too small to show in this figure.
In the full-length class I collagenase, ColG, CBD is duplicated in the C-terminal region. CBD solved in this study, S3b, is linked to another homologous CBD (S3a) at its N-terminus. It has been found that the tandem CBD–CBD (S3a3b) more efficiently binds to insoluble collagen than does CBD (S3b) alone (Matsushita et al., 2001). Recently, we determined the nucleotide sequences of collagenase genes from six more clostridial species (O.Matsushita, J.J.Wilson, S.-i.Nakamura, S.Miyata, E.Tamai and A.Okabe, manuscript in preparation), including a collagenase from Clostridium sporogenes with a triple CBD repeat. The new calcium-binding motif is almost perfectly conserved among 12 clostridial CBDs, with the only exception being C-terminal tandem CBDs from Clostridium septicum. In the presence of Ca2+, the apparent molecular mass of the ColG tandem CBDs (S3a3b) decreased by >30% versus the apparent molecular mass in the presence of EGTA. The observed change in the size of the protein suggests an associated change in quaternary structure. Therefore, we speculate that the Ca2+ dictates the relative orientation of those domains linked to the CBDs in the class I collagenase and homologous collagenases. Our sequence analyses show that the calcium-binding pattern EXEXN[DN]X(4)AX(15,25) [DNS]XXD, based on homology with other clostridial collagenases, is found in human APOBEC-1 complementation factor as well as a few HSP70 molecules and a NARG protein from Staphylococcus carnosus. The dual calcium-binding motif found in CBD may be involved in domain reorientation in diverse organisms.
As a footnote, strong electron density appears near the calcium-binding residues in apo-CBD. Due to the extremely short distances between these peaks and known calcium-binding residues (1.9–2.1 Å), and their near tetrahedral coordination, a monovalent ion such as lithium may be occupying the position. Both mutagenesis studies and structural results support the idea that the calcium-binding residues enable a compact conformation on binding calcium to facilitate the interaction with collagen; however, a small monovalent cation may support a more flexible conformation due to the absence of high order coordination. A competition between divalent and monovalent ions could be controlling the secondary structure of the linker.
Collagen–CBD interaction
Extensive mutagenesis of the conserved surface residues and other surface residues in conjunction with the collagen-binding studies were carried out to identify the protein’s collagen-binding surface (Table III). As mentioned above, the linker-free CBD, Δ905, binds securely to collagen and, therefore, the linker is relatively unimportant in collagen interaction. Multiple sequence alignment identified conserved tyrosine residues 990, 994 and 996 as potentially being solvent exposed and important in collagen-binding activity (Figure 5). In the structure all of the conserved residues are located on the tyrosine-rich face (Figure 8A). Although well conserved, the buried Tyr990 probably contributes little to collagen binding; the Y990A mutation had no effect (Table III). Five residues (Thr957, Tyr970, Leu992, Tyr994 and Tyr996) that showed a significant reduction in collagen-binding activity on mutation were all centrally located on one face of the CBD structure (Figure 8A). Interestingly, Leu992 is not conserved among homologous proteins and Thr957 is only marginally conserved. The helix-to-sheet structure change clearly removes the N-terminal region from the collagen-binding face (Figure 2).
Fig. 8. Proposed CBD–collagen interactions. The five key residues showing >5-fold reduction in activity upon mutation are in red, and yellow residues showed <2-fold. The collagen helix is in a purple stick rendering. The image is ∼90° rotated on a vertical axis from that in Figure 2B. (A) CPK rendering of holo-CBD with important residues labeled. (B–D) Conolley surface representation of holo-CBD with collagen docking models. The orientation of collagen in (C) is nearly 180° from that in (B).
Mutations of Tyr994, absolutely conserved among clostridial CBDs, showed the greatest influence on collagen binding. Loss of the tyrosine hydroxyl group in Y994F decreased activity by ∼12-fold, while removal of the aromatic side chain in Y994A reduced activity to immeasurable levels. Since the triple-helical peptides (Pro-Hyp-Gly)8 and (Pro-Pro-Gly)8 bind tightly to CBD, the hydroxyl group of the Tyr994 is likely to be hydrogen binding to the main-chain atoms rather than to the hydroxyl group of Hyp as expected for a protein–collagen complex (Perona et al., 1997). Mutagenesis has allowed identification of Tyr994 as the ‘hot-spot’ for the protein– protein interface. According to Bogan and Thorn (1998), the protein–protein interface hot-spot is composed of a small set of important residues often enriched in tryptophan, tyrosine and arginine surrounded by energetically less important residues. Alanine mutations of less important residues whose role is to occlude bulk solvent from the hot-spot often do not significantly affect binding.
Of the three residues shown to increase collagen-binding activity upon mutation, only Arg929 is a surface residue. In holo-CBD Arg929 adopts one of the two orientations found in the apo-CBD structure, and mutation to alanine may reduce unfavorable interactions with collagen due to reduced functional group rotational freedom and steric hindrance. For the non-conserved core-oriented residue Phe952, the mutation to alanine most likely rearranges core packing by giving neighboring Lys948 and Lys995 more room. The weakly conserved Val978 is also core oriented, and the V978A mutation probably improves packing.
Protein docking was used to predict possible orientations of collagen across CBD. A model tri-peptide (PPG)6 was created and docked to holo-CBD. The solutions were filtered to select binding models consistent with the five key residues known to interact with collagen. Of the top ten solutions, the highest scoring orientation appeared eight times with only slight variations (Figure 8B). Another of the top ten solutions placed the collagen molecule in the opposite direction from the major solution (Figure 8C). The final orientation among the top ten allowed collagen to interact with His959 in a skewed orientation (Figure 8D). When the collagen mimic was docked to the apo-CBD structure, six of the top twenty solutions placed collagen in an orientation similar to that in Figure 8D with the collagen triple helix terminating into the protein’s α-helix. For apo-CBD the orientations in Figure 8B and C were observed eight and six times, respectively. Unfortunately, the calculation cannot take into account a possible contribution from bridging H2O nor the flexibility of the N-terminus in the absence of calcium.
Other collagen-binding proteins
CBD shares no sequence or structural homology to any eukaryotic collagen-binding proteins. For wound repair, both the inserted domain from multiple integrin α-chains (α1, α2, α10 and α11) and the homologous von Willebrand Factor (vWF) A3 domain adopt a ‘dinucleotide-binding’ fold (Emsley et al., 1997, 1998, 2000; Huizinga et al., 1997; Legge et al., 2000). The integrin α-I domain proteins contain a collagen-binding edge with a metal ion-dependent adhesion site (MIDAS) motif on the top face (Emsley et al., 2000). Tertiary structural changes upon the change of metal coordination aid collagen-binding activity and extend to the far side of the domain. vWF also binds collagen but lacks the MIDAS motif found in integrin.
Prokaryotic organisms also produce proteins with CBDs, but only one structure besides CBD has been solved and characterized for collagen interaction. As a surface protein, the Cna protein allows Staphylococcus aureus to adhere tightly to connective tissues (Symersky et al., 1997; Rich et al., 1999). The S.aureus Cna A domain binds collagen via a trench across a β-sandwich in Greek-key motif and divalent ions do not affect Cna A domain collagen-binding activity (Symersky et al., 1997; Rich et al., 1999). While CBD has a central hot-spot, the Cna A domain has collagen-binding residues spread across the binding surface. Although they are both β-sandwiches, CBD and the Cna A domain do not share the same fold nor any common collagen-binding residues and, therefore, are likely to be evolutionarily unrelated. Clearly, diverse proteins independently evolved to interact with the collagen molecule which is relatively simple and ordered in structure.
Conclusions
Calcium causes a dramatic change in the secondary structure of CBD. In the absence of Ca2+, the CBD reveals a β-sheet sandwich fold with the N-terminal linker adopting an α-helix. The addition of Ca2+ unwinds the linker and anchors it to the backside of the sandwich as a β-strand. One Ca2+ is coordinated to the protein in a typical pentagonal bipyramidal orientation, while the other is coordinated in an atypical square antiprismatic geometry. In vitro, the N-terminal linker acts as a calcium-activated molecular switch that triggers domain reorientation for the mature collagenase. The estimated calcium-binding constant for CBD suggests that in vivo, extracellular calcium could trigger the switch. Tyr994 is the critical residue, ‘hot-spot’, in collagen interaction, and it is surrounded by four energetically less critical residues whose role might be to occlude solvent molecules from the hot-spot. Mutagenesis results of CBD and collagen-like peptides allow us to suggest that the strictly conserved Tyr994 most likely forms a hydrogen bond to a collagen backbone.
Materials and methods
Production and purification of collagen-binding domain
Monomeric and tandem CBDs (S3b, Asn894–Lys1008; S3a3b, Thr773–Lys1008) derived from the C.histolyticum class I collagenase (ColG) were expressed as glutathione S-transferase (GST)-fusion proteins as described previously (Matsushita et al., 2001). For gel-filtration, fluorescence and collagen-binding studies, the flow-through fraction was dialyzed four times against one liter of 10 mM HEPES, 150 mM NaCl, 0.2 mM EGTA pH 7.4 and concentrated by ultrafiltration using Centriprep 3 devices (Millipore, Bedford, MA). For X-ray crystallography, the flow-through CBD (S3b) fraction was further purified by ion-exchange chromatography. The fraction was dialyzed four times against 1 l of 50 mM Tris–HCl (pH 7.5), and then applied to a Q–Sepharose column (bed volume, 2 ml; Pharmacia) equilibrated with the same buffer. The flow-through fractions were pooled, concentrated by ultrafiltration using a Centriprep 3 device, and stored at –80°C till use. Apo-CBD crystals were obtained when an additional dialysis against phosphate-buffered saline was performed prior to the concentration. Labeling of CBD protein with selenomethionine (Se-MET) was carried out as described by Van Duyne et al. (1993), except that the recombinant E.coli cells were grown in Tanaka minimal medium containing glucose (0.2%) and ampicillin (100 µg/ml). Purification of the labeled protein, thrombin cleavage and removal of GST were carried out as described above.
Mutagenesis
Various site-directed mutations were introduced to the CBD-encoding gene by PCR using a plasmid DNA encoding GST–CBD as a template, and 5′- and 3′-pGEX primers (Pharmacia). A list for mutagenic primers is available upon request. The mutated CBD-encoding fragment was digested with SmaI and XhoI, and inserted between the corresponding sites in pGEX4T-2 vector (Pharmacia). After the mutation was confirmed by nucleotide sequencing, the recombinant plasmid was introduced to E.coli BL21 to produce mutated CBD proteins as described above. CBD(Y990A) was purified for X-ray crystallography as described above. N-terminal truncate Δ905, starting from the numbered residue, was obtained by PCR using the same template, corresponding primer, and 3′-pGEX primer. The amplified fragment was digested with appropriate restriction enzymes and inserted between the corresponding sites in the same vector.
Analytical methods
Protein concentrations were determined by using BCA protein assay reagents (Pierce, Rockford, IL). Calcium buffer with varying pCa (2.0–8.0) contained 10 mM HEPES, 150 mM NaCl, 0.2 mM EGTA (pH 7.4), and varying concentrations of CaCl2 calculated by assuming an apparent binding constant (Kapp) value of EGTA toward Ca2+ in HEPES buffer at pH 7.4 to be 5.13 × 106 M (McGuigan et al., 1991), being designated as HBS-Ca.
Collagen-binding assay
The effect of various mutations on the binding of CBD to insoluble collagen was determined as follows; 10 mg of collagen (acid-soluble collagen, Sigma type I) was washed three times with 400 µl of HBS supplemented with 1 mM CaCl2. Then collagen was incubated in protein solution at room temperature for 30 min. The solution (100 µl) contained 2.76 µg (0.2 nmol) of CBD in the same buffer. The water phase was examined by SDS–PAGE. The effect of calcium on binding of wild-type CBD to insoluble collagen was determined in the same manner except for the buffer, where HBS-Ca (pCa 2.0–8.0) was used.
Quantitative analysis of the binding to a collagenous peptide
Binding of various CBD proteins to a collagenous peptide was measured by surface plasmon resonance using a BIACORE apparatus (Biacore, Uppsala, Sweden) with a sensor chip (CM5, Biacore) on which a peptide, Gly-(Pro-Hyp-Gly)8, was covalently immobilized. For the mutated CBDs, resonance was measured in 10 mM Na–HEPES (pH 7.4), 150 mM NaCl, 1 mM CaCl2, 0.005% Tween-20 with a flow rate of 20 µl/min at 25°C. After each cycle the chip was regenerated with a 180 s pulse of 0.1 M HCl. Values for apparent dissociation constants, KD(app), were calculated from equilibrium binding data at eight protein concentrations (100 nM–300 µM) by directly fitting to a theoretical equation (Matsushita et al., 2001). In order to access the effect of calcium for the wild-type CBD, resonance was measured in the same conditions described above except for the buffer, where HBS-Ca (pCa 2.0–8.0) supplemented with 0.005% Tween-20 was used.
Stokes radius and fluorescence of CBD
Size exclusion chromatography was performed at room temperature on a HPLC system equipped with a Superdex 75 column (1 × 30 cm, Pharmacia) at a flow rate of 0.5 ml/min. Prior to analysis, the column was equilibrated with 3.5 volume of the HBS-Ca (pCa 2.0–8.0) or HBS supplemented with 1 mM CaCl2 or 1 mM EGTA. CBD protein (20 µg) was dissolved in 50 µl of the same buffer and chromatographed. The following proteins were used as molecular mass standards: bovine serum albumin, 67.0 kDa; chicken ovalbumin, 43.0 kDa; and ribonuclease A, 13.7 kDa (Pharmacia). The measurement was carried out in triplicate. Internal fluorescence was measured by using an excitation wavelength of 280 nm with emission from 250 to 400 nm on a fluorescence spectrophotometer (model F-2000; Hitachi, Tokyo, Japan). CBD protein was dissolved at a concentration of 25 µg/ml in HBS-Ca (pCa 2.0–8.0) or HBS supplemented with 0.2 mM EGTA.
Effect of calcium on the stability of CBD
CBD protein was dissolved at a concentration of 25 µg/ml in HBS supplemented with 0.2 mM EGTA or 1.0 mM excess CaCl2 containing varying concentrations (0–9 M) of urea. Fluorescence was measured by using an excitation wavelength of 290 nm with emission at 350 nm as described above. Fluorescence intensity of each buffer was also measured as the negative control. The measurement was carried out in triplicate.
Crystal structure determination
Recombinant CBD crystals were grown by hanging drop vapor diffusion from 25–27% PEG 3350, 100 mM sodium acetate pH 4.6 and 600 mM LiCl at 4°C. Crystals belong to the monoclinic space group P21, with cell dimensions a = 40.64 Å, b = 57.71 Å, c = 46.32 Å and β = 95.69°. Single crystal data were collected at the IMCA 17-ID beamline at the Advanced Photon Source, Argonne National Laboratories. Data reduction was carried out using Brukers’ SAINT (Table I). Recombinant Se-MET derivative CBD crystals were grown by hanging drop vapor diffusion from 22–25% PEG 3350, 100 mM sodium acetate pH 4.6, 600 mM LiCl, 10% glycerol at 4°C. The Se-MET crystals were non-isomorphous, belonging to the space group P21, with cell dimensions a = 40.89 Å, b = 59.67 Å, c = 49.60 Å and β = 100.81°. Single crystal MAD data were collected at beamline 5.0.2 at the Advanced Light Source, Lawrence Berkeley National Laboratories. Data reduction was carried out using HKL2000 (Otwinowski and Minor, 1997). The positions of the two seleniums per asymmetric unit were identified and refined using CNS (Kuriyan et al., 1989). Two-fold non-crystallographic symmetry was initially used to aid in tracing the protein backbone using O (Jones et al., 1991). When ∼80% of the structure had been solved, rigid body refinement was used to apply the MAD-derived structure to the native dataset. Removing NCS, residues L897 to A909 and S962 to N965 were then resolved in one molecule but not found in the other. Phases were refined, and water molecules were located using SHELX97 (Sheldrick, 1990). The ultra-high resolution data allowed us to refine the structure using six anisotropic B-factor parameters and riding hydrogen atoms. The final R factor was 14.2% for all data to 1.00 Å (Rfree = 16.6%) (see Table II). When two independent molecules in the ASU were superimposed, their main-chain r.m.s.d. was 0.36 Å. The differences occur in a loop region and the N-terminal linker. However, one molecule has residues 894–908 unresolved and 962–966 poorly unresolved. Residues 962–966 fall in a loop region and are very mobile preventing clear identification. In the same molecule, residues 894–908 comprising the N-terminal linker are missing. Among the non-glycine and non-proline residues, 94.4% have φ, ψ values in the ‘most favored regions’ of the Ramachandran plot with no outliers (Laskowski et al., 1993).
Mutant CBD (Y990A) crystals were grown and found to be in the same space group with cell dimensions a = 41.47 Å, b = 59.75 Å, c = 48.03 Å and β = 96.33°. Data were collected in-house and data reduction was carried out using HKL version 1.9 (Otwinowski and Minor, 1997). Because the Y990A crystals were non-isomorphous to the native crystals, the native structure was refined against the Y990A data using the rigid-body option. No electron density was seen for the N-terminal linker. Instead, residues E895 to K898 form a new β-strand, and two Ca2+ ions were found. Neither molecule of the ASU showed clear density for a loop formed by residues N963 to D966. The four highest peaks in the difference Fourier maps (two per chain) were assigned as Ca2+ ions based in their density and coordination environment. The structure was named holo-Y990A. SHELX97 was used for phase refinement and water divination (Table I). A second dataset was obtained at the Cornell High Energy Synchrotron Source beamline F2. The crystal had unit cell dimensions a = 41.29 Å, b = 59.78 Å, c = 47.74 Å and β = 96.85°. The structure was named apo-Y990A since no calciums were observed. The structure was refined as above (Table I).
The calcium-free apo-Y990A is virtually identical to the apo-CBD molecule in which the N-terminal helix is not observed. The holo-Y990A mutant structure is similar to that of holo-CBD with the coordination of two Ca2+ ions. Both the apo- and holo-Y990A structures are poorly resolved in the 962–966 loop region. No significant differences in structure are observed between the Y990A and CBD molecules, and the main-chain r.m.s.d. between the two is only 0.44 Å for holo and 0.45 Å for apo. Since the Y990A data are of lower resolution, the average main-chain B-factor for holo-Y990A is significantly higher than that of holo-CBD (35.5 versus 16.9 Å2).
To ensure incorporation of calcium into the wild-type protein, crystals of CBD were grown by vapor diffusion with the inclusion of 100 mM Ca(NO3)2 in the hanging drop. The crystals were isomorphous to holo-Y990A with cell dimensions a = 40.87 Å, b = 59.40 Å, c = 48.57 Å and β = 98.73°. Data were collected at Brookhaven National Laboratories National Synchrotron Light Source beamline X25, and it was reduced using HKL2000. The holo-Y990A structure was used for a molecular replacement model in CNS. O was used for density fitting, and SHELX97 was used for refinement (Table I). Two Ca2+ ions per molecule were located, and the ions were refined anisotropically. In one molecule of the ASU, residues 890–892 comprising part of the N-terminal fusion product are observed, and residues 963–966 are unresolved. The other molecule only shows density beginning at residue 895, and 963–965 are unresolved.
The full coordinates of apo- and holo-CBD (1NQJ and 1NQD, respectively) and their corresponding structure factor amplitudes have been deposited in the Protein Data Bank (PDB) for immediate release.
Collagen docking
The 3D-Dock Suite was used to predict the likely binding orientation of collagen across CBD (Katchalski-Katzir et al., 1992). Since the program does not recognize hydroxyproline, a tripeptide (Pro-Pro-Gly)6 was created which has been shown to bind tightly with CBD (Matsushita et al., 2001). The crystal structure of (Pro-Hyp-Gly)4 Pro-Hyp-Ala (Pro-Hyp-Gly)5, PDB code 1CGD (Bella et al., 1995), with all water molecules removed was used as a starting model. To avoid the bulge created by Ala, the final four POG repeats were used, and all hydroxyprolines were edited to prolines. This (PPG)4 construct was extended to (PPG)6 via least squares superimposition. To reduce manual editing errors, geometry optimization to convergence was run on the triple-helix (PPG)6 construct using Hyperchem (Hypercube, Inc.).
The optimized (PPG)6 was used as the mobile model and one molecule of holo-CBD was used as the static model in FTDock, followed by residue level pair potential scoring using RPScore. Orientations that placed (PPG)6 within 4.5 Å of T957, Y970, L992, Y994 and Y996 were selected. In addition, a 6 Å filter was used for the weaker interacting H959 and R929. The filtering reduced the number of solutions from 10 000 to 177. Solutions that produced an end-on binding model or where collagen did not extend completely across CBD were removed, and the top ten solutions were compared.
The above procedure was also used to dock the collagen model to apo-CBD. Since R929 adopts two orientations, the calculation was performed with both orientations separately. Using the same distance filters, the number of solutions was reduced from 20 000 to 448. Solutions that produced an end-on binding model were removed, and the top ten solutions from each orientation of R929 were compared.
Acknowledgments
Acknowledgements
We are especially grateful to Dr Andy Howard for his assistance in collecting the ultra-high resolution data set. The work was supported by NIH-COBRE 1P20RR1556901, NIH-BRIN, Arkansas Biosciences Inst., USDA-CSREES and a Grant-in Aid for Scientific Research (C) from Japan Society for the Promotion of Science. Use of the APS was supported by the DOE. The IMCA-CAT facilities are supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Illinois Institute of Technology. The ALS is supported by the DOE at Lawrence Berkeley National Laboratory. CHESS is supported by the NSF and the NIH. Finally, NSLS’s support comes principally from the DOE/NIH.
References
- Altschul S.F., Madden,T.L., Schäffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bella J., Brodsky,B. and Berman,H.M. (1995) Hydration structure of a collagen peptide. Structure, 3, 893–906. [DOI] [PubMed] [Google Scholar]
- Bogan A.A. and Thorn,K.S. (1998) Anatomy of hot spots in protein interfaces. J. Mol. Biol., 280, 1–9. [DOI] [PubMed] [Google Scholar]
- Brown E.M., Vassilev,P.M. and Hebert,S.C. (1995) Calcium ions as extracellular messengers. Cell, 83, 679–682. [DOI] [PubMed] [Google Scholar]
- Busch E., Hohenester,E., Timpl,R., Paulsson,M. and Maurer,P. (2000) Calcium affinity, cooperativity and domain interactions of extracellular EF-hands present in BM-40. J. Biol. Chem., 275, 25508–25515. [DOI] [PubMed] [Google Scholar]
- Emsley J., King,S.L., Bergelson,J.M. and Liddington,R.C. (1997) Crystal structure of the I domain from integrin α2β1. J. Biol. Chem., 272, 28512–28517. [DOI] [PubMed] [Google Scholar]
- Emsley J., Cruz,M., Handin,R. and Liddington,R. (1998) Crystal structure of the von Willebrand factor A1 domain and implications for the binding of platelet glycoprotein Ib. J. Biol. Chem., 273, 10396–10401. [DOI] [PubMed] [Google Scholar]
- Emsley J., Knight,C.G., Farndale,R.W., Barnes,M.J. and Liddington,R.C. (2000) Structural basis of collagen recognition by integrin α2β1. Cell, 101, 47–56. [DOI] [PubMed] [Google Scholar]
- Gouet P., Courcelle,E., Stuart,D.I. and Metoz,F. (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics, 15, 305–308. [DOI] [PubMed] [Google Scholar]
- Holland I.B., Jones,H.E., Campbell,A.K. and Jacq,A. (1999) An assessment of the role of intracellular free Ca2+ in E.coli. Biochimie, 81, 901–907. [DOI] [PubMed] [Google Scholar]
- Huizinga E.G., Martijn van der Plas,R., Kroon,J., Sixma,J.J. and Gros,P. (1997) Crystal structure of the A3 domain of human von Willebrand factor: implications for collagen binding. Structure, 5, 1147–1156. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Zou,J.Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Katchalski-Katzir E., Shariv,I., Eisenstein,M., Friesem,A.A., Aflalo,C. and Vakser,I.A. (1992) Molecular surface recognition: determination of geometric fit between proteins and their ligands by correlation techniques. Proc. Natl Acad. Sci. USA, 89, 2195–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraulis P. (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr., 24, 946–950. [Google Scholar]
- Kuriyan J., Brunger,A.T., Karplus,M. and Hendrickson,W.A. (1989) X-ray refinement of protein structures by simulated annealing: test of the method on myohemerythrin. Acta Crystallogr. A, 45, 396–409. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., MacArthur,M.W., Moss,D.S. and Thornton,J.M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr., 26, 283–291. [Google Scholar]
- Legge G.B., Kriwacki,R.W., Chung,J., Hommel,U., Ramage,P., Case,D.A., Dyson,H.J. and Wright,P.E. (2000) NMR solution structure of the inserted domain of human leukocyte function associated antigen-1. J. Mol. Biol., 295, 1251–1264. [DOI] [PubMed] [Google Scholar]
- Matsushita O., Jung,C.M., Minami,J., Katayama,S., Nishi,N. and Okabe,A. (1998) A study of the collagen-binding domain of a 116-kDa Clostridium histolyticum collagenase. J. Biol. Chem., 273, 3643–3648. [DOI] [PubMed] [Google Scholar]
- Matsushita O., Jung,C.M., Katayama,S., Minami,J., Takahashi,Y. and Okabe,A. (1999) Gene duplication and multiplicity of collagenases in Clostridium histolyticum. J. Bacteriol., 181, 923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita O., Koide,T., Kobayashi,R., Nagata,K. and Okabe,A. (2001) Substrate recognition by the collagen-binding domain of Clostridium histolyticum class I collagenase. J. Biol. Chem., 276, 8761–8770. [DOI] [PubMed] [Google Scholar]
- Maurer P. and Hohenester,E. (1997) Structural and functional aspects of calcium binding in extracellular matrix proteins. Matrix Biol., 15, 569–581. [DOI] [PubMed] [Google Scholar]
- McGuigan J.A., Luthi,D. and Buri,A. (1991) Calcium buffer solutions and how to make them: a do it yourself guide. Can. J. Physiol. Pharmacol., 69, 1733–1749. [DOI] [PubMed] [Google Scholar]
- McPhalen C.A., Strynadka,N.C. and James,M.N. (1991) Calcium-binding sites in proteins: a structural perspective. Adv. Protein. Chem., 42, 77–144. [DOI] [PubMed] [Google Scholar]
- Mookhtiar K.A. and van Wart,H.E. (1992) Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl., 1, 116–126. [PubMed] [Google Scholar]
- Nicholson C. (1980) Modulation of extracellular calcium and its functional implications. Fed. Proc., 39, 1519–1523. [PubMed] [Google Scholar]
- Nishi N., Matsushita,O., Yuube,K., Miyanaka,H., Okabe,A. and Wada,F. (1998) Collagen-binding growth factors: production and characterization of functional fusion proteins having a collagen-binding domain. Proc. Natl Acad. Sci. USA, 95, 7018–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z. and Minor,W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol., 276, 307–326. [DOI] [PubMed] [Google Scholar]
- Perona J.J., Tsu,C.A., Craik,C.S. and Fletterick,R.J. (1997) Crystal structure of an ecotin–collagenase complex suggests a model for recognition and cleavage of the collagen triple helix. Biochemistry, 36, 5381–5392. [DOI] [PubMed] [Google Scholar]
- Pilcher B.K., Wang,M., Qin,X.J., Parks,W.C., Senior,R.M. and Welgus,H.G. (1999) Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann. N. Y. Acad. Sci., 878, 12–24. [DOI] [PubMed] [Google Scholar]
- Rich R.L., Deivanayagam,C.C., Owens,R.T., Carson,M., Hook,A., Moore,D., Symersky,J., Yang,V.W., Narayana,S.V. and Hook,M. (1999) Trench-shaped binding sites promote multiple classes of interactions between collagen and the adherence receptors, α1β1integrin and Staphylococcus aureus Cna MSCRAMM. J. Biol. Chem., 274, 24906–24913. [DOI] [PubMed] [Google Scholar]
- Sheldrick G.M. (1990) Phase annealing in SHELX-90: direct methods for larger structures. Acta Crystallogr. A, 46, 467–473. [Google Scholar]
- Symersky J. et al. (1997) Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat. Struct. Biol., 4, 833–838. [DOI] [PubMed] [Google Scholar]
- Toyoshima T., Matsushita,O., Minami,J., Nishi,N., Okabe,A. and Itano,T. (2001) Collagen-binding domain of a Clostridium histolyticum collagenase exhibits a broad substrate spectrum both in vitro and in vivo. Connect. Tissue Res., 42, 281–290. [DOI] [PubMed] [Google Scholar]
- Van Duyne G.D., Standaert,R.F., Karplus,P.A., Schreiber,S.L. and Clardy,J. (1993) Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol., 229, 105–124. [DOI] [PubMed] [Google Scholar]
- Vogel W.F. (2001) Collagen-receptor signaling in health and disease. Eur. J. Dermatol., 11, 506–514. [PubMed] [Google Scholar]
- Worthington Biochemical Co . (1988) Worthington Enzyme Manual: Enzymes and Related Biochemicals. Freehold, NJ.
- Wu Y.D. and Zhao,Y.L. (2001) A theoretical study on the origin of cooperativity in the formation of 3(10)- and α-helices. J. Am. Chem. Soc., 123, 5313–5319. [DOI] [PubMed] [Google Scholar]
- Yoshihara K., Matsushita,O., Minami,J. and Okabe,A. (1994) Cloning and nucleotide sequence analysis of the colH gene from Clostridium histolyticum encoding a collagenase and a gelatinase. J. Bacteriol., 176, 6489–6496. [DOI] [PMC free article] [PubMed] [Google Scholar]