Abstract
In Gram-negative bacteria, lipopolysaccharide and phospholipid biosynthesis takes place at the inner membrane. How the completed lipid molecules are subsequently transported to the outer membrane remains unknown. Omp85 of Neisseria meningitidis is representative for a family of outer membrane proteins conserved among Gram-negative bacteria. We first demonstrated that the omp85 gene is co-transcribed with genes involved in lipid biosynthesis, suggesting an involvement in lipid assembly. A meningococcal strain was constructed in which Omp85 expression could be switched on or off through a tac promoter-controlled omp85 gene. We demonstrated that the presence of Omp85 is essential for viability. Depletion of Omp85 leads to accumulation of electron-dense amorphous material and vesicular structures in the periplasm. We demonstrated, by fractionation of inner and outer membranes, that lipopolysaccharide and phospholipids mostly disappeared from the outer membrane and instead accumulated in the inner membrane, upon depletion of Omp85. Omp85 depletion did not affect localization of integral outer membrane proteins PorA and Opa. These results provide compelling evidence for a role for Omp85 in lipid transport to the outer membrane.
Keywords: lipopolysaccharide and phospholipid export/Neisseria meningitidis/Omp85/outer membrane
Introduction
The envelope of Gram-negative bacteria consists of an inner membrane (IM), the peptidoglycan layer and an outer membrane (OM) (Nikaido, 1996). The latter is an asymmetrical bilayer with phospholipids (PLs) in the inner leaflet and lipid A, the hydrophobic anchor of lipopolysaccharide (LPS) in the outer leaflet. LPS has a cell surface-exposed oligosaccharide chain, which in many organisms is linked further to a longer O antigen chain composed of repeating sugar subunits (Raetz and Whitfield, 2002). LPS and PL biosynthesis takes place in both the cytoplasm and IM of the bacterial cell. The enzymes responsible for the lipid A biosynthesis pathway have been well characterized in Escherichia coli (Raetz and Whitfield, 2002) and are targets for the design of novel antibacterial agents (Onishi et al., 1996; McMurry et al., 1998).
During the past 20 years, both genetic and biochemical studies have led to the description of LPS composition, biosynthesis and regulation. The formation of LPS is a complex process involving the synthesis of activated precursors in the cytoplasm, followed by the assembly of the lipid A core at the IM (Raetz and Whitfield, 2002). In E.coli, the lipid A biosynthetic pathway has been elucidated entirely. Conditionally lethal mutants have been used to isolate and characterize genes involved in the early steps of lipid A biosynthesis. These genes (lpxA, lpxC and lpxD) were shown to be essential for viability (Beall and Lutkenhaus, 1987; Galloway and Raetz, 1990; Kelly et al., 1993). However, in Neisseria meningitidis, it is possible to make a knockout mutant of lpxA, encoding UDP-GlcNAc acyltransferase required for the first step of lipid A biosynthesis (Galloway and Raetz, 1990), while maintaining cell viability (Steeghs et al., 1998). This mutant still possesses an OM in which the PL composition is altered in comparison with the wild-type strain (Steeghs et al., 2001).
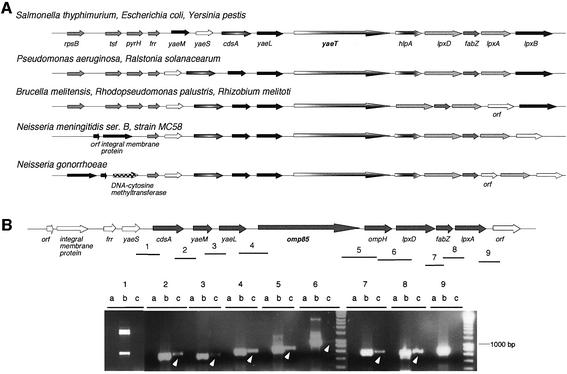
In E.coli, the lpxA gene belongs to a largely conserved cluster of genes, present in almost all Gram-negative bacteria for which genome sequences are available. Figure 1A shows the chromosomal arrangement of this locus in several Gram-negative bacteria. In addition to the lipid A biosynthesis genes lpxA, lpxD and lpxB, this cluster also includes fabZ and cdsA required for fatty acid and phospholipid synthesis, respectively (Raetz and Dowhan, 1990; Mohan et al., 1994), as well as yaeM and yaeL which are known to be involved in membrane biogenesis in E.coli: yaeM encodes 1-deoxy-d-xylulose 5-phosphate reductoisomerase involved in the synthesis of isoprenoids (Takahashi et al., 1998), which have been characterized in many diverse organisms and have been shown to serve as pigments, defensive agents, constituents of membranes or components of signal transduction networks (Sacchettini and Poulter, 1997). yaeL encodes the essential EcfE IM protease, which is involved in regulation of the heat shock response in E.coli (Dartigalongue et al., 2001). EcfE modulates the level of expression of both RpoH and RpoE σ factors, which control protein folding and degradation in the cytoplasm and extracytoplasm, respectively. Moreover, RpoE regulates lipid biogenesis in response to environmental stress (Raivio and Silhavy, 2001). The yaeT gene, encoding a putative outer membrane protein (OMP) of unknown function, is always found upstream of lpxD, in most cases associated with hlpA, which encodes a periplasmic chaperone (Chen and Henning, 1996; Missiakas et al., 1996). The presence of a gene encoding a highly conserved OMP in a cluster dedicated to LPS and PL biosynthesis suggests a possible involvement in lipid transport.
Fig. 1. (A) Schematic representation of the lpx locus of different Gram-negative bacteria. Arrows of the same shadings represent homologous genes. (B) Results of RT–PCR experiments. Top: transcriptional organization of the neisserial lpx locus showing the regions amplified by the primer pairs 1–9. Bottom: agarose gel of the RT–PCR amplification products. For each primer pair, three lanes are shown [a, negative control using RNA as template (no RT); b, positive control using genomic DNA as template; c, RT–PCR]. The cdsA-lpxA transcript is indicated with grey arrows.
The way in which completed LPS and PL are translocated to the cell surface represents the major unresolved question in their biosynthesis. Recently, a clue to the mechanism of lipid A core and PL transport within the IM has emerged from the discovery that a mutation in msbA, encoding an essential ABC transporter, results in a defect in lipid export (Doerrler et al., 2001). The MsbA protein is thought to function as an IM flippase for PL and lipid A core transport to the periplasm. To complete the LPS biosynthesis, lipid A core is attached further to the O-polysaccharide chain once both molecules are in the periplasm. The three currently known pathways for O-polysaccharide biosynthesis are distinguished by their export mechanisms (Raetz and Whitfield, 2002). The pathways are called Wzy-dependent, ABC transporter-dependent and synthase-dependent. The first two processes are widespread, while the last one has limited distribution in O-polysaccharide biosynthesis. Despite the export differences, the initiation reactions and ligation to lipid A core are similar. The molecular mechanism underlying the transport of the completed LPS molecules to the OM is still unknown. As for LPS, the mechanisms of PL transport from the IM to OM are unknown. Several hypotheses have been proposed, such as PL and LPS translocation through membrane adhesion zones (Bayer’s junctions) (Bayer, 1968), but it is still not clear whether or not these structures are physiologically relevant (Kellenberger, 1990). Another possibility is that specific proteins may serve as a shuttle for lipid transfer to the OM, but this has never been demonstrated.
Neisseria meningitidis is, to date, the only Gram-negative bacterium in which a completely LPS-deficient but still viable mutant can be obtained (Steeghs et al., 1998). This makes it a uniquely suitable organism for the study of LPS and PL transport. In the present study, we have investigated the involvement of Omp85, the neisserial YaeT homologue, in the export of lipids to the OM. We first showed that omp85, the gene encoding Omp85, is part of a transcriptional unit that starts with cdsA and ends with lpxA. We further demonstrated that Omp85 is essential for the viability of N.meningitidis and is required for LPS and PL transport in the OM. We also showed that Omp85 is not directly involved in the transport of integral OMPs such as PorA and Opa, though the depletion of Omp85 leads to increased amounts of their degradation products in the OM.
Results
The omp85 gene is transcriptionally linked to genes involved in lipid A, fatty acid and phospholipid biosynthesis
The lpx locus is highly conserved among different Gram-negative bacteria (Figure 1A), containing genes involved in lipid A, fatty acid and PL biosynthesis together with genes implicated in membrane biogenesis. In order to determine if omp85 is co-transcribed with these genes in N.meningitidis, RT–PCR assays were performed (Figure 1B). A negative control, consisting of DNase-treated RNA, and a positive control, consisting of genomic DNA, were included in the experiment. Using cDNA as template, we could amplify overlapping regions between genes of the cluster, from cdsA to lpxA. This result demonstrates that omp85 is part of a transcriptional unit that contains eight genes, starting with cdsA and ending with lpxA, which could reflect the fact that they encode proteins or enzymes involved in a common pathway.
Omp85 is essential for viability of N.meningitidis
To test directly the involvement of Omp85 in LPS and PL transport, we first attempted to make an omp85 knockout mutant in N.meningitidis wild-type strain H44/76 and its LPS-deficient lpxA mutant by allelic replacement with constructs containing either a kanamycin or chloramphenicol marker in omp85, but without any success. Moreover, we tried to delete omp85 in the HA3003 strain in which LPS expression could be switched on or off through a tac promoter-controlled lpxA gene (Steeghs et al., 2001). Again, no double crossover recombination events leading to the inactivation of the omp85 gene could be obtained, whether HA3003 expressed LPS or not, indicating an essential role for Omp85 in the viability of both the wild-type and LPS-deficient mutant.
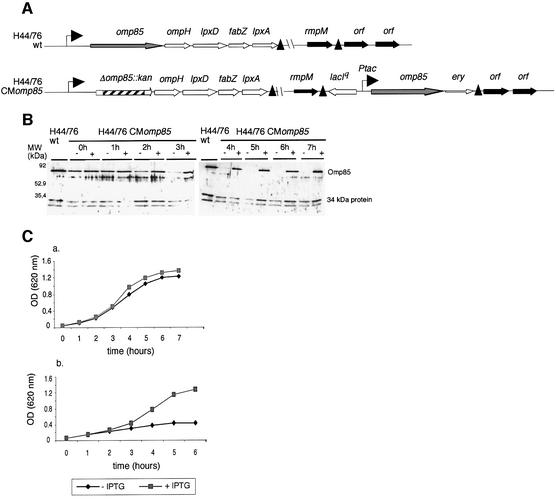
To study the function of Omp85 in spite of this limitation, we next constructed a meningococcal strain in which Omp85 expression is regulated from the tac promoter (Figure 2A). The lacIq-Ptac-regulated omp85 DNA fragment was first introduced at the rmpM locus. Secondly, the omp85 gene was deleted at its own locus using the kanamycin cassette, which does not contain a transcriptional terminator. The latter insertion did not affect expression of downstream lpx genes in the resulting omp85 conditional mutant (termed CMomp85), as was checked by RT–PCR (results not shown). We could only select kanamycin-resistant clones in the presence of isopropyl-β-d-thiogalactopyranoside (IPTG), again demonstrating that omp85 expression is essential for bacterial survival. Using this mutant, the expression of Omp85 could be regulated by the presence or absence of IPTG in the medium. Depletion of Omp85 was checked every hour of growth by western blot on whole-cell extracts using a rabbit polyclonal antiserum directed against Omp85 (Figure 2B). After 3 h of growth without IPTG, depletion of Omp85 became visible, and was apparently complete after 5 h of growth. Upon induction with IPTG, omp85 is transcribed and cells are viable (Figure 2C). When no IPTG was added in the medium, cell growth was affected. The effect of depletion of Omp85 on bacterial growth was more pronounced when, after 4 h of incubation without IPTG, the culture was diluted further into fresh medium without IPTG (see Figure 2C, panel b). This clearly demonstrates that Omp85 is essential for the viability of N.meningitidis.
Fig. 2. Omp85 is essential for viability of N.meningitidis. Construction of a meningococcal strain in which Omp85 expression is under the control of the tac promoter. (A) Chromosomal arrangement of the H44/76 wild-type and CMomp85 strains. Transcriptional terminators are indicated by black triangles. Details of cloning are explained in Materials and methods. (B) Western blot on whole-cell extracts of the H44/76 CMomp85 strain grown without (–) and with (+) 0.05 mM IPTG, and the H44/76 wild-type strain as control, at different time points of the first 7 h of growth. The antibody used is a rabbit polyclonal Omp85 antiserum. (C) Growth curves of the lacIq-Ptac-regulated omp85 strain in the absence (black diamonds) and presence of 0.05 mM IPTG (grey squares). In this panel, two growth curves are shown: in (a), colonies were picked up from IPTG-containing plates, washed and grown for 7 h. After 4 h of growth without IPTG, bacteria were diluted into new fresh medium without IPTG and grown further for 6 h. This is shown in (b).
Analysis of the ultrastructure of the cell envelope upon depletion of Omp85
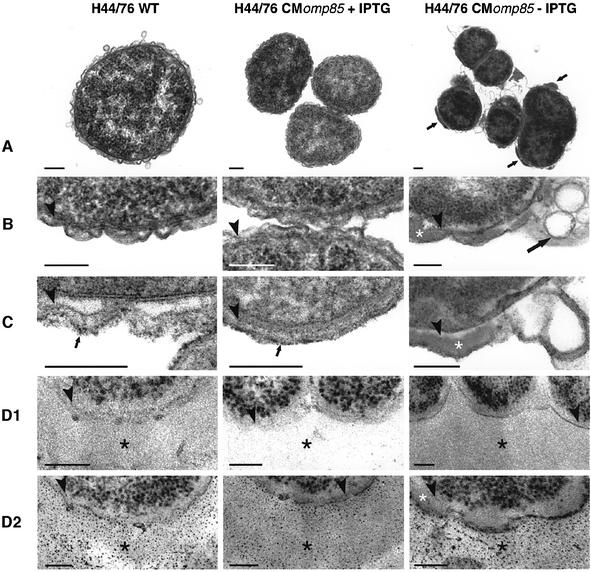
The ultrastructure of the H44/76 CMomp85 strain grown in the presence and absence of IPTG, and its parental strain was examined by transmission electron microscopy (Figure 3). We did not observe any ultrastructural difference in the cell envelope between the CMomp85 strain grown with IPTG and the wild-type strain. In contrast, cells of H44/76 CMomp85 grown without IPTG were more heterogeneous in size, and a significant proportion of cells showed signs of lysis (Figure 3A and B). A clear accumulation of both electron-dense material and vesicular structures in the periplasm was observed for these latter cells, suggesting that the depletion of Omp85 leads to a defect in transport of some component(s) that can no longer reach the OM. We further analysed ultrastructurally whether this accumulating material consists of lipids or polysaccharides. Therefore, bacteria were fixed in glutaraldehyde/osmium tetroxide solution to retain lipids and in a paraformaldehyde (PFA) solution to perform the periodic acid–Schift (PAS) reaction. H44/76 wild-type and IPTG-stimulated CMomp85 bacteria showed small lipophilic granules at the outside of the OM, whereas CMomp85 bacteria grown without IPTG did not show these lipophilic granules. The accumulating material in these cells was also not reactive with osmium and is therefore considered as not lipophilic (Figure 3C). A cytochemical reaction for polysaccharides revealed many granules in a broad band directly outside these cells, presumably representing the capsule. No reaction was seen within the periplasmic space (Figure 3D). These findings suggest that the accumulating electron-dense material in the periplasm is of a proteinaceous nature.
Fig. 3. Electron micrographs of ultrathin sections of H44/76 wild-type and CMomp85 grown in the presence and absence of 0.05 mM IPTG. (A) Overview and (B–D) high magnification of the cell envelope of the different strains. Bacteria were fixed in a Karnovsky solution and contrasted according to standard procedures (A and B), or they were fixed in a combined glutaraldehyde and OsO4 solution to retain lipids and contrasted to standard procedures (C), and fixed in PFA, followed directly by embedding without contrasting (D1/2). Sections in (D1) were not post-stained and all the contrast was imparted by the embedding medium. A cytochemical reaction for the presence of PAS-positive material on ultrathin sections is illustrated in (D2). Depletion of Omp85 leads to accumulation of electron-dense amorphous material (small arrows in A, white asterisk in B) and to vesicular structures (large arrow in B) in the periplasmic space. This amorphous material is not lipophilic or cytochemically stained for polysaccharides (white asterisk in C and D2). The small arrows in (C) mark the osmiophilic granules outside the OM. The black asterisks in (D1) and (D2) denote a layer of PAS-positive material outside the cells, presumably the capsular polysaccharide. Arrowheads in (B–D) mark the peptidoglycan layer. The bars represent 200 µm in (A) and 100 µm in (B–D).
IM and OM separation of the H44/76 CMomp85 strain
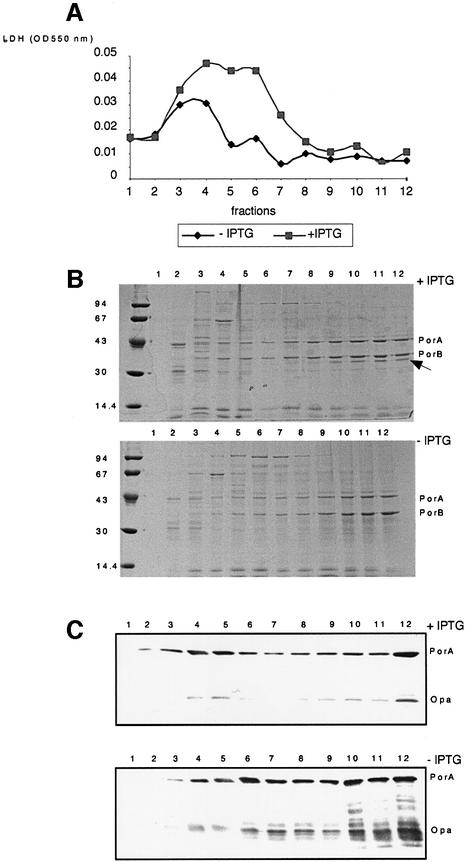
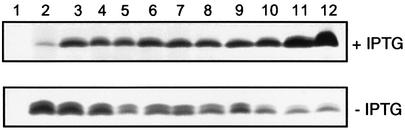
The cell envelope composition of the H44/76 CMomp85 strain, grown for 4 and 6 h in the presence and absence of IPTG, was examined by IM and OM separation through isopycnic sucrose gradient centrifugation (Steeghs et al., 2001). We will only present the data on the collected fractions after 6 h of growth. Gradient fractions of 1 ml were collected, protein quantified and analysed for the presence of lactate dehydrogenase (LDH) activity as a marker for the IM (Osborn et al., 1972), and for the presence of PorA and Opa as markers for the OM (Figure 4). LDH activity peaked in fractions 3 and 4 when the H44/76 CMomp85 strain was grown without IPTG (Figure 4A). In contrast, in the presence of IPTG, LDH activity was higher and peaked in fractions 3–6. In both cases, no LDH activity was detected in fractions 8–12, which are therefore regarded as pure OM fractions. We analysed the protein content on each collected fraction by SDS–PAGE followed by Coomassie Blue staining (Figure 4B). The protein profile for these fractions is the same for bacteria grown in the presence or absence of IPTG, with the exception of a band (marked with a black arrow) at a molecular weight of 35 kDa that disappears when bacteria are not induced with IPTG. We performed a western blot experiment using monoclonal antibodies directed against PorA and Opa, two integral OMPs (Figure 4C). PorA and Opa proteins are visible in almost all the fractions, but with strong enrichment of these proteins in the OM fractions. This was observed previously using the same procedures for the IM and OM separation of the wild-type strain and the LPS-deficient mutant (Steeghs et al., 2001). The Opa protein remains present in the OM fractions of the strain grown without IPTG, suggesting that the 35 kDa band that disappears upon depletion of Omp85 does not correspond to Opa. Moreover, depletion of Omp85 did not lead to accumulation of PorA and Opa in the IM fractions, indicating that Omp85 is not directly involved in the transport of these proteins to the OM. The only effect is a somewhat increased amount of PorA and Opa degradation products in the OM fractions. Degradation of OMPs has also been reported in the LPS-deficient mutant (Steeghs et al., 2001).
Fig. 4. Analysis of fractions 1–12, collected after isopycnic sucrose gradient centrifugation, to separate the membranes of the H44/76 CMomp85 strain grown for 6 h in the presence and absence of 0.05 mM IPTG. (A) LDH activity per 5 µg of proteins as a marker for the IM. (B) SDS–PAGE analysis of the fractions. The positions of molecular weight standard proteins are indicated on the left in kDa; the positions of the porins PorA and PorB are indicated on the right. The black arrow shows the 35 kDa band. (C) Western blot using PorA (MN5C11G) and Opa (15-1-P5.5 + αD2) monoclonal antibodies as markers for the OM.
Omp85 is required for LPS transport to the OM
To study the effect of Omp85 depletion on LPS localization, the LPS content of each proteinase K-treated fraction was analysed by Tricine-SDS–PAGE followed by silver staining (Lesse et al., 1990) (Figure 5). When Omp85 was present, LPS was observed mainly in the OM fractions. In contrast, when Omp85 was depleted, LPS mostly disappeared from the OM fractions and instead accumulated in the IM fractions. Moreover, a two band pattern was observed, suggesting that depletion of Omp85 in addition leads to the relative increase in a modified form of LPS with slightly lower gel mobility. After 4 h of growth without IPTG, the same result was observed, though the effect of depletion of Omp85 on LPS accumulation in IM fractions was less marked, suggesting that LPS accumulation in the IM occurs directly and progressively upon depletion of Omp85.
Fig. 5. Omp85 is required for LPS export to the OM. Silver-stained Tricine-SDS–polyacrylamide gel showing the LPS pattern of proteinase K-treated fractions containing 5 µg of protein of the H44/76 CMomp85 strain grown in the presence (top) and absence (bottom) of 0.05 mM IPTG.
Omp85 is required for PL transport to the OM
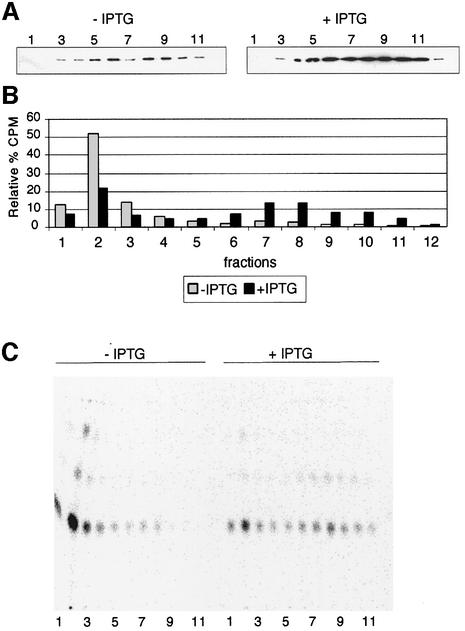
The results shown in Figure 5 indicate a role for Omp85 in LPS export. However, as a knockout omp85 mutant could not be isolated in the LPS-deficient mutant, Omp85 might also be required for another function in membrane biogenesis. Therefore, we examined the effect of Omp85 depletion on PL transport to the OM (Figure 6). Bacteria were grown for 6 h as previously described, but in the presence of [1,2-14C]acetic acid. Bacterial cell fractionation was performed by another method, based on the lysis of lysozyme–EDTA spheroblasts (Osborn et al., 1972). IMs and OMs were separated on a sucrose gradient. Each fraction was analysed for the presence of PorA as a marker for the OM (Figure 6A). Compared with the previous membrane separation, we observed a slightly different distribution of OM fractions in this experiment: PorA is distributed in fractions 4–10. This might be explained by the use of lysozyme, which degrades the peptidoglycan layer, leading to a shift of OM to the lower density fractions of the gradient. This has been observed previously in the sucrose gradient fractionation of membranes from Salmonella typhimurium using the same procedures (Osborn et al., 1972). The N.meningitidis OM localizes in the same density region of the gradient (38–43% sucrose) as the ‘H’ band representing the S.typhimurium OM. PLs were extracted and analysed for their c.p.m. value as shown in Figure 6B. We calculated the relative percentage of each PL extract compared with the sum of the c.p.m. of all extracts. The distribution of PLs can also be visualized after their separation on thin-layer chromatography (TLC) as shown in Figure 6C. Compared with the strain grown with IPTG, PLs are decreased in the OM (fractions 6–10) and instead accumulate in the IM (particularly in fraction 2) after depletion of Omp85 (–IPTG). These results demonstrate that Omp85 is also required for PL transport to the OM. Moreover, this might explain why no omp85 knockout mutant could be isolated in the LPS-deficient mutant.
Fig. 6. Omp85 is required for PL export to the OM. The H44/76 CMomp85 strain was grown in the presence of [1,2-14C]acetic acid for 6 h in the presence and absence of 0.05 mM IPTG. (A) Collected fractions 1–12 were analysed by western blot using PorA monoclonal antibody as a marker for the OM. (B) Relative percentage of the c.p.m. value in each fraction [(c.p.m. value FX/total c.p.m. value) × 100]. (C) TLC separation of PLs. PLs were extracted using the method of Bligh and Dyer (1959) and separated on TLC plates as explained in Materials and methods.
Discussion
In the present study, we have investigated the potential involvement of Omp85 in transport of lipids to the OM of N.meningitidis. In contrast to most Gram-negative bacteria, N.meningitidis is viable without LPS, making it a uniquely suitable organism to study lipid transport.
The omp85 gene has a conserved location in all Gram-negative bacterial genomes sequenced to date. We demonstrated that, in N.meningitidis, omp85 is part of a transcriptional unit that starts with cdsA and ends with lpxA. The association of omp85 with genes encoding proteins involved in LPS and PL biosynthesis and cell envelope assembly suggested a common involvement in lipid biosynthesis and membrane biogenesis pathways.
The omp85 gene is essential for survival of N.meningitidis wild-type and its LPS-deficient mutant. We constructed a derivative strain of H44/76, designated H44/76 CMomp85, which carries an IPTG-inducible omp85 gene on the chromosome, allowing us to study the function of Omp85 after its gradual depletion. The analysis of the ultrastructure of the bacterial envelope by electron microscopy revealed that together with depletion of Omp85, an electron-dense amorphous material, as well as vesicular structures are accumulating between the IM and OM. Different staining techniques were used to demonstrate that the electron-dense material probably consists of protein. The vesicular structures are reminiscent of the IM reduplications seen in the E.coli msbA mutant (Doerrler et al., 2001), and suggest a block in lipid export. IM and OM separation of the CMomp85 strain, grown in the presence and absence of IPTG, demonstrated that Omp85 is required for LPS transport to the OM. Analysis of the LPS content in the IM and OM revealed a more pronounced two-band pattern in the mutant, indicating that depletion of Omp85 leads to some modification of LPS. This could be explained by increased sialylation of LPS in the mutant. In Neisseria, sialylation is catalysed by an α-2,3-sialyltransferase (Lst) that monosialylates the terminal galactose of LOS (for lipooligosaccharide) by using 5′-cytidine monophospho-N-acetylneuraminic acid as donor (Mandrell et al., 1990). Recently, it has been shown that Lst is a surface-exposed OMP with its active site possibly facing the periplasm (Shell et al., 2002). In the CMomp85 strain grown without IPTG, LPS is accumulating in a compartment where it could be subjected to increased sialylation by Lst. To check whether the accumulating upper band in the non-induced cells corresponds to intensive sialylation of LPS, these samples were subjected to neuraminidase treatment. The upper band is disappearing following this treatment, indicating that this band corresponds to a sialylated form of LPS (results not shown).
Transport of the integral OMPs PorA and Opa to the OM is not impaired when Omp85 is depleted, though an increased amount of PorA and Opa degradation products is observed in the OM fractions. Various studies have shown that in E.coli, LPS is required for the correct assembly of OMP (de Cock and Tommassen, 1996). In contrast, in N.meningitidis, the trimerization of porins is not affected in the LPS-deficient mutant (Steeghs et al., 2001), though some degradation products of these OMPs were observed.
We have shown that in the absence of Omp85, LPS accumulates in the IM; this is probably toxic for the bacteria and would explain why an omp85 knockout mutant could not be isolated. The isolation of an omp85 knockout mutant in the LPS-deficient mutant would have demonstrated that Omp85 is only involved in LPS transport to the OM. Using different constructs containing either a kanamycin or chloramphenicol marker inserted into omp85, it was not possible to isolate such a mutant, suggesting that Omp85 might have an additional function. We therefore checked if depletion of Omp85 also has an effect on PL transport to the OM. This turned out to be the case, explaining why we could not isolate a double omp85-lpxA mutant. Consistently, homologues of Omp85 have also been found in the genomes of Treponema pallidum and Borrelia burgdorferi (Fraser et al., 1997, 1998), which are spirochetes that do not contain LPS in their OM (Hardy and Levin, 1983). In these genomes, no lpx genes are found and the omp85 homologues are associated with ompH encoding a periplasmic chaperone. Nevertheless, other glycolipids as constituents of the OM of LPS-lacking bacteria have been described (Schultz et al., 1998), and it would be interesting to study the probable involvement in lipid transport of the Omp85 homologues in these organisms.
Under Omp85 depletion conditions, growth is affected and the bacteria will eventually start to lyse. This makes it critical to find the right timing for analysing membrane composition. If bacteria are growing for too short a time without IPTG, the Omp85 depletion is not complete; if the depletion time is too long, bacteria start to lyse, making membrane fractionation difficult to achieve. We used different growth conditions in this work: after 4 h of growth without IPTG, we could see an effect on LPS localization (data not shown), but it was less marked than after 6 h of growth. However, after longer depletion times, the OM in particular became difficult to isolate, presumably due to its disintegration when the lipid content becomes too low (results not shown). These disadvantages are inherent with mutants with regulated expression, and can only be circumvented by using a temperature-sensitive mutant of Omp85, which may, however, be difficult to isolate. This strategy has been successful in the characterization of the MsbA protein involved in the transport of lipids through the IM (Doerrler et al., 2001).
During the revision of our manuscript, another study on the role of meningococcal Omp85 in OM biogenesis was published (Voulhoux et al., 2003). These authors reported that Omp85 appeared to be essential for viability, and that unassembled forms of various OMPs accumulated upon Omp85 depletion. However, they analysed the effect of Omp85 depletion after 20 h of incubation under non-induced conditions, and by this time the observed defect in OMP assembly could very well be a secondary result due to a block in lipid export to the OM. Indeed, they report a reduction in LPS content of cell envelopes of 60% upon Omp85 depletion. The authors argue against this possibility by quoting our previous work on the LPS-deficient meningococcal lpxA mutant, which demonstrated that LPS is not required for OMP export and assembly in this organism (Steeghs et al., 2001). However, our present work shows a role for Omp85 as a more general lipid transporter and, if no new OM lipid bilayer can be formed due to Omp85 depletion, insertion of new OMPs is bound to be affected at some point. Our results with OM and IM separation at earlier time points (4 and 6 h) demonstrate that the effect on lipid export precedes any effect on OMP localization.
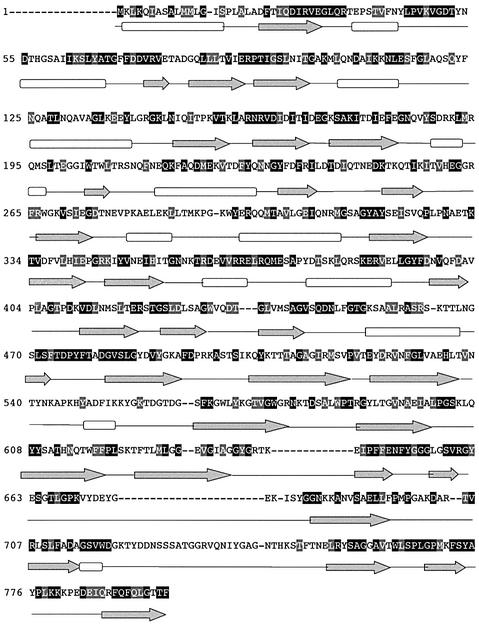
A topological model of Omp85 was elaborated to investigate how an OMP could be involved in lipid transport. We performed ClustalW alignment between Omp85 and its homologues in Neisseria gonorrhoeae, Salmonella enterica, E.coli, Haemophilus influenzae, Pasteurella multocida and Brucella abortus (Higgins et al., 1996). Figure 7 shows the results for the Omp85 sequence of N.meningitidis, with black boxes for conserved identical residues and grey boxes for conserved similar residues. Conservation in amino acid sequences is observed throughout, with some defined boxes corresponding to conserved secondary structures in the N-terminal part of the protein. Secondary structure prediction methods, such as PsiPred (Jones, 1999) and PHD (Rost and Sander, 1993), predict at least two domains. The N-terminal domain consists of β-strands and α-helices and is very well conserved among all Omp85 homologues. According to secondary structure predictions, a motif of three β-strands and two α-helices is repeated five times in the N-terminal domain. The C-terminal domain mainly consists of β-strands and could form a β-barrel for anchorage in the OM. This model thus reveals features characteristic of a transporter, as described for other OMPs belonging to the family of iron transporters, FepA (Buchanan et al., 1999) and FhuA (Ferguson et al., 1998). Conceivably, the C-terminal β-barrel domain could be involved in transport while the N-terminal domain interacts with other proteins in the periplasm. Indeed, Manning et al. (1998) reported surface cross-linking experiments that suggest that Omp85 is surface exposed and interacts with other proteins. Among them is a 34 kDa protein, homologous to IAP34, a GTP-binding protein belonging to the chloroplast import-associated protein (IAP) complex (Schnell et al., 1994).
Fig. 7. Secondary structure predictions for Omp85 in N.meningitidis and its homologues in other Gram-negative bacteria (N.gonorrhoeae, S.enterica, E.coli, H.influenzae, P.multocida and B.abortus). Multiple sequence alignments were performed using the ClustalW program. Shown is the N.meningitidis Omp85 sequence. Black and grey squares represent identical and similar residues in the alignment, respectively. Predicted β-strands and α-helices are represented by grey arrows and white rectangles, respectively.
In conclusion, we have demonstrated that Omp85 is required for LPS and PL transport to the OM. In the same way that MsbA functions in the IM to transport lipid A core and PL actively to the periplasm (Chang and Roth, 2001; Doerrler et al., 2001), Omp85 could be a general lipid transporter in the OM. LPS transport to the OM is likely to involve two steps: first, from the periplasm to the inner leaflet of the OM and, secondly, from the inner leaflet of the OM to the outer surface of the OM (Raetz and Whitfield, 2002). Our data strongly suggest an essential role for Omp85 in this process. Whether Omp85 directly functions as a transporter, or has a more indirect effect due to its interaction with other components required for this export pathway, remains to be established. However, its nature as an OMP potentially forming a β-barrel is consistent with a transport function within the OM, i.e. the last step in the pathway mentioned above. The present work demonstrates its essential role in lipid transport and, subsequently, opens the door to further elucidation of this pathway. In addition, because Omp85 is a surface-exposed and highly conserved protein essential for viability, it will be a good target for the design of new antibiotics.
Materials and methods
Bacterial strains and growth conditions
Escherichia coli NM522 (Promega), used for propagation of plasmids, was grown in LB medium containing ampicillin (100 µg/ml), kanamycin (100 µg/ml) and erythromycin (200 µg/ml) at 37°C. Neisseria meningitidis strains were grown overnight at 37°C on GC medium base (Difco Laboratories) supplemented with IsoVitaleX (Becton Dickinson) in a humid atmosphere containing 5% CO2. Neisseria meningitidis H44/76 CMomp85 strain was grown on GC plates in the same conditions but with addition of 0.01 mM IPTG (Roche). For the characterization of the CMomp85 mutant, bacteria were grown in Meningococcal Medium (van der Ley et al., 1993) without IPTG to impose depletion of Omp85. As control, bacteria were grown with IPTG at a concentration of 0.05 mM. Bacteria were grown for 4 or 6 h (log phase) and inactivated by incubation for 2 h with 200 µg/ml tetracycline in 2% ethanol (final concentration) at 37°C under aeration. For PL characterization, bacteria were grown for 6 h in the presence of 1 µCi/ml [1,2-14C]acetic acid (specific activity 50–120 mCi/mmol, ICN Biochemicals). Transformation of N.meningitidis was done as described previously (van der Ley and Poolman, 1992) with selection of erythromycin (5 µg/ml) or kanamycin (100 µg/ml).
RNA isolation and RT–PCR assays
Neisseria meningitidis cultures (100 ml) were grown to mid-exponential growth phase in TSB (Difco Laboratories). Bacterial cells were harvested by centrifugation for 15 min at 2500 r.p.m., washed and resuspended in 2 ml of TE (Tris 10 mM, EDTA 1 mM pH 8) containing 1% SDS and proteinase K (200 µg/ml final concentration) and incubated for 1 h at 37°C. Total RNA was then extracted using the RNeasy Total RNA Midi kit (Qiagen). Contaminating DNA was digested with RQ1 DNase I (Promega). This enzyme was later eliminated by extraction twice with phenol/chloroform/isoamylalcohol (25:24:1) followed by ethanol precipitation. RNA was resuspended in 20 µl of RNase-free water. RNaseOUT ribonuclease inhibitor (Life Technologies) and random primers were added to 2 µl of this DNase-treated RNA and denatured by heating to 70°C for 10 min. Reverse transcription was performed with Superscript II reverse transcriptase (Life Technologies), in a final volume of 20 µl containing 10 mM dithiothreitol, 1 mM dNTP (Eurogentec), 50 U of reverse transcriptase and the manufacturer’s buffer (provided with the enzyme). This mixture was incubated at 42°C for 50 min and the enzyme was inactivated by heating to 70°C for 15 min. A control reaction containing the same components but no reverse transcriptase was included to check for DNA contamination. The cDNA products (2 µl) were then used in a PCR performed in a final volume of 50 µl containing 1 U of Biotools DNA polymerase (B&M Labs SA), 1 mM dNTP, manufacturer’s buffer (provided with the enzyme) and 10 pmol of each primer. A positive control in which N.meningitidis genomic DNA was used as a template for PCR was included. The amplification programme consisted of one cycle of 5 min at 95°C followed by 30 cycles of 45 s at 95°C, 45 s at annealing temperature (depending on the primer used), 1 min at 72°C and a final step of 10 min at 72°C.
Construction of plasmids
Plasmids were constructed by using standard recombinant DNA techniques (Sambrook and Russel, 2001). Plasmid DNA was isolated using the Wizard kit (Promega). Plasmid pGEM-Teasy-omp85 is a pGEM-T easy derivative (Promega) containing the omp85 gene amplified with Expand High Fidelity polymerase (Roche) with primers omp85F (5′-ctcatgatgctgatgatggc-3′) and omp85R.3 (5′-aaacgggtcatggtaaaactcc-3′). This plasmid was the basis for the construction of plasmid pCF3ery-CMomp85 which was used to make the conditional omp85 mutant. The omp85 gene was cloned from pGEM-Teasy-omp85 in pJF118EH in EcoRI sites, in such a way so as to place the omp85 gene under the control of the tac promoter (Furste et al., 1986). In parallel, an 85mer linker (5′-cgatgatgtgaaacaaacccccgcttttgcggggtttgtttttttgctcgcgagtagcccaagcttgggaaccgctcgagcggat-3′) containing NruI, HindIII and XhoI sites (underlined) possesses ClaI overhangs and contains the 42 bp transcriptional terminator of the rmpM gene. It was cloned in the ClaI site of the pCF3 plasmid (van der Ley et al., 1995), downstream of the rmpM gene and its transcriptional terminator. The orientation of the linker in pCF3 was checked by PCR and sequence analysis with an Applied Biosystems automatic sequencer 310 on double-stranded plasmid DNA templates with a cycle sequencing protocol and primer hybridizing with the rmpM gene. The erythromycin cassette was cloned in HindIII–XhoI sites to obtain the pCF3ery plasmid. Subsequently, the lacIq-tac-omp85 region released from plasmid pJF118EHomp85 by an NruI–HindIII digestion was inserted into the unique NruI–HindIII sites of the pCF3ery plasmid, resulting in plasmid pCF3ery-CMomp85. The orientation of the lacIq-tac-omp85 region was checked by restriction analysis with KpnI, which is present both in the original plasmid and in the omp85 gene. Plasmid pGEM-Teasy-Δomp85-Kan was used to delete omp85 at its own locus. We first amplified upstream (439 bp) and downstream (539 bp) parts of omp85 with primers omp85F2SB (5′-gttcctgcaggcggatccgctggatggtgaagtcggcaa-3′) and orfR (5′-aacgcctccgtcagccatat-3′) for the upstream part, and primers omp85R2SB (5′-gcggatccgcctgcaggaacgaagacgaaatccaacgctt-3′) and ompHF.1 (5′-aatgacatcgtaaccttcctgt-3′) for the downstream part. Primers omp85F2SB and omp85R2SB contain BamHI sites at their 5′ ends and region of 20 bp that anneal to each other. We used a site-directed mutagenesis approach to amplify and clone both fragments together in the pGEM-Teasy vector. Subsequently, we cloned in the BamHI site the Kan cassette released by BamHI restriction from pUC4K. The orientation of the Kan cassette was checked by ClaI restriction analysis. We first transformed N.meningitidis H44/76 with pCF3ery-CMomp85 plasmid. Erythromycin-resistant clones were transformed with pGEM-Teasy-Δomp85 and selected on kanamycin only upon addition of IPTG to the media. The strain obtained was called N.meningitidis H44/76 CMomp85.
We used plasmid pGEM-T-easy-ΔlpxA-ery to delete lpxA at its own locus. We used the same strategy as explained above for the deletion of omp85 to delete lpxA. The upstream part of lpxA was amplified using primers lpxA.2 (5′-cggtccgcacgccgtcatcaac-3′) and lpxA-UP (5′-aattcccccggggaagcttccggtaacgtgtccggcaa-3′) containing AvaI and HindIII sites (underlined). The downstream part of lpxA was amplified using primers lpxAcom.2US (5′-ccgatgccgtctgaaacgtacggtcagcggatgatgccg-3′) containing the uptake sequence (underlined), and lpxA-DOWN (5′-ggaagcttccccgggggaattccaattctgccgcatcggc-3′) containing HindIII and AvaI sites (underlined).
The resulting clone allowed us to transform with pGEM-T-easy-Δomp85-Kan in an assay to isolate double mutant ΔlpxA-Δomp85.
Electron microscopy
Meningococci were grown with or without 0.05 mM IPTG for 6 h, harvested, fixed in three different solutions, washed in cacodylate buffer, pelleted for 5 min at 2000 g at 20°C, taken up in agar [2% (w/v)] and embedded in epoxy resin glycidether 100 (Merck). The following three procedures were used. (i) Bacteria were fixed in Karnovsky solution (2% PFA and 2.5% glutaraldehyde in 0.1 M cacodylate/HCl pH 7.4) and post-fixed in osmium tetroxide (OsO4) [1% (w/v)] with 1.5% ferrocyanide in 0.1 M cacodylate buffer. Ultrathin sections were contrasted with aqueous uranyl acetate [2% (w/v)] and lead citrate (1% w/v). (ii) A procedure in which lipids were retained: bacteria were fixed in freshly prepared ice-cold glutaraldehyde [2% (w/v)] with OsO4 [1% (w/v)] in 0.1 M cacodylate/HCl pH 7.4 buffer (Hayat, 1981). Sections were contrasted with aqueous uranyl acetate [2% (w/v)] and lead citrate [1% (w/v)]. (iii) A procedure in which polysaccharides were detected cytochemically: bacteria were fixed in PFA [4% (w/v)] in 0.1 M cacodylate/HCl pH 7.4. Ultrathin sections were stained cytochemically for polysaccharides (Ainsworth et al., 1972). Ultrathin sections were examined in a Philips TECHNAI 12 electron microscope (Philips/FEI, Eindhoven, The Netherlands). Images were digitally acquired with an SIS MegaView II CCD-camera and processed with SIS NT-DOCU TEM analysis software (Soft Imaging System, Münster, Germany).
Membrane separation
For LPS and OMP characterization, IMs and OMs were isolated and separated by sucrose density gradient centrifugation as described (Steeghs et al., 2001).
For PL characterization, bacterial cell fractionation was performed by another method, based on the lysis of lysozyme–EDTA spheroblasts (Osborn et al., 1972). The lysed bacteria were loaded directly on to the gradient without a sonication step.
Lactate dehydrogenase activity assay
LDH activity was measured as described (Osborn et al., 1972).
SDS–PAGE and western blotting
Prior to electrophoresis, whole-cell extracts or fractions were incubated in sample loading buffer (Lugtenberg et al., 1975) containing 2% SDS (final concentration). Incubations were performed for 10 min at 100°C. SDS–polyacrylamide gels were prepared according to Lugtenberg et al. (1975). Western blots were performed as described (Towbin et al., 1979). The PorA-specific monoclonal antibody was MN5C11G (P1.16). The Opa-specific monoclonal antibodies were 15-1-P5.5 and αD2. The Omp85 rabbit polyclonal antiserum is a generous gift from Dr E.Wedege and Dr J.Holst (Fredriksen et al., 1994).
LPS analysis
Tricine-SDS–PAGE was performed in 4% stacking and 16% separating gels as described (Lesse et al., 1990). Proteinase K-treated and boiled bacterial cells were used as samples (Apicella et al., 1994). The gel was run for 16–17 h at a constant current of 20 mA, and silver stained (Tsai and Frasch, 1982).
PL analysis
PLs were extracted from protein quantified fractions according to the method of Bligh and Dyer (1959). The amount of 14C-labelled PL was determined by liquid scintillation counting of the PL extracts. PLs were separated by TLC on silica gel 60 plates (Merck) using CHCl3/MeOH/HAc, 65:25:10 (by vol.) as eluent. The radioactive spots were analysed with an Instant Imager (Packard Bioscience).
Acknowledgments
Acknowledgements
We thank Christian Didembourg, Hendrik Jan Hamstra, Besty Kuipers and Marina Burger for technical support, Maria-Laura Boschiroli for help in RNA manipulation and RT–PCR experiments, and Elizabeth Wedege and Johan Holst (National Institute of Public Health, Oslo, Norway) for providing the rabbit polyclonal Omp85 antiserum. S.G. was a recipient of a fellowship from the Fonds pour la Recherche dans l’Industrie et l’Agriculture (FRIA, Belgium). This work was supported by FNRS, EMBO and FEMS grants.
References
- Ainsworth S.K., Ito,S. and Karnovsky,M.J. (1972) Alkaline bismuth reagent for high resolution ultrastructural demonstration of periodate-reactive sites. J. Histochem. Cytochem., 20, 995–1005. [DOI] [PubMed] [Google Scholar]
- Apicella M.A., Griffiss,J.M. and Schneider,H. (1994) Isolation and characterization of lipopolysaccharides, lipooligosaccharides and lipid A. Methods Enzymol., 235, 242–252. [DOI] [PubMed] [Google Scholar]
- Bayer M.E. (1968) Areas of adhesion between wall and membrane of Escherichia coli. J. Gen. Microbiol., 53, 395–404. [DOI] [PubMed] [Google Scholar]
- Beall B. and Lutkenhaus,J. (1987) Sequence analysis, transcriptional organization and insertional mutagenesis of the envA gene of Escherichia coli. J. Bacteriol., 169, 5408–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh E.G. and Dyer,W.J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol., 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Buchanan S.K., Smith,B.S., Venkatramani,L., Xia,D., Esser,L., Palnitkar,M., Chakraborty,R., van der Helm,D. and Deisenhofer,J. (1999) Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol., 6, 56–63. [DOI] [PubMed] [Google Scholar]
- Chang G. and Roth,C.B. (2001) Structure of MsbA from E.coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science, 293, 1793–1800. [DOI] [PubMed] [Google Scholar]
- Chen R. and Henning,U. (1996) A periplasmic protein (Skp) of Escherichia coli selectively binds a class of outer membrane protein. Mol. Microbiol., 19, 1287–1294. [DOI] [PubMed] [Google Scholar]
- Dartigalongue C., Loferer,H. and Raina,S. (2001) EcfE, a new essential inner membrane protease: its role in the regulation of heat shock response in Escherichia coli. EMBO J., 20, 5908–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cock H. and Tommassen,J. (1996) Lipopolysaccharides and divalent cations are involved in the formation of an assembly-competent intermediate of outer-membrane protein PhoE of E.coli. EMBO J., 15, 5567–5573. [PMC free article] [PubMed] [Google Scholar]
- Doerrler W.T., Reedy,M.C. and Raetz,C.R. (2001) An Escherichia coli mutant defective in lipid export. J. Biol. Chem., 276, 11461–11464. [DOI] [PubMed] [Google Scholar]
- Ferguson A.D., Hofmann,E., Coulton,J.W., Diederichs,K. and Welte,W. (1998) Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharides. Science, 282, 2215–2220. [DOI] [PubMed] [Google Scholar]
- Fraser C.M. et al. (1997) Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature, 390, 580–586. [DOI] [PubMed] [Google Scholar]
- Fraser C.M. et al. (1998) Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science, 281, 375–388. [DOI] [PubMed] [Google Scholar]
- Fredriksen J.H., Griffiths,E., Grotterod,E.M., Hoiby,E.A., Rosenqvist,E., Stevenson,P. and Wedege,E. (1994) Characterization of high molecular weight components in MenB-vaccine ‘Folkehelsa’; an outer membrane vesicle vaccine against group B meningococcal disease. In Conde-Glez,C.J., Morse,S., Rice,P., Sparling,F. and Calderon,E. (eds), Pathobiology and Immunobiology of Neisseriaceae. Proceedings of the VIII International Pathogenic Neisseria Conference. ISBN: 968-6502-13-0, pp. 818–824.
- Furste J.P., Pansegrau,W., Frank,R., Blocker,H., Scholz,P., Bagdasarian,M. and Lanka,E. (1986) Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene, 48, 119–131. [DOI] [PubMed] [Google Scholar]
- Galloway S.M. and Raetz,C.R. (1990) A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J. Biol. Chem., 265, 6394–6402. [PubMed] [Google Scholar]
- Hardy P.H. Jr and Levin,J. (1983) Lack of endotoxin in Borrelia hispanica and Treponema pallidum. Proc. Soc. Exp. Biol. Med., 174, 47–52. [DOI] [PubMed] [Google Scholar]
- Hayat M. (1981) Fixation for Electron Microscopy. Academic Press, New York.
- Higgins D.G., Thompson,J.D. and Gibson,T.J. (1996) Using CLUSTAL for multiple sequence alignments. Methods Enzymol., 266, 383–402. [DOI] [PubMed] [Google Scholar]
- Jones D.T. (1999) Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol., 292, 195–202. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. (1990) The ‘Bayer bridges’ confronted with results from improved electron microscopy methods. Mol. Microbiol., 4, 697–705. [DOI] [PubMed] [Google Scholar]
- Kelly T.M., Stachula,S.A., Raetz,C.R. and Anderson,M.S. (1993) The firA gene of Escherichia coli encodes UDP-3-O-(R-3- hydroxymyristoyl)-glucosamine N-acyltransferase. The third step of endotoxin biosynthesis. J. Biol. Chem., 268, 19866–19874. [PubMed] [Google Scholar]
- Lesse A.J., Campagnari,A.A., Bittner,W.E. and Apicella,M.A. (1990) Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate–polyacrylamide gel electrophoresis. J. Immunol. Methods, 126, 109–117. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers,J., Peters,R., van der Hoek,P. and van Alphen,L. (1975) Electrophoretic resolution of the ‘major outer membrane protein’ of Escherichia coli K12 into four bands. FEBS Lett., 58, 254–258. [DOI] [PubMed] [Google Scholar]
- Mandrell R.E. et al. (1990) In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J. Exp. Med., 171, 1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning D., Reschke,D. and Judd,R. (1998) Omp85 proteins of Neisseria gonorrhoeae and Neisseria meningitidis are similar to Haemophilus influenza D15 antigen and Pasteurella multocida Oma87. Microb. Pathog., 25, 11–21. [DOI] [PubMed] [Google Scholar]
- McMurry L.M., Oethinger,M. and Levy,S.B. (1998) Triclosan targets lipid synthesis. Nature, 394, 531–532. [DOI] [PubMed] [Google Scholar]
- Missiakas D., Betton,J.M. and Raina,S. (1996) New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol. Microbiol., 21, 871–884. [DOI] [PubMed] [Google Scholar]
- Mohan S., Kelly,T.M., Eveland,S.S., Raetz,C.R. and Anderson,M.S. (1994) An Escherichia coli gene (FabZ) encoding (3R)-hydroxymyristoyl acyl carrier protein dehydrase. Relation to fabA and suppression of mutations in lipid A biosynthesis. J. Biol. Chem., 269, 32896–32903. [PubMed] [Google Scholar]
- Nikaido H. (1996) The outer membrane. In Neidhardt,F.C. et al. (eds), Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 29–47.
- Onishi H.R. et al. (1996) Antibacterial agents that inhibit lipid A biosynthesis. Science, 274, 980–982. [DOI] [PubMed] [Google Scholar]
- Osborn M.J., Gander,J.E., Parisi,E. and Carson,J. (1972) Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem., 247, 3962–3972. [PubMed] [Google Scholar]
- Raetz C.R. and Dowhan,W. (1990) Biosynthesis and function of phospholipids in Escherichia coli. J. Biol. Chem., 265, 1235–1238. [PubMed] [Google Scholar]
- Raetz C.R. and Whitfield,C. (2002) Lipopolysaccharide endotoxins. Annu. Rev. Biochem., 71, 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio T.L. and Silhavy,T.J. (2001) Periplasmic stress and ECF σ factors. Annu. Rev. Microbiol., 55, 591–624. [DOI] [PubMed] [Google Scholar]
- Rost B. and Sander,C. (1993) Prediction of protein secondary structure at better than 70% accuracy. J. Mol. Biol., 232, 584–599. [DOI] [PubMed] [Google Scholar]
- Sacchettini J.C. and Poulter,C.D. (1997) Creating isoprenoid diversity. Science, 277, 1788–1789. [DOI] [PubMed] [Google Scholar]
- Sambrook J. and Russel,D.W. (2001) Molecular Cloning: A Laboratory Manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schnell D.J., Kessler,F. and Blobel,G. (1994) Isolation of components of the chloroplast protein import machinery. Science, 266, 1007–1012. [DOI] [PubMed] [Google Scholar]
- Schultz C.P., Wolf,V., Lange,R., Mertens,E., Wecke,J., Naumann,D. and Zahringer,U. (1998) Evidence for a new type of outer membrane lipid in oral spirochete Treponema denticola. Functioning permeation barrier without lipopolysaccharides. J. Biol. Chem., 273, 15661–15666. [DOI] [PubMed] [Google Scholar]
- Shell D.M., Chiles,L., Judd,R.C., Seal,S. and Rest,R.F. (2002) The Neisseria lipooligosaccharide-specific α-2,3-sialyltransferase is a surface-exposed outer membrane protein. Infect. Immun., 70, 3744–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeghs L., den Hartog,R., den Boer,A., Zomer,B., Roholl,P. and van der Ley,P. (1998) Meningitis bacterium is viable without endotoxin. Nature, 392, 449–450. [DOI] [PubMed] [Google Scholar]
- Steeghs L., de Cock,H., Evers,E., Zomer,B., Tommassen,J. and van Der Ley,P. (2001) Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J., 20, 6937–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Kuzuyama,T., Watanabe,H. and Seto,H. (1998) A 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc. Natl Acad. Sci. USA, 95, 9879–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin,T. and Gordon,J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA, 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.M. and Frasch,C.E. (1982) A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem., 119, 115–119. [DOI] [PubMed] [Google Scholar]
- van der Ley P. and Poolman,J.T. (1992) Construction of a multivalent meningococcal vaccine strain based on the class 1 outer membrane protein. Infect. Immun., 60, 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., van der Biezen,J., Hohenstein,P., Peeters,C. and Poolman,J.T. (1993) Use of transformation to construct antigenic hybrids of the class 1 outer membrane protein in Neisseria meningitidis. Infect. Immun., 61, 4217–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., van der Biezen,J. and Poolman,J.T. (1995) Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine, 13, 401–407. [DOI] [PubMed] [Google Scholar]
- Vieira J. and Messing,J. (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene, 19, 259–268. [DOI] [PubMed] [Google Scholar]
- Voulhoux R., Bos,M.P., Geurtsen,J., Mols,M. and Tommassen,J. (2003) Role of a highly conserved bacterial protein in outer membrane protein assembly. Science, 299, 262–265. [DOI] [PubMed] [Google Scholar]