Abstract
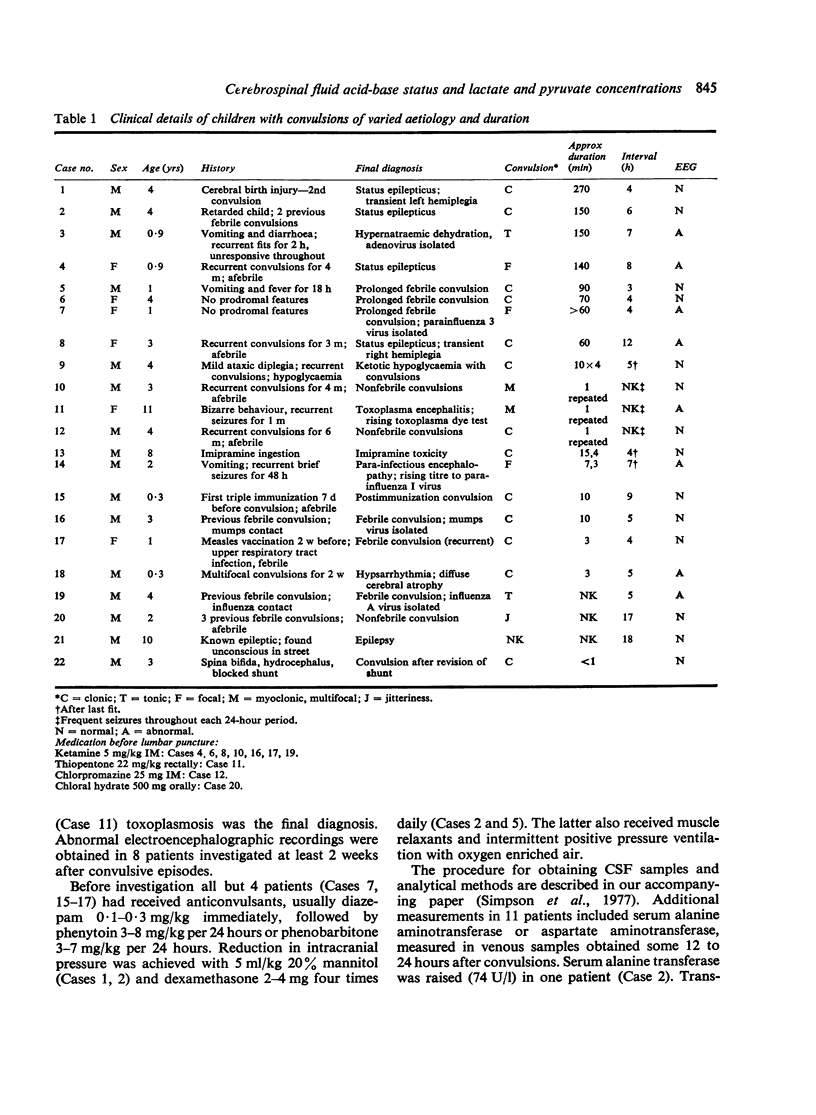
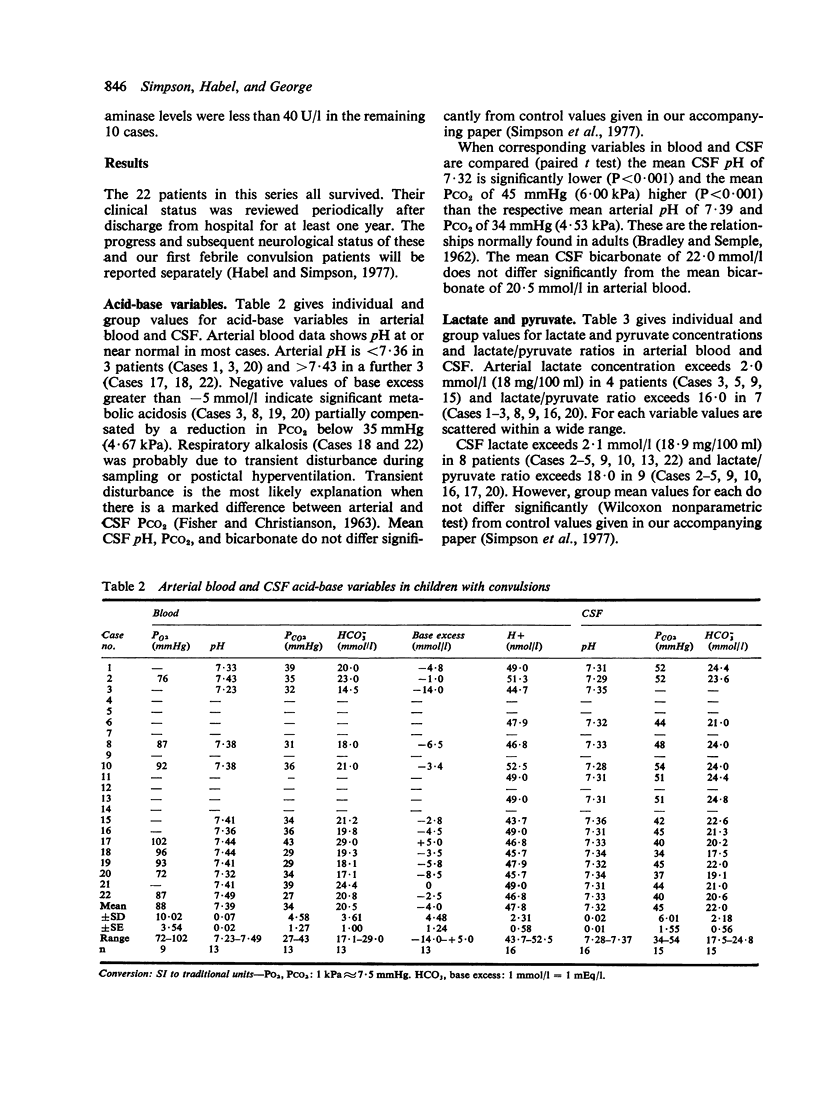
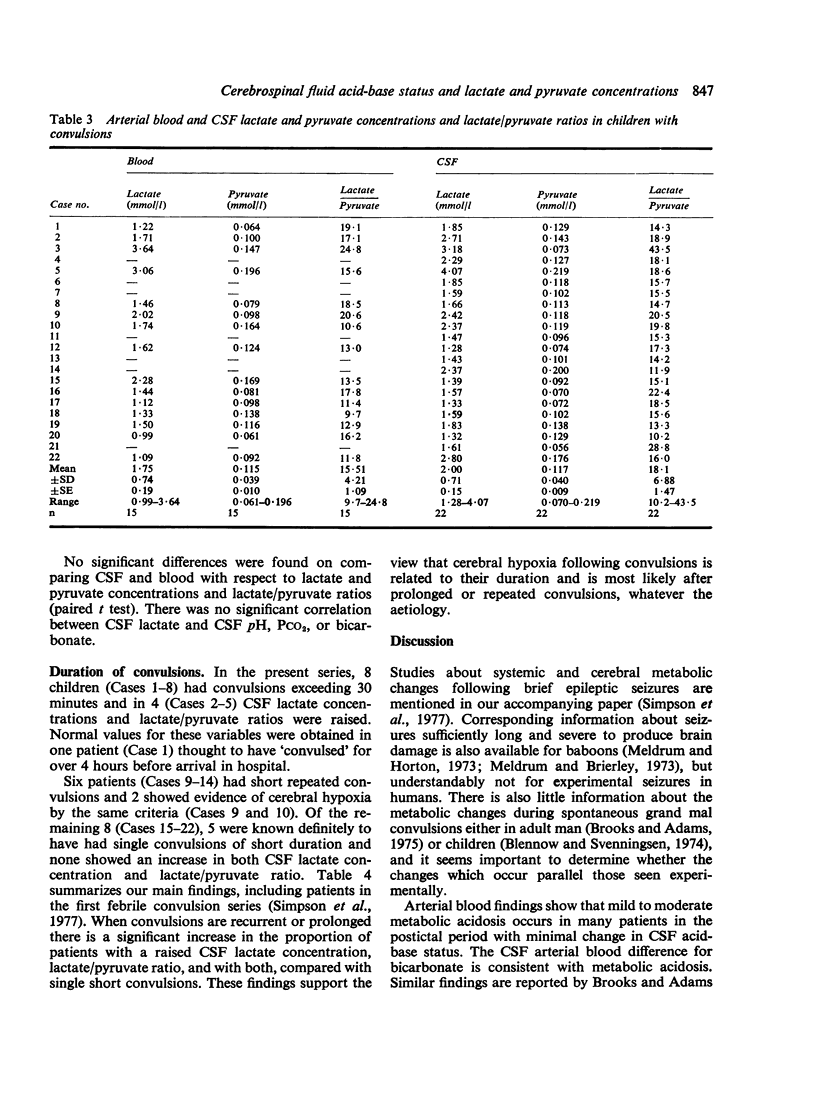
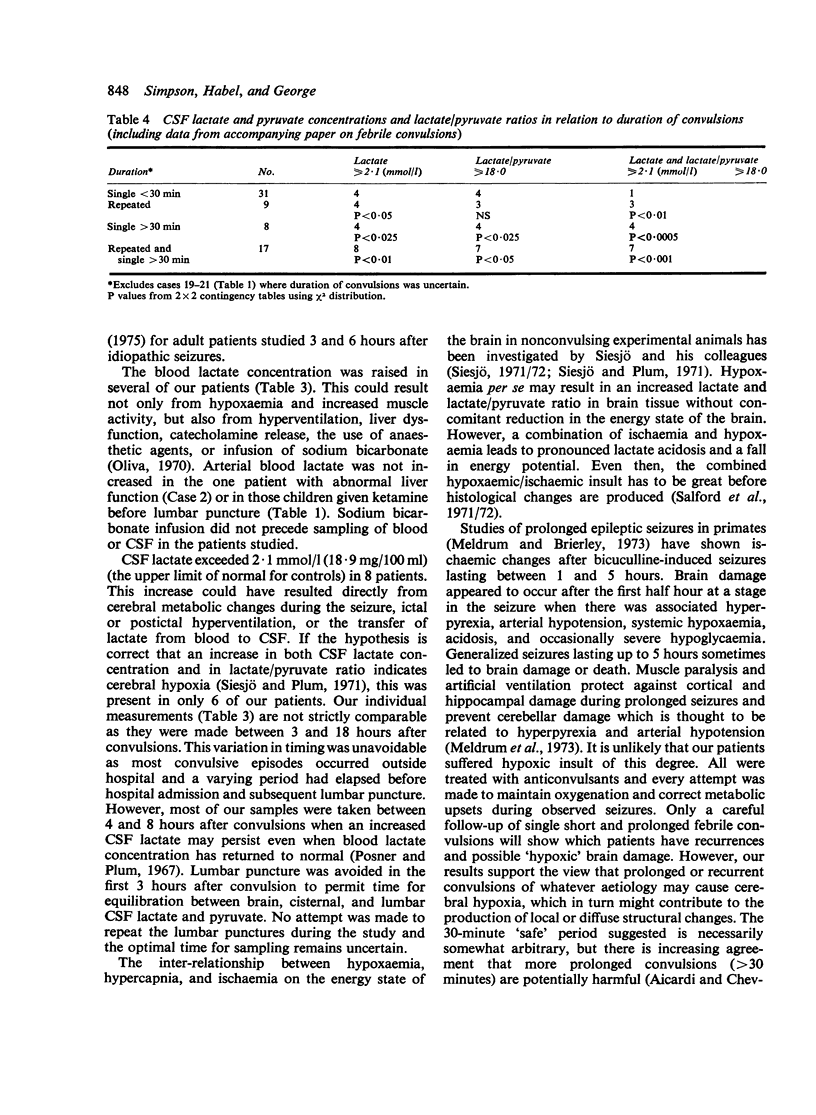
Twenty-two infants and children were studied after convulsions of varied cause and duration. Arterial and CSF acid-base variables, lactate and pyruvate concentrations, and lactate/pyruvate ratios were measured between 3 and 18 hours after convulsive episodes. Biochemical signs of cerebral hypoxia were found in 7 patients with prolonged (greater than 30 minutes) or recurrent short convulsions. These signs were absent in patients with single short convulsions. These findings indicate that cerebral hypoxia and possible brain damage is a hazard of prolonged or rapidly recurring short convulsions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aicardi J., Baraton J. A pneumoencephalographic demonstration of brain atrophy following status epilepticus. Dev Med Child Neurol. 1971 Oct;13(5):660–667. doi: 10.1111/j.1469-8749.1971.tb08333.x. [DOI] [PubMed] [Google Scholar]
- Aicardi J., Chevrie J. J. Convulsive status epilepticus in infants and children. A study of 239 cases. Epilepsia. 1970 Jun;11(2):187–197. doi: 10.1111/j.1528-1157.1970.tb03880.x. [DOI] [PubMed] [Google Scholar]
- BRADLEY R. D., SEMPLE S. J. A comparison of certain acidbase characteristics of arterial blood, jugular venous blood and cerebrospinal fluid in man, and the effect on them of some acute and chronic acid-base disturbances. J Physiol. 1962 Mar;160:381–391. doi: 10.1113/jphysiol.1962.sp006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow G., Svenningsen N. W. Cerebrospinal fluid lactate-pyruvate ratio in children with febrile convulsions. Neuropadiatrie. 1974 May;5(2):157–161. doi: 10.1055/s-0028-1091698. [DOI] [PubMed] [Google Scholar]
- Brooks B. R., Adams R. D. Cerebrospinal fluid acid-base and lactate changes after seizures in unanesthetized man. I. Idiopathic seizures. Neurology. 1975 Oct;25(10):935–942. doi: 10.1212/wnl.25.10.935. [DOI] [PubMed] [Google Scholar]
- FISHER V. J., CHRISTIANSON L. C. Cerebrospinal fluid acidbase balance during a changing ventilatory state in man. J Appl Physiol. 1963 Jul;18:712–716. doi: 10.1152/jappl.1963.18.4.712. [DOI] [PubMed] [Google Scholar]
- FOWLER M. Brain damage after febrile convulsions. Arch Dis Child. 1957 Apr;32(162):67–76. doi: 10.1136/adc.32.162.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer M. A. Genetic and related aetiological factors in temporal lobe epilepsy. A review. Epilepsia. 1971 Mar;12(1):13–31. doi: 10.1111/j.1528-1157.1971.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Meldrum B. S., Brierley J. B. Prolonged epileptic seizures in primates. Ischemic cell change and its relation to ictal physiological events. Arch Neurol. 1973 Jan;28(1):10–17. doi: 10.1001/archneur.1973.00490190028002. [DOI] [PubMed] [Google Scholar]
- Meldrum B. S., Horton R. W. Physiology of status epilepticus in primates. Arch Neurol. 1973 Jan;28(1):1–9. doi: 10.1001/archneur.1973.00490190019001. [DOI] [PubMed] [Google Scholar]
- Meldrum B. S., Vigouroux R. A., Brierley J. B. Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Arch Neurol. 1973 Aug;29(2):82–87. doi: 10.1001/archneur.1973.00490260026003. [DOI] [PubMed] [Google Scholar]
- Oliva P. B. Lactic acidosis. Am J Med. 1970 Feb;48(2):209–225. doi: 10.1016/0002-9343(70)90117-8. [DOI] [PubMed] [Google Scholar]
- Posner J. B., Plum F. Independence of blood and cerebrospinal fluid lactate. Arch Neurol. 1967 May;16(5):492–496. doi: 10.1001/archneur.1967.00470230044005. [DOI] [PubMed] [Google Scholar]
- Salford L. G., Brierley J. B., Plum F., Siesjö B. K. Energy metabolism and histology in the brain during combined hypoxemia and ischemia. Eur Neurol. 1971;6(1):329–334. doi: 10.1159/000114517. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Metabolism and flow in the hypoxic brain. Eur Neurol. 1971;6(1):43–48. doi: 10.1159/000114464. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Plum F. Cerebral energy metabolism in normoxia and in hypoxia. Acta Anaesthesiol Scand Suppl. 1971;45:81–101. doi: 10.1111/j.1399-6576.1971.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Simpson H., Habel A. H., George E. L. Cerebrospinal fluid acid-base status and lactate and pyruvate concentrations after short (less than 30 minutes) first febrile convulsions in children. Arch Dis Child. 1977 Nov;52(11):836–843. doi: 10.1136/adc.52.11.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasterlain C. G. Effects of neonatal status epilepticus on rat brain development. Neurology. 1976 Oct;26(10):975–986. doi: 10.1212/wnl.26.10.975. [DOI] [PubMed] [Google Scholar]
- Wasterlain C. G., Plum F. Vulnerability of developing rat brain to electroconvulsive seizures. Arch Neurol. 1973 Jul;29(1):38–45. doi: 10.1001/archneur.1973.00490250056006. [DOI] [PubMed] [Google Scholar]


