Abstract
Hsp90, an abundant heat shock protein that is highly expressed even under physiological conditions, is involved in the folding of key molecules of the cellular signal transduction system such as kinases and steroid receptors. It seems to contain two chaperone sites differing in substrate specificity. Binding of ATP or the antitumor drug geldanamycin alters the substrate affinity of the N-terminal chaperone site, whereas both substances show no influence on the C-terminal one. In wild-type Hsp90 the fragments containing the chaperone sites are connected by a highly charged linker of various lengths in different organisms. As this linker region represents the most striking difference between bacterial and eukaryotic Hsp90s, it may be involved in a gain of function of eukaryotic Hsp90s. Here, we have analyzed a fragment of yeast Hsp90 consisting of the N-terminal domain and the charged region (N272) in comparison with the isolated N-terminal domain (N210). We show that the charged region causes an increase in the affinity of the N-terminal domain for nonnative protein and establishes a crosstalk between peptide and ATP binding. Thus, the binding of peptide to N272 decreases its affinity for ATP and geldanamycin, whereas the ATP-binding properties of the monomeric N-terminal domain N210 are not influenced by peptide binding. We propose that the charged region connecting the two chaperone domains plays an important role in regulating chaperone function of Hsp90.
Keywords: heat shock proteins, antitumor drugs, peptide binding, steroid receptors, titration calorimetry
The Hsp90 family is one of the major heat shock proteins, which include the well-examined Hsp70 and Hsp60 classes (1). In contrast to these proteins, little is known about functional properties and mechanistic details of Hsp90, although it is the most abundant heat shock protein in eukaryotic cells (2). All known members of the Hsp90 protein family are highly conserved, especially in the N-terminal and C-terminal regions (3). These regions were recently shown to contain independent chaperone sites (4, 5) with different substrate specificity. In this context, the previously observed ATP-independent chaperone activity of Hsp90 (6–8) could be shown to be located in the dimeric C-terminal fragment, which acts as a promiscuous binding site for nonnative substrates (5). The N-terminal domain, which binds ATP (9, 10) and the antitumor drug geldanamycin (GA) (11, 12), was found to be able to interact selectively with completely unfolded proteins and small peptides (5). Binding of ATP or GA lowered the affinity of this domain for substrates, resulting in the release of bound polypeptides. ADP showed no influence on the in vitro chaperone activity of this fragment (5).
In wild-type Hsp90 the highly conserved N- and C-terminal regions are connected by a charged linker (3). Comparison of all known Hsp90 sequences revealed that this linker is of variable length and is nearly completely missing in prokaryotic Hsp90s (13) and a new eukaryotic member of the Hsp90 family named Trap-1 (14, 15). As this linker region represents the most striking difference between bacterial and eukaryotic Hsp90s, it may be involved in a gain of function of eukaryotic Hsp90s. This view was strengthened by the observation that in yeast, in which Hsp90 is essential for viability (16), Escherichia coli Hsp90 is not able to rescue Hsp90-depleted strains (17), whereas yeast Hsp90 mutants lacking some parts missing in E. coli showed no detectable influence on cell growth (18).
To address the function of the charged region, we analyzed the chaperone activity of an N-terminal fragment of yeast Hsp90 comprising the N-terminal chaperone site (N210) and the charged region. Our data reveal that the charged region increases the substrate affinity of N272 compared with the monomeric fragment N210. At the same time, peptide binding decreases the affinity of N272 for ATP.
MATERIALS AND METHODS
Protein Purifications.
Yeast Hsp90 was purified as described previously (19). The yeast Hsp90 fragments comprising the amino acids 1–210 (N210, 23,100 Da) and 262–709 (262C, 48,300 Da) were expressed and purified as described (5). The yeast Hsp90 fragment consisting of amino acids 1–272 (N272, 29,400 Da) was purified after recombinant expression in E. coli using the protocol established for N210 (5). The purity of the fragment was >97% as measured by densitometry. The concentration of N272 was determined by using the calculated extinction coefficient of 0.49 for a 1 mg/ml solution in a 1-cm cuvette at 280 nm (20).
Insulin Assay.
Insulin aggregation was followed by monitoring turbidity at 650 nm as described (5). Insulin (50 μM) was preincubated at 30°C with various concentrations of the fragments in the absence or presence of various additives as described in the figure legends.
Purification of Nucleotides.
Nucleotides were obtained from Boehringer Mannheim. All nucleotides were repurified by reversed-phase chromatography as previously described (21).
Titration Calorimetry.
Changes in temperature upon nucleotide binding to N-terminal fragments of Hsp90 were measured with a Microcal MC-2 high-sensitivity titration calorimeter as described previously (22). The data were analyzed with origin software (Microcal Software, Northampton, MD). All measurements were performed at 28°C.
High-Pressure Liquid Chromatography (HPLC).
A Superdex 200 high-resolution column (Pharmacia) was used for size exclusion chromatography carried out in 40 mM Hepes–KOH, pH 7.5/100 mM KCl/5 mM MgCl2 with a flow rate of 0.5 ml/min and a sample volume of 60 μl. Proteins were detected by fluorescence at an excitation wavelength of 280 nm and an emission wavelength of 330 nm with a Jasco FP-920 fluorescence detector.
Analytical Ultracentrifugation.
Sedimentation velocity and sedimentation equilibrium runs were performed in analytical ultracentrifuges (Beckman Spinco E and Optima XL-A). Double sector cells were used at 16,000, 18,000, 20,000, and 40,000 rpm in AnD, AnF-Ti, and AnTi 60 rotors. The data were analyzed by using the software provided by Beckman Instruments and with a program developed by G. Böhm (University of Regensburg). For calculations, a partial specific volume of 0.734 ml/g was used; all measurements were corrected for water viscosity and 20°C. Analyses were performed at protein concentrations between 0.2 and 1.8 mg/ml.
RESULTS AND DISCUSSION
The Hsp90 Fragments N210 and N272 Differ in Their Peptide-Binding Properties.
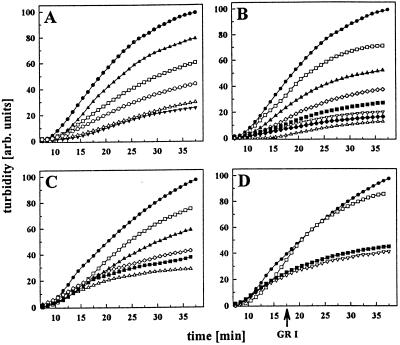
To investigate the influence of the charged region on the function of the N-terminal domain of Hsp90, a fragment, N272, comprising both the N-terminal domain and the charged region, was created by PCR and purified after recombinant expression in E. coli. We performed chaperone assays using insulin as a substrate for Hsp90. In this assay, as previously reported (5), the Hsp90 fragments N210 and 262C were active in suppressing insulin aggregation in a concentration-dependent manner (Fig. 1A and data not shown). Coincubation of the two fragments resulted in a chaperone activity comparable to that in wild-type Hsp90. In the presence of small substrate proteins such as insulin the two chaperone sites seemed to work independently in the wild-type protein (5). Here, we show that the fragment N272 also suppresses insulin aggregation in a concentration-dependent way (Fig. 1B). Interestingly, however, N272 is more active than N210, 262C, or even the wild-type protein in this assay (Fig. 1C). Thus, the presence of the charged domain in the absence of the C-terminal region seemed to increase the affinity of the fragment N272 for nonnative proteins. It is reasonable to speculate that in the wild-type protein, in the absence of partner proteins, the C-terminal domain may negatively influence the binding properties of the N-terminal domain and the charged region (see below). To further analyze the binding properties of N272, we performed competition assays as described previously (5). We preincubated N272 with a 19-aa peptide (GR1) derived from the human glucocorticoid receptor and tested whether insulin could displace GR1 from N272–GR1 complexes. Similar to its influence on N210 (5), binding of GR1 rendered N272 inactive in the insulin assay (Fig. 1D). Surprisingly, however, after preformation of the N272⋅insulin complex, GR1 was unable to compete with insulin for binding to N272 (Fig. 1D). This finding was in contrast to previous observations for N210 (5). An explanation might be that binding of substrate leads to conformational changes in N272 that prevent exchange. The observed increase in binding affinity for the fragment containing the charged region is in agreement with previous observations that purified Hsp90 from higher eukaryotes contains tightly bound peptides (23, 24). These data can hardly be explained by the peptide-binding properties of Hsp90 fragments previously detected in vitro (4, 5).
Figure 1.
Influence of Hsp90 fragments on the aggregation of insulin B-chain. Insulin aggregation (50 μM) was monitored in the absence (●) or presence of Hsp90. Hsp90 fragments or wild-type Hsp90 exhibited no detectable influence on the turbidity of the solution. All measurements with N210, 262C, and yeast Hsp90 reflect experiments repeated as in ref. 5. (A) Influence of increasing concentrations of N210 on insulin aggregation: 5 μM N210 (▴), 10 μM N210 (□), 25 μM N210 (○), 50 μM N210 (▵), and 250 μM N210 (▾). (B) Influence of increasing concentrations of N272 on insulin aggregation: 2 μM N272 (□), 3 μM N272 (▴), 6 μM N272 (⋄), 12.5 μM N272 (■), 25 μM N272 (▿), 50 μM N272 (♦), and 100 μM N272 (▵). (C) Comparison of different Hsp90 fragments and wild-type Hsp90 (6 μM each). Influence of yeast Hsp90 (⋄), N210 (▴), 262C (□), N272 (■), and a coincubation of N272 and 262C (▵). (D) The effects of N272 (5 μM) on insulin aggregation were monitored in the absence (▿) or presence (□) of the peptide GR1 (50 μM). Addition of GR1 (50 μM) to a preformed N272⋅insulin complex showed no detectable influence on the aggregation kinetics (■). All concentrations are calculated for the respective monomers.
The Charged Region Does Not Influence the Substrate Specificity of the N-Terminal Chaperone Site.
Having established that the charged region increases substrate affinity, we analyzed the substrate specificity of N272. To investigate specificity, we tested the interaction of the Hsp90 fragments with insulin in a competition assay with various peptides derived from the glucocorticoid receptor and other sources (Table 1; refs. 5 and 25). As mentioned above, preincubation of N272 with equimolar concentrations of the 19-aa GR1 rendered N272 inactive in suppressing insulin aggregation (Fig. 1D), whereas addition of GR1 to preformed N272⋅insulin complexes showed no influence on the chaperone activity of this fragment (Fig. 1D). Peptides longer than 13 aa had been found to bind most efficiently to N210, whereas peptides between 8 and 13 aa long were significantly less effective (5). Strikingly, heptapeptides, which had been shown to be sufficient for efficient binding to the Hsp70 chaperone family (26, 27), did not compete with insulin for binding to N210 (5). Investigating the same set of peptides as for 210, we found that peptides with a length of 8 aa or more were able to bind to N272 and to influence its ability to suppress insulin aggregation (Table 1). Again, as for N210, heptapeptides did not efficiently compete with insulin for N272 binding (Table 1). Therefore, we propose that the presence of the charged region does not affect the binding specificity of the N-terminal domain but increases its apparent affinity for peptides.
Table 1.
Influence of peptides on the chaperone activity of N-terminal Hisp90 fragments
| Peptide | Sequence | Turbidity
|
|
|---|---|---|---|
| N210* | N272 | ||
| None | — | 30 | 17 |
| Heptapeptides | |||
| HD 25 | SGFTFND | 35 | 23 |
| HD 86 | LRAEDMA | 39 | 21 |
| HD 131 | GPSVFPL | 53 | 22 |
| HD 132 | PSVFPLA | 38 | 19 |
| HD 133 | SVFPLAPS | 55 | 21 |
| Larger peptides | |||
| GR 3 | AKAILGLRN | 76 | 94 |
| GR 4 | LRNLHLDDQ | 65 | 91 |
| VSV 8 | RGYVYQGL | 71 | 91 |
| GR 5 | LHLDDQMTLL | 46 | 93 |
| HD 131 L | GPSVFLLAPSSKC | 95 | 93 |
| GR 1 | AKAILGLRNLHLDDQMTLL | 92 | 96 |
Aggregation of insulin (50 μM) was measured in the presence of N210 or N272 (50 μM each). The peptides (50 μM each) were incubated for 1 min with the fragments prior to the addition of insulin. The numbers represent the normalized turbidity in arbitrary units determined 35 min after reduction of insulin. The HD peptides were derived from the antibody 3E6 (5, 25).
The results for heptapeptides and N210 are taken from ref. 5. The results for longer peptides and N210 are repeated measurements included for comparison.
Peptide Binding Decreases the Affinity of N272 for ATP and GA.
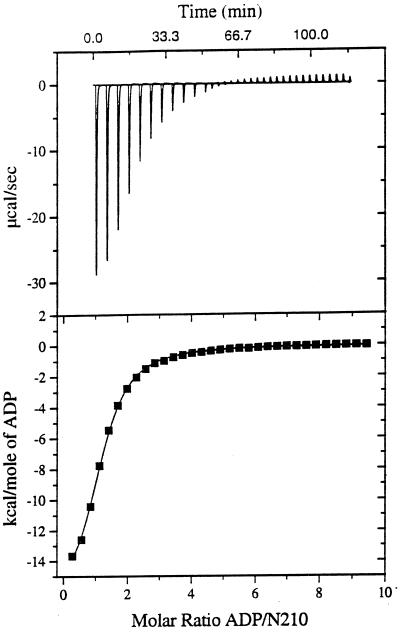
Recently, ATP binding to Hsp90 was shown biochemically (12, 28) and by x-ray crystallography (9). Furthermore, ATP binding was shown to switch the conformational state of the N-terminal domain of Hsp90 (10, 12), leading to a decrease in substrate affinity and release of bound peptides (5). To determine the ATP-binding constants of the different N-terminal Hsp90 fragments exactly we chose titration calorimetry as a rapid and accurate method for characterizing the binding reactions (22). We could confirm the previous estimations for ATP binding to Hsp90 in the micromolar range (9, 28). For N210 we determined a Kd of 98 μM for ATP and a Kd of 40 μM for ADP (Fig. 2, Table 2). For N272 similar values were obtained. The Kd for ATP was 94 μM and for ADP the Kd was 43 μM (Table 2). Thus the presence of the charged region alone does not influence nucleotide binding. We could exclude ATP hydrolysis by N210 or N272 during the calorimetric measurement, as both proteins revealed an ATP turnover number of 0.009 ± 0.001 min−1 under the buffer conditions used.
Figure 2.
Titration of N210 with ADP as detected by calorimetry. (Upper) Raw data obtained for 32 automatic injections (4 μl each) of an ADP solution (16.9 mM) into the sample cell containing N210 at 180 μM in 10 mM potassium phosphate, pH 6.9/100 mM sodium chloride/30 mM MgCl2. (Lower) Plot of processed data. The solid line corresponds to the best-fit curve obtained by least-squares deconvolution. The best values for fitting parameters are 1.03 for n (number of sites), 25,000 M−1 for K (binding constant), and −17,100 cal/mol for ΔH. The standard deviation of points from the calculated line is 0.069% of the total integral heat for saturating all sites in the N210 sample. ATP hydrolysis did not interfere with the calorimetric experiments, since the turnover number of both N210 and N272 is very low under the conditions used (cf. ref. 5; additional data not shown).
Table 2.
Dissociation constants of Hsp90 fragments for ADP and ATP
| Fragment |
Kd, μM
|
|
|---|---|---|
| ADP | ATP | |
| In the absence of peptide GR1 | ||
| N210 | 40 ± 2 | 98 ± 4 |
| N272 | 43 ± 3 | 94 ± 1 |
| In the presence of peptide GR1 | ||
| N210 | ND | 101 ± 6 |
| N272 | ND | 287 ± 27 |
The dissociation constants of ADP/ATP and N210/N272 were determined as described in the text and the legend of Fig. 2 in the absence or presence of GR1 (500 μM). The numbers represent the obtained dissociation constants with their calculated errors. ND, not determined.
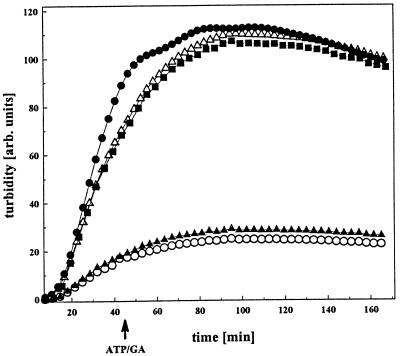
To investigate the effect of ATP on the function of N272, we formed an ATP⋅N272 complex and investigated its influence on insulin aggregation. As in the case of N210 (5), the ATP⋅N272 complex showed a decreased affinity for insulin in the aggregation assay (Fig. 3). The antitumor drug GA, which binds to the ATP-binding pocket of Hsp90 (9, 11), also decreased the affinity of either N210 or N272 for insulin in a manner very similar to that of ATP (Fig. 3). ADP did not exhibit any influence on the chaperone function of N272 or N210 (data not shown). Surprisingly, however, addition of ATP or GA to a preformed N272⋅insulin complex did not affect the binding properties of N272 (Fig. 3). This result was strikingly different from that with N210, as addition of ATP or GA to a preformed N210⋅insulin complex resulted in the slow release and subsequent aggregation of insulin (5). Since ATP had identical effects on the binding properties of uncomplexed N210 and N272, this finding suggested that substrate binding influenced ATP binding. A potential explanation for this phenomenon is that the binding of a peptide causes conformational changes that decrease the affinity of N272 for ATP. To test this possibility, we determined the dissociation constant for ATP of preformed N210⋅GR1 and N272⋅GR1 complexes by titration calorimetry (Table 2). These experiments showed that the ATP-binding properties of N210 were not influenced by bound GR1. In contrast, N272 exhibited a decreased affinity for ATP in the presence of GR1, with a Kd for ATP of 287 μM (Table 2). Taken together, our data suggest that the presence of the charged domain is important for the cross-talk between peptide binding and ATP binding, thus increasing the level of regulation.
Figure 3.
ATP dependence of the interaction between N272 and insulin. The effects of N272 (10 μM) on the aggregation of insulin B-chain (50 μM) (●) were monitored in the absence (○) and presence of 10 mM ATP and 20 mM MgCl2 (■) or 150 μM GA (▵). Addition of MgATP/GA to a preformed N272⋅insulin complex showed no detectable effect in this assay (▴).
Influence of the Charged Region on the Structure of the N-Terminal Domain of Hsp90.
Hsp90 has been shown to exist mainly in a homodimeric form (29, 30). The region responsible for its dimerization is thought to be localized in the C-terminal 50–100 amino acids, as deletion of these led to monomerization of the protein (30, 31).
In the crystal structure of an N-terminal construct of yeast Hsp90 (containing amino acids 1–219), the domain was found to be dimeric (32, 33). The authors suggested that interaction between residues of the charged region are important for dimerization and that dimerization leads to the formation of a “molecular clamp” enclosing the nonnative substrate with the opposing faces of the β-sheets in the dimer interface defining the potential peptide-binding cleft.
In apparent agreement with these findings, the analysis of the quaternary structure of N210 and N272 by size-exclusion HPLC suggested that N272 is dimeric, since it eluted at a position characteristic for a protein of 60-kDa molecular mass—i.e., about double the size observed for N210 (data not shown). To further analyze the oligomeric state of N272, analytical ultracentrifugation and chemical crosslinking were used as independent methods. Chemical crosslinking using the N-hydroxysuccinimide/arylazide cross-linker sulfosuccinimidyl (4-azidophenyldithio)propionate with a spacer length of 13.9 Å suggested that N272 has some tendency to form oligomeric species in a temperature-dependent way. At 30°C, the protein was completely shifted to dimeric and oligomeric species, whereas at lower temperatures mostly monomeric species could be detected.
Analytical ultracentrifugation allows one to determine the apparent molecular mass in solution. Analysis of sedimentation velocity and sedimentation equilibrium runs of N272 performed at different velocities and temperatures gave highly reproducible results (Table 3). In all cases, there was no indication of a significantly populated dimeric species. The numbers determined for the apparent molecular masses and for the s values vary between 28.4 and 34.8 kDa and 1.88 and 2.5 S, respectively. This is in good agreement with the calculated molecular mass of 29.4 kDa for N272. Thus, under the experimental conditions used in the functional assays, N272 is monomeric. Additional experiments performed in the presence of the peptide GR1 did not show an increase in the molecular mass. In this case, molecular masses of between 30.7 and 32.2 kDa were observed (Table 3). Therefore, a stable peptide-induced dimerization of N272 can be excluded. The dramatic functional differences compared with N210 must therefore be due to conformational changes in the monomer.
Table 3.
Analysis of the quaternary structure of Hsp90 fragment N272
| Sedimentation velocity
|
Sedimentation
equilibrium
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Velocity, rpm | Centrifuge | Temp., °C |
s20,w, S
|
Velocity, rpm | Centrifuge | Temp., °C | Mass, kDa
|
||
| N272 | N272 + GR1 | N272 | N272 + GR1 | ||||||
| 44,000 | Spinco | 20 | 2.50 ± 0.06 | ND | 18,000 | Spinco | 20 | 28,400 ± 1,770 | ND |
| 60,000 | Spinco | 18 | 1.99 ± 0.04 | ND | 20,000 | Spinco | 20 | 32,150 ± 1,630 | 31,920 ± 1,480 |
| 40,000 | Optima | 20 | 1.88 ± 0.04 | 1.73 ± 0.08 | 16,000 | Optima | 20 | 34,920 ± 1,130 | 30,170 ± 1,310 |
| 40,000 | Optima | 30 | 2.07 ± 0.07 | 1.91 ± 0.14 | 16,000 | Optima | 30 | 33,650 ± 1,570 | 32,170 ± 1,720 |
Analytical ultracentrifugation was performed as described in the text. A molar excess of peptide GR1 was added to N272 to determine the influence of peptides on the quaternary structure. The analytical ultracentrifuge used were the Spinco model E and the Optima X-LA. ND, not determined.
Implications.
Previously, we described a functional cycle for an ATP-dependent substrate interaction of the monomeric N-terminal domain N210 lacking the charged region (5). In the absence of ATP or in the presence of ADP, N210 has a high-affinity binding state for substrate. Addition of nonnative proteins results in efficient complex formation. Binding of ATP leads to a conformational change and decreases the affinity of N210 for substrate. As a consequence, immediate dissociation of the substrate is observed. To restore the high-affinity binding site, ATP can be either released or hydrolyzed (5).
The charged region markedly changes the properties of the N-terminal domain concerning peptide binding and the interplay between peptide binding and ATP binding.
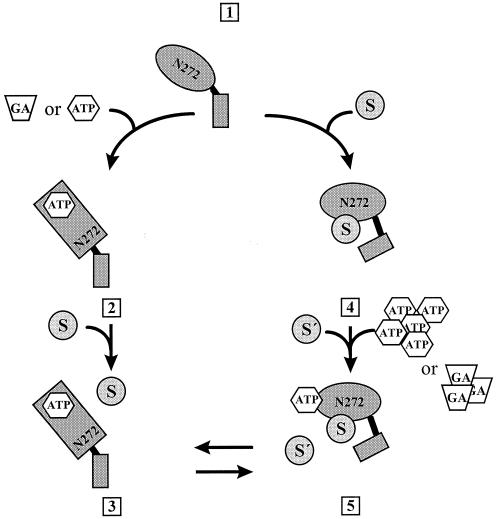
In the absence of peptide, ATP, or GA, N272 has an apparent high affinity for protein ligands. As for N210, ATP binding decreased the affinity of the N272 for nonnative polypeptides. The substrate specificity was not changed significantly in N272 compared with N210, but, surprisingly, an increase in affinity could be detected. In contrast to N210, binding of peptides to N272 leads to a conformational change resulting in a largely decreased affinity for ATP and GA (Fig. 4). Thus, the binding site for peptides is interacting with the ATP-binding site, and binding of peptides prevents further binding of other substrates.
Figure 4.
Model for the chaperone activity of N272. The model describes different functional states of the N-terminal peptide-binding site of Hsp90. In the absence of substrate, ATP, or GA, the fragment has a high affinity for these ligands [1]. The presence of ADP does not influence the affinity for the ligand (not shown). Binding of ATP or GA to N272 results in a conformational change [2] that decreases the affinity of N272 for substrates [3]. This is in accordance with the previously described substrate binding cycle of N210 (5). Binding of substrate is followed by a conformational switch [4], as the N272⋅substrate complex showed a decreased binding affinity for ATP/GA [5]. Furthermore, after the change in conformation, the bound substrate does not exchange with peptides free in solution [5]. The existence of the complex [5] is hypothetical. S and S′, substrates.
The crystal structure of the N-terminal domain of yeast Hsp90 comprising part of the charged region revealed a tendency to dimerize at least at the high concentrations occurring during crystallization (9). In agreement with this finding, our experiments showed that dimers and higher oligomers of N272 can be trapped by chemical cross-linking in a temperature-dependent way. In contrast, a detailed analysis of the quaternary structure by analytical ultracentrifugation did not reveal a dimeric species, even in the presence of peptide. Taken together, these results suggest that N272 has a tendency for low-affinity association. However, under the conditions used in this study, the fragment is present in its monomeric form.
The low-affinity peptide-binding form of the full-length Hsp90 dimer exhibits an extended conformation in which only the C-terminal domain is involved in dimerization (31). It has been suggested that Hsp90 changes conformation and becomes activated at heat shock temperatures (34–36). Since N272 seems to be more active than the respective binding site in the full-length protein or the shorter fragment, we propose that the charged region enhances the chaperone activity of the N-terminal domain and, furthermore, that under certain conditions, the N-terminal domains in full-length Hsp90 are negatively influenced by the C-terminal part of the protein. It is known that Hsp90 performs part of its functions in specific complexes with partner proteins or cochaperones (2, 37). Therefore, it is reasonable to speculate that, in addition to environmental conditions, one of these partners may directly influence the function of Hsp90. Binding of a partner protein to Hsp90 may restore the high-affinity binding site of the N-terminal domain. One possible factor in this context might be p23, which was previously shown to associate with Hsp90 in an ATP-dependent manner (38). Our findings on the function of the charged domain of Hsp90 are a further step in understanding a complicated molecule that seems to fulfill a large variety of different functions.
Acknowledgments
We thank Prof. Joachim Seelig for his kind support of the titration calorimetry and Dr. Susanne Modrow for peptides. We are grateful to Christian Mayr and Stefan Bell for critically reading the manuscript. We thank the Experimental Drug Division of the National Institutes of Health for providing GA. This work was supported by the Deutsche Forschungsgemeinschaft, the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, and the Fonds der Chemischen Industrie.
ABBREVIATIONS
- GA
geldanamycin
- GR
glucocorticoid receptor
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Beissinger M, Buchner J. Biol Chem Hoppe-Seyler. 1998;379:245–259. [PubMed] [Google Scholar]
- 2.Scheibel T, Buchner J. Biochem Pharmacol. 1998;56:675–682. doi: 10.1016/s0006-2952(98)00120-8. [DOI] [PubMed] [Google Scholar]
- 3.Scheibel T, Buchner J. In: Guidebook to Chaperones. Gething M J, editor. Oxford: Oxford Univ. Press; 1997. pp. 147–150. [Google Scholar]
- 4.Young J C, Schneider C, Hartl F U. FEBS Lett. 1997;418:139–143. doi: 10.1016/s0014-5793(97)01363-x. [DOI] [PubMed] [Google Scholar]
- 5.Scheibel T, Weikl T, Buchner J. Proc Natl Acad Sci USA. 1998;95:1495–1499. doi: 10.1073/pnas.95.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiech H, Buchner J, Zimmermann R, Jakob U. Nature (London) 1992;358:169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- 7.Jakob U, Lilie H, Meyer I, Buchner J. J Biol Chem. 1995;270:7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- 8.Freeman B C, Morimoto R I. EMBO J. 1996;15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- 9.Prodromou C, Roe S M, O’Brian R, Ladbury J E, Piper P W, Pearl L H. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan W, Stensgard B, Caucutt G, Bartha B, McMahon N, Alnemri E S, Litwack G, Toft D O. J Biol Chem. 1997;272:8007–8012. doi: 10.1074/jbc.272.12.8007. [DOI] [PubMed] [Google Scholar]
- 11.Stebbins C E, Russo A A, Schneider C, Rosen N, Hartl F U, Pavletich N P. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 12.Grenert J P, Sullivan W P, Fadden P, Haystead T A J, Clark J, Mimnaugh E, Krutzsch H, Ochel H J, Schulte T W, Sausville E, et al. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 13.Bardwell J C, Craig E A. Proc Natl Acad Sci USA. 1987;84:5177–5181. doi: 10.1073/pnas.84.15.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H Y, Dunbar J D, Zhang Y X, Guo D, Donner D B. J Biol Chem. 1995;270:3574–3581. [PubMed] [Google Scholar]
- 15.Chen C F, Chen Y, Dai K, Chen P L, Riley D J, Lee W H. Mol Cell Biol. 1996;16:4691–4699. doi: 10.1128/mcb.16.9.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borkovich K A, Farrelly F W, Finkelstein D B, Taulien J, Lindquist S. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer G, Louvion J F, Tibbetts R S, Engman D M, Picard D. Mol Biochem Parasitol. 1995;70:199–202. doi: 10.1016/0166-6851(95)00007-n. [DOI] [PubMed] [Google Scholar]
- 18.Louvion J F, Warth R, Picard D. Proc Natl Acad Sci USA. 1996;93:13937–13942. doi: 10.1073/pnas.93.24.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchner J, Bose S, Mayr C, Jakob U. Methods Enzymol. 1998;290:409–418. doi: 10.1016/s0076-6879(98)90034-9. [DOI] [PubMed] [Google Scholar]
- 20.Wetlaufer D B. Adv Protein Chem. 1962;17:303–390. doi: 10.1016/s0065-3233(08)60518-5. [DOI] [PubMed] [Google Scholar]
- 21.Horst M, Oppliger W, Feifel B, Schatz G, Glick B S. Protein Sci. 1996;5:759–767. doi: 10.1002/pro.5560050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiseman T, Williston S, Brandts J F, Lin L N. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava P K, Udono H, Blachere N E, Li Z. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 24.Blachere N E, Li Z, Chandawarkar R Y, Suto R, Jaikaria N S, Basu S, Udono H, Srivastava P K. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knarr G, Gething M-J, Modrow S, Buchner J. J Biol Chem. 1995;270:27589–27594. doi: 10.1074/jbc.270.46.27589. [DOI] [PubMed] [Google Scholar]
- 26.Flynn G C, Pohl J, Flocco M T, Rorthman J E. Nature (London) 1991;353:726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- 27.Blond-Elguindi S, Cwirla S E, Dower W J, Lipshutz R J, Sprang S R, Sambrook J F, Gething M J. Cell. 1993;75:717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- 28.Scheibel T, Neuhofen S, Weikl T, Mayr C, Reinstein J, Vogel P D, Buchner J. J Biol Chem. 1997;272:18608–18613. doi: 10.1074/jbc.272.30.18608. [DOI] [PubMed] [Google Scholar]
- 29.Minami Y, Kawasaki H, Suzuki K, Yahara I. J Biol Chem. 1993;268:9604–9610. [PubMed] [Google Scholar]
- 30.Meng X, Devin J, Sullivan W P, Toft D O, Baulieu E E, Catelli M G. J Cell Sci. 1996;109:1677–1687. doi: 10.1242/jcs.109.7.1677. [DOI] [PubMed] [Google Scholar]
- 31.Minami Y, Kimura Y, Kawasaki H, Suzuki K, Yahara I. Mol Cell Biol. 1994;14:1459–1464. doi: 10.1128/mcb.14.2.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prodromou C, Roe S M, Piper P W, Pearl L. Nat Struct Biol. 1997;4:477–482. doi: 10.1038/nsb0697-477. [DOI] [PubMed] [Google Scholar]
- 33.Joachimiak A. Nat Struct Biol. 1997;4:430–434. doi: 10.1038/nsb0697-430. [DOI] [PubMed] [Google Scholar]
- 34.Koyasu S, Nishida E, Kadowaki T, Matsuzaki F, Iida K, Harada F, Kasuga M, Sakai H, Yahara I. Proc Natl Acad Sci USA. 1986;83:8054–8058. doi: 10.1073/pnas.83.21.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonehara M, Minami Y, Kawata Y, Nagai J, Yahara I. J Biol Chem. 1996;271:2641–2645. doi: 10.1074/jbc.271.5.2641. [DOI] [PubMed] [Google Scholar]
- 36.Lanks K W, London E, Dong D L. Biochem Biophys Res Commun. 1992;184:394–399. doi: 10.1016/0006-291x(92)91206-6. [DOI] [PubMed] [Google Scholar]
- 37.Smith D F. Sci Med. 1995;2:38–47. [Google Scholar]
- 38.Johnson J J, Toft D O. Mol Endocrinol. 1995;9:670–678. doi: 10.1210/mend.9.6.8592513. [DOI] [PubMed] [Google Scholar]