Abstract
Cell cultures of Catharanthus roseus were supplied with [2-13C,3-2H]-deoxyxylulose or [2-13C,4-2H]1-deoxyxylulose. Lutein and chlorophylls were isolated from the cell mass, and hydrolysis of the chlorophyll mixtures afforded phytol. Isotope labeling patterns of phytol and lutein were determined by 2H NMR and 1H,2H-decoupled 13C NMR. From the data it must be concluded that the deuterium atom in position 3 of deoxyxylulose was incorporated into both isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate with a rate of 75% (with respect to the internal 13C label). The detected stereochemical signature implies that the label is located preferentially in the (E)-hydrogen atom of IPP. This preferential labeling, in turn, rules out dimethylallyl pyrophosphate as the compulsory precursor of IPP. In the experiment with [2-13C,4-2H]1-deoxyxylulose, the 13C label was efficiently transferred to the terpenoids whereas the 2H label was completely washed out, most probably after IPP formation as a consequence of the isomerization and elongation process. In addition, the data cast light on the stereochemical course of the dehydrogenation and cyclization steps involved in the biosynthesis of lutein.
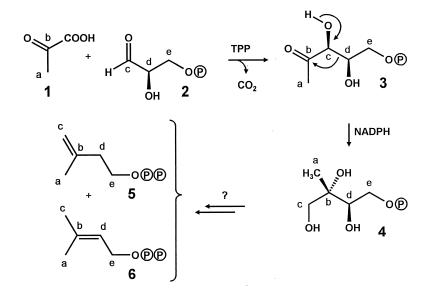
The mevalonate pathway has been considered as the unique pathway of terpene biosynthesis for many years despite an abundance of experimental data that could not be explained (for reviews see refs. 1–4). Recently, independent studies done by the groups of Rohmer, Sahm, and D.A. showed that many bacteria generate isoprenoids by a different pathway (Fig. 1) using glyceraldehyde 3-phosphate (2) and a two-carbon moiety derived from pyruvate (5–7). D.A.’s research group also could show the occurrence of this pathway in plants. Thus, ginkgolides are biosynthesized via the alternative pathway in seedlings of Ginkgo biloba, whereas sterols are synthesized via the classical mevalonate pathway (8). Subsequently, D.A.’s research group identified 1-deoxyxylulose or its 5-phosphate (3) as the first committed precursor in the alternative pathway (7), and a rearrangement of the carbohydrate was shown to be responsible for the formation of the branched five-carbon skeleton of the terpenoid precursors (9–11). More recently, numerous plant terpenoids have been shown to be formed via the deoxyxylulose pathway (for review see refs. 12 and 26).
Figure 1.
Biosynthesis of the terpenoid precursors IPP and DMAPP via the deoxyxylulose pathway (for review see refs. 12 and 27).
Enzymes catalyzing the formation of deoxyxylulose 5-phosphate from glyceraldehyde phosphate and pyruvate have been characterized in Escherichia coli (13, 14) and Mentha piperita (15). More recently, an E. coli enzyme converting 1-deoxyxylulose 5-phosphate into 2-C-methylerythritol 4-phosphate (4) has been reported (16). To date, no other intermediates on the way to isopentenyl pyrophosphate (IPP) (5) and dimethylallyl pyrophosphate (DMAPP) (6) have been identified.
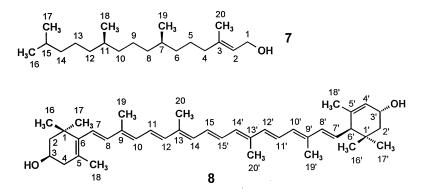
Phytol (7, Fig. 2), β-carotene, and lutein (8) are biosynthesized in Catharanthus roseus from DMAPP and IPP stemming largely, if not exclusively, from deoxyxylulose (9). Specifically, DMAPP serves as a starter unit, which is converted into the common precursor geranylgeranyl pyrophosphate by sequential reactions with three IPP units. To obtain information on the nature and sequence of the unknown steps that lead from 1-deoxyxylulose to the two C5 building blocks of terpene biosynthesis we decided to follow the fate of individual hydrogen atoms of the precursor by using samples labeled both with 13C in position 2 and with 2H at either position 3 or position 4. This strategy makes it possible to follow the joint transfer of the two labels by the detection of the up-field shifts caused by the presence of deuterium on the 13C-signals of the enriched carbon centers.
Figure 2.
Structures and atom numbering of phytol (7) and lutein (8).
EXPERIMENTAL PROCEDURES
Materials.
[2-13C]pyruvate (99% 13C enrichment) was purchased from Isotec. [1-2H]d-glyceraldehyde was synthesized from 2,3-O-isopropylidene-d-glyceric acid methyl ester through reduction with LiAlD4 (98 atom % 2H) and subsequent Swern oxidation of the resulting alcohol. The isopropylidene group was removed by using Dowex 50/H+. [5-2H]d-glucose was prepared according to ref. 17. [2-2H]d-glyceraldehyde was prepared from [5-2H]d-glucose as described (18). [2-13C,3-2H]deoxyxylulose and [2-13C,4-2H]deoxyxylulose were prepared enzymatically by using [2-13C]pyruvate and [1-2H]d-glyceraldehyde, respectively, [2-2H]d-glyceraldehyde, as substrates (19). The samples were purified by HPLC on a column of RPM Monosaccharide (8 μm, 300 × 7.8 mm, Phenomenex, Belmont, CA) using water as eluent and a refractometer as detecting system. The retention volume of deoxyxylulose was 12 ml. The following 1H NMR data were obtained for [2-13C,3-2H]deoxyxylulose (ring-open form) in D2O: δ 2.04 (d, H-1, 13C coupling to C-2, 5.9 Hz), 3.42 (dd, H-5a, 1H coupling to H-5b, 11.0 Hz, 1H coupling to H-4, 7.1 Hz), 3.48 (dd, H-5b, 1H coupling to H-5a, 11.0 Hz, 1H coupling to H-4, 5.6 Hz), 3.95 (m, H-4). The following 1H NMR data were obtained for [2-13C,4-2H]deoxyxylulose in D2O: δ 2.04 (d, H-1, 13C coupling to C-2, 5.9 Hz), 3.41 (d, H-5a, 1H coupling to H-5b, 11.2 Hz), 3.47 (d, H-5b, 1H coupling to H-5a, 11.2 Hz), 4.16 (d, H-3, 13C coupling to C-2, 3.4 Hz).
Plant Cell Culture and Isolation of Terpenoids.
Cultures of C. roseus cells were grown as described (9). [2-13C,3-2H]1-deoxyxylulose (99% 13C enrichment, 93% 2H enrichment) or [2-13C,4-2H]1-deoxyxylulose (99% 13C enrichment, 75% 2H enrichment) was added to a final concentration of 44 mg/liter of culture fluid. The cultures were incubated for 7 days with continuous illumination. The cells were harvested (25 g, dry weight), and carotenoids and chlorophylls were isolated. Hydrolysis of chlorophylls afforded phytol as described (9). Further purification of phytol and lutein was achieved by HPLC on a column of Hypersil-RP18 (5 μm, 250 × 4.5 mm) using 95% (vol/vol) aqueous methanol as eluent. The effluent was monitored at 214 nm. The retention volumes were 10 ml for phytol and 6 ml for lutein. Typically, 0.4 mg of phytol and 0.06 mg of lutein were obtained per g of cell mass (dry weight), respectively.
NMR Spectroscopy.
1H and 13C NMR spectra of phytol and lutein were recorded at 17°C in CDCl3 and 2H NMR spectra were recorded in CHCl3 without lock by using a Bruker DRX500 spectrometer. 2H decoupling was achieved by using a lock switch unit. Two-dimensional experiments were performed according to standard Bruker software (xwinnmr 1.3). 13C Abundance in terpenoids was analyzed by quantitative NMR analysis as described (9).
RESULTS
Lutein and phytol were isolated from cell mass and analyzed by 1H, 2H, and 13C NMR spectroscopy. 13C NMR signal assignments for phytol were published earlier (9). 1H and 13C NMR signals of lutein and 1H NMR signals of phytol were assigned by two-dimensional correlated spectroscopy, nuclear Overhauser effect spectroscopy, heteronuclear multiple quantum correlation, and heteronuclear multiple bond correlation experiments (Tables 1 and 2).
Table 1.
NMR analysis of phytol from a cell culture of C. roseus supplied with [2-13C,3-2H]- and [2-13C,4-2H]1-deoxyxylulose
| Chemical shift,* δ, ppm
|
Proferred deoxyxylulose | ||||
|---|---|---|---|---|---|
| [2-13C,3-2H]
|
[2-13C,4-2H]
|
||||
| Position | 13C | 1H | % 13C | % 2H† | % 13C |
| 1 | 59.39 | 4.14(d) | 1.20 | 1.48 | |
| 2 | 123.09 | 5.39(t) | 1.20 | 1.00 | |
| 3 | 140.23 | 22.75 | 23.40 | ||
| 4 | 39.85 | 1.97(m) | 1.09 | 14.6 | 0.99 |
| 5 | 25.12 | 1.40(m) | 1.20 | 1.30 | |
| 1.36(m) | |||||
| 6 | 36.65 | 1.24(m,HSi) | 1.04 | 0.86 | |
| 1.05(m,HRe) | |||||
| 7 | 32.67 | 1.35(m) | 19.63 | 18.73 | |
| 8 | 37.35 | 1.23(m,HRe) | 13.0 | 1.10 | |
| 1.03(m,HSi) | |||||
| 9 | 24.45 | 1.29(m) | 1.10 | 1.17 | |
| 1.15(m) | |||||
| 10 | 37.41 | 1.23(m,HSi) | 1.07 | 0.97 | |
| 1.03(m,HRe) | |||||
| 11 | 32.77 | 1.35(m) | 17.89 | 17.09 | |
| 12 | 37.28 | 1.23(m,HRe) | 1.03 | 12.0 | 1.08 |
| 1.03(m,HSi) | |||||
| 13 | 24.78 | 1.25(m) | 1.13 | 1.39 | |
| 14 | 39.35 | 1.11(m) | 1.01 | 1.02 | |
| 1.03(m) | |||||
| 15 | 27.95 | 1.50(hp) | 21.18 | 15.53 | |
| 16 | 22.60 | 0.84(d) | 1.05 | 14.2 | 1.00 |
| 17 | 22.69 | 0.84(d) | 1.06 | 1.02 | |
| 18‡ | 19.69 | 0.83(d) | 1.00 | 0.95 | |
| 19‡ | 19.72 | 0.82(d) | 1.07 | 1.05 | |
| 20 | 16.14 | 1.65(s) | 1.30 | 1.21 | |
Referenced to solvent signals at 7.24 and 77.0 ppm, respectively; multiplicities of the observed 1H NMR are indicated in parentheses (s, singlet; d, doublet; t, triplet; hp, heptet; m, multiplet)
Calculated as the fraction of the 2H-shifted satellite signal in the global 13C NMR intensity of the adjacent atom. These values were used to calculate the cotransfer of 13C and 2H after correction for the presence of molecules with natural 13C abundance.
Assignments may be interchanged.
Table 2.
NMR analysis of lutein from a cell culture of C. roseus supplied with [2-13C,3-2H]- and [2-13C,4-2H]1-deoxyxylulose
| Chemical shift,* δ,
ppm
|
Proffered deoxyxylulose | ||||
|---|---|---|---|---|---|
| [2-13C,3-2H]
|
[2-13C,4-2H]
|
||||
| Position | 13C | 1H | % 13C | % 2H† | % 13C |
| 1 | 37.09 | 21.01 | 12.36 | ||
| 1′ | 34.01 | 19.28 | 10.86 | ||
| 2 | 48.30 | 1.75(α,ddd) | 1.0 | 1.20 | |
| 1.46(β,t) | |||||
| 2′ | 44.60 | 1.34(α,dd) | 0.94 | 0.97 | |
| 1.82(β,dd) | |||||
| 3 | 65.04 | 3.98(m) | 0.99 | 1.27 | |
| 3′ | 65.89 | 4.23(m) | 0.91 | 1.09 | |
| 4 | 42.46 | 2.36(α,ddd) | 1.01 | 1.14 | |
| 2.02(β,dd) | 12.43 | ||||
| 4′ | 124.39 | 5.53(s) | 1.12 | 10.97 | 1.07 |
| 5 | 126.13 | 18.59 | 15.94 | ||
| 5′ | 137.98 | 21.03 | 15.27 | ||
| 6 | 137.68 | 1.13 | 1.18 | ||
| 6′ | 54.87 | 2.38(d) | 0.93 | 1.07 | |
| 7 | 125.53 | 6.11(nd) | 1.20 | 1.36 | |
| 7′ | 128.71 | 5.41(dd) | 1.00 | 0.88 | |
| 8 | 138.47 | 6.11(nd) | 1.28 | 7.80 | 1.12 |
| 8′ | 137.68 | 6.11(nd) | 1.13 | 9.18 | 1.18 |
| 9 | 135.68 | 21.83 | 16.16 | ||
| 9′ | 135.06 | 24.23 | 15.88 | ||
| 10 | 131.28 | 6.11(nd) | 1.13 | 1.13 | |
| 10′ | 130.77 | 6.11(nd) | 1.04 | 0.93 | |
| 11 | 124.90 | 6.61(nd) | 1.56 | 1.32 | |
| 11′ | 124.77 | 6.61(nd) | 1.46 | 0.99 | |
| 12,12′ | 137.53 | 6.34(d) | 1.07 | 2.95 | 1.11 |
| 13 | 136.40 | 21.22 | 16.60 | ||
| 13′ | 136.48 | 21.46 | 16.02 | ||
| 14,14′ | 132.56 | 6.23(d) | 1.24 | 1.12 | |
| 15 | 130.05 | 6.61(nd) | 1.41 | 1.25 | |
| 15′ | 130.01 | 6.61(nd) | 1.19 | 1.53 | |
| 16 | 30.22 | 1.05(s) | 0.99 | 0.95 | |
| 16′ | 24.17 | 0.83(s) | 1.03 | 1.07 | |
| 17 | 28.68 | 1.059(s) | 0.99 | 14.05 | 1.21 |
| 17′ | 29.46 | 0.98(s) | 1.21 | 12.76 | 0.93 |
| 18 | 21.63 | 1.72(s) | 1.06 | 1.01 | |
| 18′ | 22.88 | 1.60(s) | 0.98 | 1.07 | |
| 19 | 12.76 | 1.95(s) | 1.02 | 0.94 | |
| 19′ | 13.11 | 1.89(s) | 0.85 | 0.75 | |
| 20,20′ | 12.81 | 1.95(s) | 1.04 | 0.99 | |
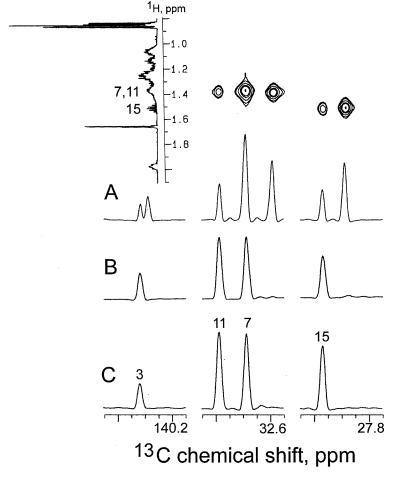
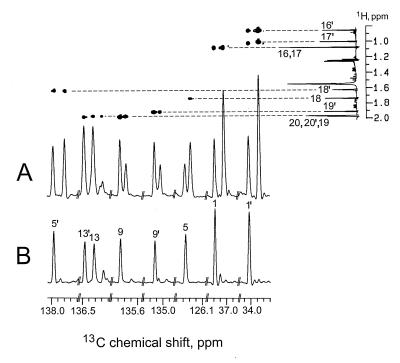
In the experiment with [2-13C,3-2H]1-deoxyxylulose, four 13C signals of phytol (carbon atoms 3, 7, 11, and 15) and eight 13C signals of lutein (carbon atoms 1, 1′, 5, 5′, 9, 9′, 13, and 13′) displayed increased intensities, as expected on the basis of previous results (9). By comparison with the spectra of the unlabeled molecule, an average 13C content of 20.8 ± 1.8% was detected for the labeled centers (Tables 1 and 2).
As indicated in Figs. 3A and 4A each of the enriched signals is accompanied by high-field shifted satellites (Δδ = −26 to −110 ppb, Tables 1 and 2) shown to belong to the same molecule rather than to impurities by CH-correlated spectroscopy and correlated spectroscopy via long-range coupling (COLOC) experiments in which a 1H-13C correlation of both the low- and high-field component with the same hydrogen atoms can be detected. The magnitude of the observed shifts is consistent with the presence of deuterium at positions adjacent to the 13C-enriched centers. The cotransfer of 13C and 2H can be assessed quantitatively from the relative intensities of the shifted vs. nonshifted signals after correction for the presence of the known amount of molecules with natural 13C abundance. A cotransfer value of about 75% was detected for all the deuterium-labeled centers of phytol as well as for the centers 4, 17, and 17′ in lutein; this value was used as a standard for normalizing the deuterium content of specific positions in terms of atomic ratios.
Figure 3.
13C NMR signals of phytol. (A) From the experiment with [2-13C,3-2H]deoxyxylulose; signals from a two-dimensional CH- correlated spectroscopy experiment are displayed (Upper). (B) From the experiment with [2-13C,4-2H]deoxyxylulose. (C) From a sample with natural 13C abundance.
Figure 4.
13C NMR signals of lutein. (A) From the experiment with [2-13C,3-2H]deoxyxylulose; signals from a two-dimensional correlated spectroscopy via long-range coupling (COLOC) experiment are displayed (Upper). (B) From the experiment with [2-13C,4-2H]deoxyxylulose.
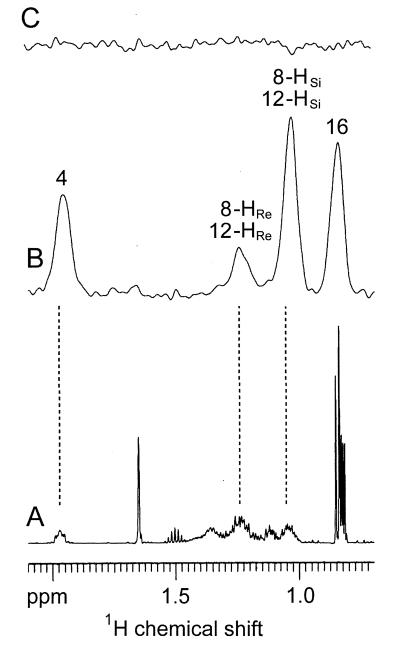
The 2H NMR spectrum of phytol provides evidence for the presence of deuterium at C-4 (δ = 1.96 ppm) and in one of the methyl groups (δ = 0.85 ppm), which can be identified as C-16 because it is the only one in the molecule not derived from the methyl group of deoxyxylulose (9). The remaining signals at 1.24 and. 1.04 ppm are the result of the pairwise overlap of signals belonging to two methylene groups adjacent to C-7 and C-11, respectively (Fig. 5B). By comparison with the biogenetically equivalent positions of lutein, the labeled methylene groups can be localized at C-8 and C-12. These methylene groups are known from the 13C NMR data to be labeled with the same amount of deuterium; the 3:1 ratio observed for the intensity of the signals at 1.04 ppm and 1.24 ppm in the 2H NMR spectrum must reflect the partitioning of the heavy atom among the two diastereotopic positions within each of the methylene groups. With reference to the spectroscopic work recently described by Eguchi et al. (20), the more intense high-field signal can be tentatively assigned to the Ha-positions (HSi-8; HSi-12) indicated in Fig. 6; this assignment is vindicated by the results obtained for the lutein probe generated in the same experiment.
Figure 5.
Part of the 1H NMR spectrum (A) and 2H NMR spectrum (B) of phytol from the experiment with [2-13C,3-2H]deoxyxylulose. 2H NMR spectrum (C) of phytol from the experiment with [2-13C,4-2H]deoxyxylulose.
Figure 6.
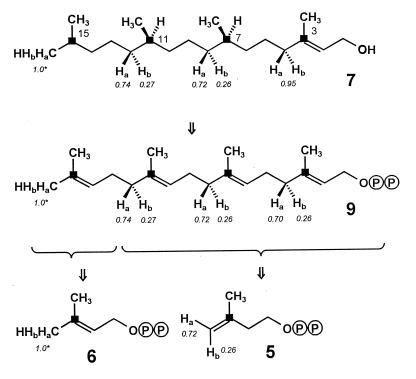
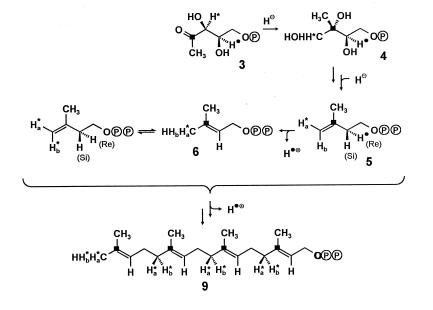
Labeling pattern of phytol (7) from the experiment with [2-13C,3-2H]deoxyxylulose. The labeling patterns of geranylgeranyl pyrophosphate (9), DMAPP (6), and IPP (5) are reconstructed retrobiosynthetically. ■ indicates 13C enrichment from 13C-2 of deoxyxylulose. Ha and Hb indicate positions of 2H enrichment from 2H-3 of the deoxyxylulose precursor. The numbers in italics indicate atomic ratios with respect to the value for the 16 position arbitrarily set at 1.
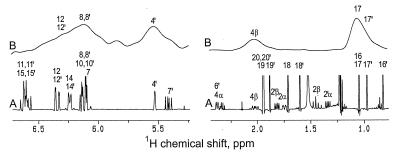
Two overlapping signals at 1.0 ppm and 1.1 ppm in the 2H NMR spectrum of lutein (Fig. 7B) demonstrate the presence of deuterium in the 17′ methyl group and in one of the two isochronous methyl groups linked to C-1. However, the 13C signals of the two groups are anisochronous and their stereochemical assignment already had been settled by Britton et al. (21). Hence, a choice in favor of the 17 methyl group can be made on the basis of the previous demonstration that this group (much as its 17′ counterpart) stems specifically from C-3 rather than from the methyl group of the deoxyxylulose precursor (9). The 2H NMR spectrum of lutein also discloses the presence of deuterium at the 4 and 4′ positions. The C-4 methylene group of lutein is biogenetically equivalent to C-12 of phytol and has the same deuterium content; the preponderance of the label in the Ha position (H-4β of lutein) is evident from the spectrum of the compound, but the low intensity signal expected for the Hb position is hardly discernible in this spectrum, probably as a consequence of low resolution. The complex signal at 6–6.5 ppm is consistent with the presence of deuterium requested by the 13C NMR data for positions 8, 8′, 12, and 12′. The labeling patterns of phytol and lutein from the experiment with [2-13C,3-2H]deoxyxylulose are summarized in Figs. 6 and 8.
Figure 7.
Parts of the 1H NMR (A) and 2H NMR (B) of lutein from the experiment with [2-13C,3-2H]deoxyxylulose.
Figure 8.
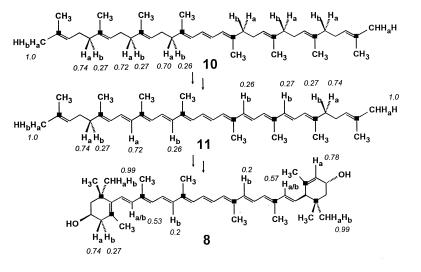
Labeling pattern of lutein (8) from the experiment with [2-13C,3-2H]deoxyxylulose. The labeling pattern of phytoene (10) was reconstructed from the labeling pattern of phytol (see also Fig. 6). The labeling pattern of lycopene (11) was adjusted to accommodate the observed labeling pattern of lutein. For other details see Fig. 6 and text.
In the experiment with [2-13C,4-2H]deoxyxylulose an average content of 16.1 ± 3.1% was observed for the 13C-enriched carbon atoms of phytol and lutein (Tables 1 and 2). The lack of high-field shifted satellite lines in the 13C NMR spectra (Figs. 3B and 4B) and the absence of signals in the 2H NMR spectra of phytol (Fig. 5C) and lutein demonstrate that the deuterium label of the precursor is not incorporated to any significant extent into the isoprenoids under investigation.
DISCUSSION
The labeling patterns observed for phytol (7) and lutein (8) in the experiment with [2-13C,3-2H]deoxyxylulose are consistent with the intermediate formation of a geranylgeranyl pyrophosphate (9), which had retained (with respect to the internal C-13 standard) 75% of the original deuterium label in its (E)-methyl group as well as in all other positions corresponding to the terminal methylene group of IPP (5); within the labeled methylene groups the label is distributed between the two heterotopic positions in a ratio of ca. 3:1 in favor of the Ha alternative (cf. Figs. 6, 8, and 9). This label distribution is preserved in phytol, whereas part of the deuterium is lost in later stages of lutein biosynthesis. The observed regiospecificity of deuterium incorporation matches the one recently reported for the formation of the octaprenyl side chain of ubiquinone in E. coli (22). In addition, the demonstration that deuterium label from C-3 of the precursor resides predominantly in the Ha-position of the labeled methylene groups implies a corresponding asymmetry for the label distribution within the terminal methylene group of the IPP intermediate. From the known stereochemical course of the chain elongation step in terpene biosynthesis (23) it is possible to reconstruct for the preponderant form of the intermediate the labeling pattern indicated in Fig. 9. The observed partial scrambling of label is easily explained by the subsequent and reversible action of the enyzme IPP-DMAPP isomerase on a significant fraction of IPP molecules originally labeled exclusively in their Ha position. The maintenance of a stereochemical signature evidenced by the incorporation results rules out DMAPP conclusively as a compulsory precursor of IPP in the deoxyxylulose pathway.
Figure 9.
Biosynthetic origin of hydrogen atoms in geranylgeranyl pyrophosphate (9) derived from IPP (5) and DMAPP (6) assembled via the deoxyxylulose pathway. ∗ and ● indicate positions of deuterium label. For details see Fig. 6 and text.
The sizable loss of deuterium (approximately 25%) from C-3 of the precursor, which accompanies the formation of both IPP and DMAPP, can be explained, in part, as a further consequence of the enzyme-catalyzed interconversion that is responsible for the scrambling of the deuterium label in IPP, but the magnitude of the loss seems to exceed the figure, which can be estimated from the observed degree of label randomization. Spontaneous enolization of the precursor in solution is ruled out by the result of a control experiment, in which the starting material was recovered unchanged at the end of an incubation carried out under identical conditions in the absence of plant cells. Among the many remaining possibilities, the one of enzyme-catalyzed enolation of the precursor deserves specific mention, because it is known to operate in E. coli as the first and most probably reversible step in the well-documented transformation of 1-deoxyxylulose into 5-hydroxypentane-2,3-dione (24).
Because of the C2 symmetry of lycopene (11, Fig. 8), the last acyclic precursor of lutein, each carbon center of lutein corresponds biosynthetically to a pair of carbon centers of the precursor, which are homotopically related and hence indistinguishable. As a consequence, any deuterium label appended to these centers in the precursor will be distributed statistically among the two corresponding centers of the derived lutein, unless this label is affected specifically after the asymmetrization process. The identical or nearly identical deuteration degrees detected in lutein for such positions therefore is not fortuitous and should be viewed instead as a test for the accuracy of the analytical tools that led to their detection. In this perspective, it is clear that formation of both the Δ12 and the Δ12′ double bonds of lycopene involves specific removal of Ha atoms from phytoene (10), whereas the deuterium values observed for C-8 and C-8′ in lutein are highly suggestive of a double dehydrogenation sequence involving specific removal of Ha in one step and of Hb in the other, respectively (Fig. 8). These results match only in part previous findings on the biosynthesis of carotenoids in Flavobacterium, which were interpreted as evidence for an exclusive loss of Ha in each of the four dehydrogenation steps (25).
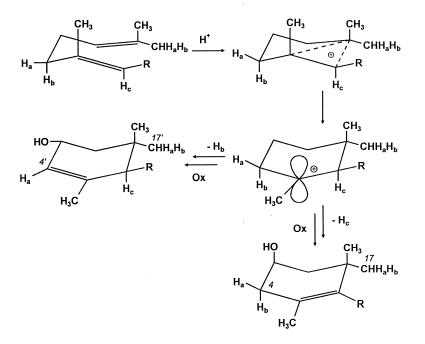
In connection with the known origin of C-17′, which stems from C-4 of IPP via the (E)-methyl group of DMAPP, it is now possible to exploit the data pertaining to C-4′ of lutein for reconstructing the stereochemical course of the cyclization process responsible for the formation of the A′-ring of lutein. Starting with the chair conformation indicated in Fig. 10, addition of a proton triggers the formation of a cyclic cation, in which Hb resides in an axial position meeting the stereoelectronic requirement necessary for its subsequent elimination. Within the same scheme the alternative elimination of HC, which is similarly oriented with respect to the adjacent electronically deficient center, accounts for the formation of an A-ring with the correct stereochemical arrangement of the two biogenetically nonequivalent methyl groups at C-1.
Figure 10.
Stereochemical course of ring formation processes during biosynthesis of lutein.
In the experiment with [2-13C,4-2H]deoxyxylulose no deuterium transfer to phytol or lutein could be detected; the absence of label that must be inferred in the Catharanthus system for the DMAPP starter unit as well as for all the C5 units derived from IPP in the elongation process is in striking contrast with the situation in E. coli, where it was shown that during formation of the octaprenyl side chain of ubiquinone deuterium from C-4 of deoxyxylulose is retained specifically in the vinylic position of the DMAPP starter unit (23). This finding suggests that (i) in both systems the deuterium from C-4 of the precursor is incorporated stereospecifically into the C-2 methylene group of IPP and subsequently lost in the elimination step of the elongation process and (ii) the two systems differ in the stereospecificity of their IPP-DMAPP isomerase, the Catharanthus enzyme operating on the same proton that is involved in the elongation step and the anomalous E. coli enzyme affecting instead the enantiotopic H(Si) position (Fig. 9). Alternative explanations, e.g., independent formation of IPP and DMAPP from a common intermediate along the deoxyxylulose pathway, seem less plausible but cannot be ruled out. It is therefore important to secure by independent means that the C-4 hydrogen of the precursor is indeed preserved during formation of IPP, because this retention would impose a further stringent requirement on the mechanism of the still unknown steps of the alternative pathway.
Acknowledgments
We thank A. Werner, H. Wagner, and F. Wendling for help with the preparation of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 369), the Fonds der Chemischen Industrie, and the Hans-Fischer-Gesellschaft. Financial support by Novartis International AG Basel (to D.A.) is gratefully acknowledged.
ABBREVIATIONS
- IPP
isopentenyl pyrophosphate
- DMAPP
dimethylallyl pyrophosphate
References
- 1.Qureshi N, Porter J W. In: Biosynthesis of Isoprenoid Compounds. Porter J W, Spurgeon S L, editors. Vol. 1. New York: Wiley; 1981. pp. 47–94. [Google Scholar]
- 2.Banthorpe D V, Charlwood B V, Francis M J O. Chem Rev. 1972;72:115–155. doi: 10.1021/cr60276a002. [DOI] [PubMed] [Google Scholar]
- 3.Bloch K. Steroids. 1992;57:378–382. doi: 10.1016/0039-128x(92)90081-j. [DOI] [PubMed] [Google Scholar]
- 4.Bach T J. Lipids. 1995;30:191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- 5.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. J Am Chem Soc. 1996;118:2564–2566. [Google Scholar]
- 7.Broers S T J. Ph.D. thesis. Zurich, Switzerland.: Eidgenössische Technische Hochschule; 1994. [Google Scholar]
- 8.Schwarz M K. Ph.D. thesis. Zurich, Switzerland.: Eidgenössische Technische Hochschule; 1994. [Google Scholar]
- 9.Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk M H. Proc Natl Acad Sci USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putra S R, Lois L M, Campos N, Boronat A, Rohmer M. Tetrahedron Lett. 1998;39:23–26. [Google Scholar]
- 11.Sagner S, Eisenreich W, Fellermeier M, Latzel C, Bacher A, Zenk M H. Tetrahedron Lett. 1998;39:2091–2094. [Google Scholar]
- 12.Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk M H, Bacher A. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- 13.Sprenger G A, Schörken U, Wiegert T, Grolle S, de Graaf A A, Taylor S V, Begley T P, Bringer-Meyer S, Sahm H. Proc Natl Acad Sci USA. 1997;94:12857–12862. doi: 10.1073/pnas.94.24.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lois L M, Campos N, Putra S R, Danielsen K, Rohmer M, Boronat A. Proc Natl Acad Sci USA. 1998;95:2105–2110. doi: 10.1073/pnas.95.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange B M, Wildung M R, McCaskill D, Croteau R. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi S, Kuzuyama T, Watanabe H, Seto H. Proc Natl Acad Sci USA. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardick D J, Hutchinson D W. Bioorg Med Chem Lett. 1994;4:409–414. [Google Scholar]
- 18.Schöpf C, Wild H. Chem Ber. 1954;87:1571–1575. [Google Scholar]
- 19.Shimizu M, Shogawa H, Matsuzawa T, Yonezawa S, Hayshi T, Arisawa M, Suzuki S, Yoshizaki M, Morita N, Ferro E, et al. Chem Pharm Bull. 1990;38:2283–2284. doi: 10.1248/cpb.38.2283. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi T, Morita M, Kakinuma K. J Am Chem Soc. 1998;120:5427–5433. [Google Scholar]
- 21.Britton, G., Goodwin, T. W., Lockley, W. J. S., Mundy, A. P., Patel, N. J. & Englert, G. (1979) J. Chem. Soc. Chem. Commun. 27–28.
- 22.Giner, J.-L., Jaun, B. & Arigoni, D. (1998) J. Chem. Soc. Chem. Commun. 1857–1858.
- 23.Poulter C D, Rilling H C. In: Biosynthesis of Isoprenoid Compounds. Porter J W, Spurgeon S L, editors. Vol. 1. New York: Wiley; 1981. pp. 161–224. [Google Scholar]
- 24.Putra S R, Charon L, Danielsen K, Pale-Grosdemange C, Lois L-M, Campos N, Boronat A, Rohmer M. Tetrahedron Lett. 1998;39:6185–6188. [Google Scholar]
- 25.McDermott J C B, Britton G, Goodwin T W. Biochem J. 1973;134:1115–1117. doi: 10.1042/bj1341115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohmer M. Prog Drug Res. 1998;50:135–154. doi: 10.1007/978-3-0348-8833-2_3. [DOI] [PubMed] [Google Scholar]