Abstract
A retrospective analysis was made of the incidence and severity of the idiopathic respiratory distress syndrome (RDS) in babies of less than 35 weeks' gestation born at this hospital from January 1967-December 1974. There was a lower incidence of RDS in babies born after pregnancies complicated only by prolonged rupture of membranes (PRM) (19%) and in babies born vaginally after pregnancies complicated only by pre-eclamptic toxaemia (PET) (18%) compared with the incidence of RDS after uncomplicated pregnancies (35%). Babies born vaginally who developed RDS after pregnancies complicated by PRM or PET had less severe disease compared with those who developed RDS after uncomplicated pregnancies. Mortality in babies who developed severe RDS was not influenced by the occurrence of PRM or PET. The biological implication of the study is that certain complications of pregnancy may accelerate pulmonary surfactant production in preterm babies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLANC W. A. Pathways of fetal and early neonatal infection. Viral placentitis, bacterial and fungal chorioamnionitis. J Pediatr. 1961 Oct;59:473–496. doi: 10.1016/s0022-3476(61)80232-1. [DOI] [PubMed] [Google Scholar]
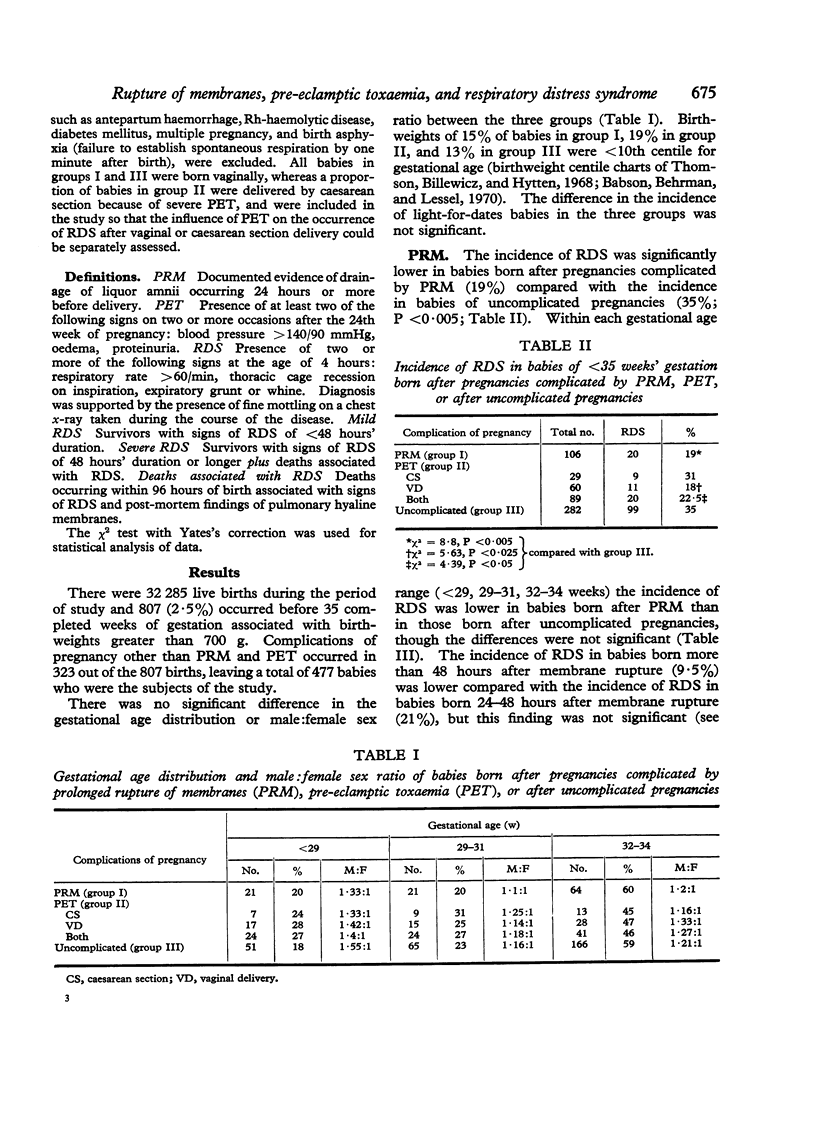
- Babson S. G., Behrman R. E., Lessel R. Fetal growth. Liveborn birth weights for gestational age of white middle class infants. Pediatrics. 1970 Jun;45(6):937–944. [PubMed] [Google Scholar]
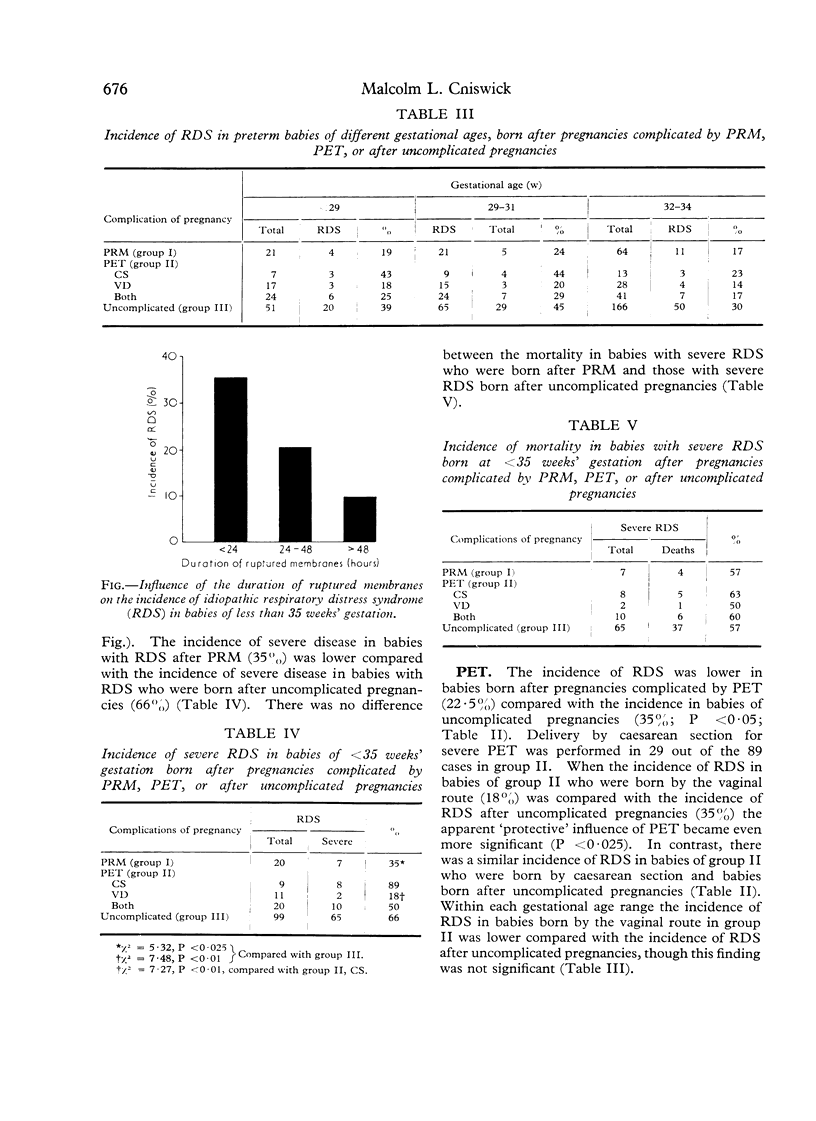
- Bauer C. R., Stern L., Colle E. Prolonged rupture of membranes associated with a decreased incidence of respiratory distress syndrome. Pediatrics. 1974 Jan;53(1):7–12. [PubMed] [Google Scholar]
- Blackburn W. R., Travers H., Potter D. M. The role of the pituitary-adrenal-thyroid axes in lung differentiation. I. Studies of the cytology and physical properties of anencephalic fetal rat lung. Lab Invest. 1972 Mar;26(3):306–318. [PubMed] [Google Scholar]
- Chiswick M. L., Ahmed A., Jack P. M., Milner R. D. Control of fetal lung development in the rabbit. Arch Dis Child. 1973 Sep;48(9):709–713. doi: 10.1136/adc.48.9.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H., Langley F. A. Leukocytic infiltration of the placenta and umbilical cord. A clinico-pathologic study. Obstet Gynecol. 1971 Mar;37(3):451–458. [PubMed] [Google Scholar]
- Genazzani A. R., Fioretti P., Lemarchand-Beraud T. Plasma thyrotrophin levels during pregnancy. J Obstet Gynaecol Br Commonw. 1971 Feb;78(2):117–122. doi: 10.1111/j.1471-0528.1971.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Gluck L., Kulovich M. V., Eidelman A. I., Cordero L., Khazin A. F. Biochemical development of surface activity in mammalian lung. IV. Pulmonary lecithin synthesis in the human fetus and newborn and etiology of the respiratory distress syndrome. Pediatr Res. 1972 Feb;6(2):81–99. doi: 10.1203/00006450-197202000-00002. [DOI] [PubMed] [Google Scholar]
- Gluck L., Kulovich M. V. Lecithin-sphingomyelin ratios in amniotic fluid in normal and abnormal pregnancy. Am J Obstet Gynecol. 1973 Feb 15;115(4):539–546. doi: 10.1016/0002-9378(73)90404-3. [DOI] [PubMed] [Google Scholar]
- Hendricks C. H., Brenner W. E. Toxemia of pregnancy: relationship between fetal weight, fetal survival, and the maternal state. Am J Obstet Gynecol. 1971 Jan 15;109(2):225–233. doi: 10.1016/0002-9378(71)90871-4. [DOI] [PubMed] [Google Scholar]
- Hershman J. M., Starnes W. R. Extraction and characterization of a thyrotropic material from the human placenta. J Clin Invest. 1969 May;48(5):923–929. doi: 10.1172/JCI106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. D., Jr, Burd L. I., Bowes W. A., Jr, Battaglia F. C., Lubchenco L. O. Failure of association of premature rupture of membranes with respiratory-distress syndrome. N Engl J Med. 1975 Jun 12;292(24):1253–1257. doi: 10.1056/NEJM197506122922401. [DOI] [PubMed] [Google Scholar]
- Kelsall G. R., Barter R. A., Manessis C. Prospective bacteriological studies in inflammation of the placenta, cord and membranes. J Obstet Gynaecol Br Commonw. 1967 Jun;74(3):401–411. doi: 10.1111/j.1471-0528.1967.tb03964.x. [DOI] [PubMed] [Google Scholar]
- Kopelman J. J., Levitz M. Plasma cortisol levels and cortisol binding in normal and pre-eclamptic pregnancies. Am J Obstet Gynecol. 1970 Nov 15;108(6):925–930. doi: 10.1016/0002-9378(70)90335-2. [DOI] [PubMed] [Google Scholar]
- Kotas R. V. Accelerated pulmonary surfactant after untrauterine infection in the fetal rabbit. Pediatrics. 1973 Apr;51(4):655–659. [PubMed] [Google Scholar]
- Kotas R. V., Avery M. E. Accelerated appearance of pulmonary surfactant in the fetal rabbit. J Appl Physiol. 1971 Mar;30(3):358–361. doi: 10.1152/jappl.1971.30.3.358. [DOI] [PubMed] [Google Scholar]
- Liggins G. C., Howie R. N. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972 Oct;50(4):515–525. [PubMed] [Google Scholar]
- Liggins G. C. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969 Dec;45(4):515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- Mukherjee K., Swyer G. I. Plasma cortisol and adrenocorticotrophic hormone in normal men and non-pregnant women, normal pregnant women and women with pre-eclampsia. J Obstet Gynaecol Br Commonw. 1972 Jun;79(6):504–512. doi: 10.1111/j.1471-0528.1972.tb14192.x. [DOI] [PubMed] [Google Scholar]
- Naeye R. L., Harcke H. T., Jr, Blanc W. A. Adrenal gland structure and the development of hyaline membrane disease. Pediatrics. 1971 Apr;47(4):650–657. [PubMed] [Google Scholar]
- Osburn B. I., Drost M., Stabenfeldt G. H. Response of fetal adrenal cortex to congenital infections. Am J Obstet Gynecol. 1972 Nov 1;114(5):622–627. doi: 10.1016/0002-9378(72)90839-3. [DOI] [PubMed] [Google Scholar]
- Redding R. A., Douglas W. H., Stein M. Thyroid hormone influence upon lung surfactant metabolism. Science. 1972 Mar 3;175(4025):994–996. doi: 10.1126/science.175.4025.994. [DOI] [PubMed] [Google Scholar]
- Sprague A. D., Duhring J. L., Moser R. J., Hollingsworth D. Maternal and fetal levels of HCS in preeclampsia. Obstet Gynecol. 1973 May;41(5):770–773. [PubMed] [Google Scholar]
- Thomson A. M., Billewicz W. Z., Hytten F. E. The assessment of fetal growth. J Obstet Gynaecol Br Commonw. 1968 Sep;75(9):903–916. doi: 10.1111/j.1471-0528.1968.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Wu B., Kikkawa Y., Orzalesi M. M., Motoyama E. K., Kaibara M., Zigas C. J., Cook C. D. The effect of thyroxine on the maturation of fetal rabbit lungs. Biol Neonate. 1973;22(3):161–168. doi: 10.1159/000240550. [DOI] [PubMed] [Google Scholar]
- Yoon J. J., Harper R. G. Observations on the relationship between duration of rupture of the membranes and the development of idiopathic respiratory distress syndrome. Pediatrics. 1973 Aug;52(2):161–168. [PubMed] [Google Scholar]
- de Sa D. J. Adrenal changes in chorioamnionitis. Arch Dis Child. 1974 Feb;49(2):149–151. doi: 10.1136/adc.49.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]


