Abstract
Saccharomyces cerevisiae was engineered to express different amount of heavy (H)- and light (L)-chain subunits of human ferritin by using a low-copy integrative vector (YIp) and a high-copy episomal vector (YEp). In addition to pep4::HIS3 allele, the expression host strain was bred to have the selection markers leu2− and ura3− for YIplac128 and YEp352, respectively. The heterologous expression of phytase was used to determine the expression capability of the host strain. Expression in the new host strain (2805-a7) was as high as that in the parental strain (2805), which expresses high levels of several foreign genes. Following transformation, Northern and Western blot analyses demonstrated the expression of H- and L-chain genes. The recombinant yeast was more iron tolerant, in that transformed cells formed colonies on plates containing more than 25 mM ferric citrate, whereas none of the recipient strain cells did. Prussian blue staining indicated that the expressed isoferritins were assembled in vivo into a complex that bound iron. The expressed subunits showed a clear preference for the formation of heteropolymers over homopolymers. The molar ratio of H to L chains was estimated to be 1:6.8. The gel-purified heteropolymer took up iron faster than the L homopolymer, and it took up more iron than the H homopolymer did. The iron concentrations in transformants expressing the heteropolymer, L homopolymer, and H homopolymer were 1,004, 760, and 500 μg per g (dry weight) of recombinant yeast cells, respectively. The results indicate that heterologously expressed H and L subunits coassemble into a heteropolymer in vivo and that the iron-carrying capacity of yeast is further enhanced by the expression of heteropolymeric isoferritin.
Iron is an essential element in most living organisms. However, its availability is limited by the low solubility of Fe(III) and the ability of intracellular free iron to produce toxic radicals. Consequently, once iron enters a cell, it must be stored in an intracellular form that is soluble, nontoxic, and bioavailable (9, 27).
Ferritin (apoferritin-Fe complex) is an iron storage protein found in most living organisms (25). It is a spherical macromolecule with a protein coat made of 24 structurally equivalent subunits, which can hold up to 4,500 iron atoms as a ferric oxyhydroxide polymer in its central core (26). The major roles of ferritin are to provide iron for the synthesis of iron-containing proteins and to prevent damage by the free radicals produced in iron-dioxygen interactions (28). Ferritin has two main subunits: heavy (heart [H]) and light (liver [L]). Various combinations of the two subunits give rise to isoferritins, many of which are associated with specific pathologies or are located in discrete tissues (5). Generally, L-rich ferritins are characteristic of organs that store relatively large amounts of iron (≥1,500 Fe atoms/molecule), while H-rich ferritins are found in organs with a low average iron content (≤1,000 Fe atoms/molecule). No H homopolymers have been isolated in nature, whereas human serum ferritin is devoid of H chains (1) and an L-homopolymer fraction has been extracted from horse spleen ferritin (10). Since H homopolymer is a relatively poor iron core former and L homopolymer lacks an intrasubunit ferroxidase center, heteropolymeric ferritin stores and releases iron more efficiently than the homopolymer. Natural isoferritins are mostly heteropolymers formed by the coassembly of the two subunits. The subunits can be joined in various ratios, and only a limited number of H chains are required to maximize the iron uptake (22). Native horse spleen ferritin, one of the best-characterized and most common sources of ferritin, appears to have on average about 3 H chains/molecule.
The food yeast Saccharomyces cerevisiae, which is known as a GRAS (generally recognized as safe) organism, is grown to produce biomass rich in high-quality proteins and vitamins. As a consequence, it is used in feeds for fish, poultry, and fur-bearing animals and as a food supplement for human consumption (3). Increasing the nutritional quality of feed additives and food supplements is an efficient way to enrich feed and food with specific nutrients. In S. cerevisiae, extracellular Fe3+ is first reduced to the more soluble Fe2+ form by a plasma membrane Fe3+-reductase, and the resulting Fe2+ product is then taken up by either of two Fe2+-specific transport systems, depending on the extracellular iron concentration (4). The intracellular iron, which is extremely toxic at high concentrations, must be compartmented or stored as a safe, bioavailable form in the cytosolic ferritin.
The yeast ferritin-like protein has a very low binding affinity to cytosolic iron, which prevents yeast from utilizing it as an efficient source of bioavailable iron. In a previous study, we showed that S. cerevisiae can express the gene encoding the heavy subunit of tadpole ferritin and that the protein product can assemble into iron-storing apoferritin, improving the iron storage capability of the recombinant yeast (24). In this study, we further increased the iron-storing capability of the recombinant yeast via the expression of heteropolymeric apoferritin. The recombinant was designed to differentially express the H and L subunits of human ferritin in order to obtain heteropolymers that contained a limited number of H chains by coexpressing a low-copy integrative vector and a high-copy episomal vector for the H and L subunits, respectively. We also expressed homopolymers consisting of either H or L subunits as controls to compare the biochemical characteristics and demonstrated that the improved iron-storing capability of the recombinant yeast is mainly due to expression of heteropolymeric ferritin.
MATERIALS AND METHODS
Strains and culture conditions.
Plasmids were maintained and propagated in Escherichia coli HB101 or DH5α as described by Sambrook et al. (21). S. cerevisiae 2805 (MATα pep4::HIS3 prb1-δ Can1 GAL2 his3 ura3-52) (19) and S. cerevisiae 15Dau (MATa ade1 his2 leu2-3,112 trp1-1 ura3Δns) (29) were used to breed ascospore progeny with double selection markers for ura− and leu− and a concomitant pep4− for heteropolymeric ferritin expression. Mating, sporulation induction, and tetrad analysis were conducted as described elsewhere (18). Briefly, two strains of opposite mating types were mated on a yeast extract-peptone-dextrose (YEPD) plate, and the diploid was sporulated. The resulting tetrads were dissected onto YEPD plates, and the progeny that required both uracil and leucine were selected. Uracil-deficient (Ura−) selection medium (0.67% yeast nitrogen base without amino acids [Sigma], 30 mg of adenine and tryptophan per liter, 0.5% Casamino Acids, 2% dextrose, and 2% agar) and leucine-deficient (Leu−) selection medium (0.67% yeast nitrogen base without amino acids [Sigma], 0.14% yeast synthetic drop-out medium [Sigma], 30 mg of tryptophan per liter, 2% dextrose, and 2% agar) were used to screen the mating progenies and transformants.
S. cerevisiae was maintained in YEPD medium at 30°C. A primary inoculum was prepared from 5 ml of the Ura− selection medium and cultured for 24 h, and 107 cells were inoculated into a 300-ml Erlenmeyer flask containing 40 ml of YEPD medium. Expression cultures were grown at 30°C with continuous agitation (200 rpm), after which the cells were harvested and examined for the expression of ferritin.
To verify iron tolerance, transformants were grown and CFU on Ura− selection plates supplemented with ferric citrate were counted.
Vector construction.
In order to express different levels of H and L chains, a low-copy integrative vector, YIplac128, (6), and a high-copy episomal vector, YEp352 (19), were engineered to express the H and L chains, respectively. We used the same promoter for both vectors to minimize the discrepancy due to differences in promoter strength. Briefly, we cloned the human ferritin H-chain gene (HuFH; accession no. BC016009) from 41 bp upstream from the translational start codon to 142 bp downstream from the translational stop codon, between the ADH2-GPD hybrid promoter and the galactose-1-phosphate uridyl transferase (GAL7) terminator, and the resulting expression cassette was ligated to EcoRI and HindIII sites of the integrative vector YIplac128. In addition, the human ferritin L-chain gene (HuFL; accession no. BC004245) from 41 bp upstream from the translational start codon to 45 bp downstream from the translational stop codon was also placed between the hybrid promoter and GAL7 terminator of the episomal vector YEp352 (19). The restriction maps of both the integrative and episomal recombinant plasmids are shown in Fig. 1. In order to produce L and H homopolymers, only the high-copy episomal vector YEp352 was used as the expression vector.
FIG. 1.
Schematic diagram of the recombinant integrative plasmid (A) and episomal plasmids with HuFH (B) and HuFL (C). The boxes represent the genes or their corresponding functional domains. pAG and tGAL7 represent the ADH2-GPD hybrid promoter and terminator of galactose-1-phosphate uridyl transferase, respectively. The lower panels in each panel show the sequence covering the links of promoter-ferritin gene-terminator. The transcription initiation sites are marked with asterisks, and translation start and stop codons are in boldface.
The S. cerevisiae strain was transformed as previously reported (6, 11). The transformed cells were selected on either Leu− or Ura− selection medium, and the presence of the transforming plasmid was confirmed by either Southern blot analysis or back transformation of E. coli with DNA prepared from the putative transformants.
The stability of the introduced plasmids in yeast was measured by using appropriate selection media according to a previously described procedure (24).
Northern blot analysis.
Transformants were grown in YEPD medium, and total RNA was prepared (17). Following electrophoresis on a 1.2% agarose gel in the presence of formaldehyde and transfer to a nylon membrane, Northern blots were probed with 32P-labeled HuFH and HuFL (21).
Preparation of crude cell extract of yeast.
Cells were grown for 3 days, harvested, washed twice with extraction buffer (50 mM Tris-HCl, 2 mM EDTA), and ground three times in a Bead Beater (Biospec Products Inc.) for 1 min. The lysate was centrifuged (10 min at 10,000 × g), and the supernatant was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (see below). Since ferritin is thermostable (12) and the host strain is protease deficient, all of the preparation steps were performed at room temperature (∼25°C).
PAGE.
SDS-PAGE was conducted according to the method of Laemmli (13), with 12% acrylamide separating gels and 5% stacking gels, each containing 1% SDS. Samples were heated at 100°C for 10 min in 125 mM Tris-glycine buffer (pH 6.8) containing 1% SDS and 1% β-mercaptoethanol (8). Electrophoresis was carried out at a constant current of 10 mA for 3 h with a running buffer of 25 mM Tris-glycine (pH 8.8) containing 0.1% SDS. After electrophoresis, the gel was stained with 0.025% Coomassie brilliant blue R-250.
We used staining with Prussian blue (12) to determine whether the expressed ferritin H-chain subunits assemble into a large complex that can store iron. The cell extracts were heated at 75°C for 15 min, resolved by 6.0% native PAGE under nondenaturing conditions (8), and then stained with a mixture of 2% K4Fe(CN)6 and 2% 11.6 M HCl (1:1, vol/vol) prepared immediately before use (12). Electrophoresis was carried out at a constant current of 25 mA for 5 h with a running buffer of 25 mM Tris-glycine (pH 8.5). Horse spleen ferritin purchased from Sigma was included as a control.
Protein purification.
The expressed protein products were purified by native gel filtration with 6.0% preparative native PAGE under nondenaturing conditions and then stained with copper chloride (14). The bands were eluted by using a model 422 Electro-Eluter (Bio-Rad) and then concentrated by using an Amicon concentrator with a 30-kDa-molecular-mass molecular cutoff. The purity of the eluted ferritin was checked again by native PAGE, and its subunit composition was revealed by SDS-PAGE. The biochemical characteristics of the purified ferritin product were then determined in further experiments.
Phytase expression.
Since several studies have shown that parental strain 2805 is good at expressing foreign genes, the heterologous expression capability of the progeny was compared with that of strain 2805 by using phytase as a reporter enzyme. The phytase expression plasmid obtained from our previous study (Fig. 1C) (24) was introduced into selected ascospore progenies, and the resulting transformants were analyzed to compare phytase activity with that of parental strain 2805. The phytase activity was measured as described previously (15), and 1 U of enzyme activity was defined as the amount of activity that released 1 μmol of phosphate per min at 58°C. The measurements were performed in triplicate, and all experiments were carried out at least three times and had similar results.
Atomic absorption spectrometry.
We conducted an iron uptake assay with samples grown in Ura− selection medium supplemented with ferric citrate. Cells were grown in Ura− selection medium supplemented with ferric citrate at 30°C for 4 days, harvested by centrifugation (10 min, 3,500 × g), washed three times with H2O, and then dried at 60°C for 48 h. The dried cells were digested with 6 ml of 14 M nitric acid-10 M perchloric acid (2:1, vol/vol) in a glass tube at 250°C for 8 h. The iron concentration was determined by atomic absorption spectrometry (AAS 208; Hitachi Inc., Tokyo, Japan) (16).
Statistical analyses were performed with the t test procedure from the Statistical Analysis System (version 6.0 for PC; SAS/STAT Institute Inc.) (23).
RESULTS
Breeding of a host strain.
In order to produce a coexpression system using YIplac128 and YEp352, the host strain requires at least two selection markers, leu2− and ura3−, respectively. Since S. cerevisiae strain 2805 expresses several foreign genes well, including a tadpole ferritin H-chain gene (24), it was desirable to incorporate an additional leu2− marker into strain 2805. Therefore, S. cerevisiae strain 15Dau, which is an opposite mating type containing the leu2− marker, was selected as the mating strain. Ascospore progenies were screened for the double mutant phenotypes. Fifty-eight (25.8%) of 224 ascospore progeny showed auxotrophic phenotypes for both leucine and uracil. In order to protect the expressed foreign protein, protease deficiency (pep4::HIS3) in strain 2805 was preferred. Therefore, deletion of the wild-type PEP4 allele was examined by PCR amplification with a primer pair in which one sequence (5′-TGGGCAGCAGCATAGAAC-3′) corresponded to the PEP4 gene and the other (5′-TCACTACATAAGAACACCTT-3′) corresponded to the HIS3 gene. Of the 58 progeny screened, 19 showed an expected 2.0-kb PCR amplicon indicating the presence of a mutated pep4 allele. In addition, phytase expression revealed that 10 out of 19 progeny containing leu2−, ura3−, and pep4::HIS3 alleles showed phytase activity equivalent to that of parental strain 2805. Consequently, ascospore progeny 2805-a7 was selected as the expression host based on the characteristics of double selection markers, pep4::HIS3 allele, and high expression capability.
Expression of heteropolymeric ferritin in recombinant yeast.
Since a high proportion of L chain is preferred, a high-copy episomal vector, YEp352, was engineered to construct the expression plasmid for the L chain. Northern blot analysis of the transformants showed that the introduced HuFL gene was strongly expressed under the control of the hybrid promoter. Transformant TYEFLAG-1, which expressed the highest levels among the 10 transformants, was selected for subsequent transformation with an H-chain gene. After transformation of TYEFLAG-1 with a low-copy integrative vector (YIplac128) containing an H-chain gene, transformant TYFHLAG-1 was selected from 10 candidates that showed similar levels of H-chain gene transcript expression (data not shown).
In order to produce homopolymeric ferritins, transformants TYEFHAG-1 and TYEFLAG-1, which contained episomal vectors containing the H- and L-chain genes, respectively, were selected.
The plasmid stability of the episomal vector was good, and more than 80% of the plated cells appeared to carry the plasmid 72 h after liquid culture. In addition, no loss of an integrative plasmid was observed (data not shown). When grown in YEPD medium, all transformants showed growth curves similar to that of the host strain, suggesting that no growth defect resulted from expression of the ferritin gene.
In previous studies, iron tolerance was one of the characteristics of recombinant yeast expressing ferritin subunit genes (24). Iron tolerance was measured by spreading cells on Ura− selection medium containing 25 mM ferric citrate. After a 48-h incubation, all transformants, regardless of whether they produced homo- or heteroferritin, formed distinctive colonies, while the untransformed host did not, which suggested the presence of functional homo- and heteroferritins in the recombinant yeasts.
Characteristics of heterologously expressed isoferritins.
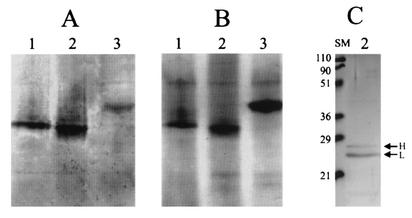
The iron-binding capability of the expressed ferritin was analyzed by Prussian blue staining (Fig. 2A). After staining with ferrocyanide, characteristic blue bands were observed in cell extracts from all transformants, indicating that the expressed ferritin subunits had assembled into a functional protein that could bind iron. Before the sample was loaded on a nondenaturing gel, a longer incubation (≥4 h) with freshly made ferrous ammonium sulfate solution was necessary for the cell extract from the L-homopolymeric transformant, while less than 20 min was sufficient for the cell extract from either the H-homopolymeric or heteropolymeric transformants. The heteropolymeric ferritin moved as a single monomeric band more slowly than either homopolymer in nondenaturing gel electrophoresis (Fig. 2A). This single monomeric band pattern of the heteropolymer was maintained throughout culture, and no change in relative mobility, compared with the homopolymers, was observed (data not shown). Neither homopolymeric band was detected.
FIG. 2.
Prussian blue staining of isoferritins. (A) Prussian blue-stained nondenaturing PAGE after preincubation of the cell extract of yeast with 1 mM ferrous ammonium sulfate. (B) Coomassie blue-stained nondenaturing PAGE without preincubation with ferrous ammonium sulfate. (C) Coomassie blue-stained SDS-PAGE of gel-purified heteropolymeric ferritin. Lanes: 1, H homopolymer; 2, heteropolymer; 3, L homopolymer; SM, size marker. The numbers on the left indicate estimated sizes in kilodaltons.
Since electrophoresis under nondenaturing conditions resulted in a relatively pure ferritin protein (Fig. 2B), the expressed ferritins were purified by preparative nondenaturing gel electrophoresis followed by gel elution. No difference in the mobility of Coomassie blue-stained isoferritins was observed between protein extracts incubated with and without ferrous ammonium sulfate. Therefore, none of the preparations were incubated with protein extract with ferrous ammonium sulfate, which helped to maintain the expressed isoferritins as relatively iron-free apoferritin. The purified ferritins were checked by SDS-PAGE and appeared to be electrophoretically pure (Fig. 2C). From SDS-PAGE, the molecular sizes of the yeast-expressed H and L subunits were estimated to be 27 and 24 kDa, respectively. In addition, it was confirmed that the heteropolymeric ferritin consisted of H and L subunits in a different molar ratio, based on the considerable difference in the band intensities between the two subunits. The protein concentrations of the purified H and L homopolymers and the heteropolymer were determined to be 42, 36, and 37 μg per mg of total soluble protein, respectively. These amounts were determined from at least three independent experiments, and less than 5% deviation was observed among trials. There was more H-homopolymeric isoferritin than L-homopolymeric isoferritin, and the amount of heteropolymer was intermediate between them. These results suggested that nearly equimolar expression of isoferritins occurred among transformants expressing the H and L homopolymers and heteropolymer. The purified heteropolymer was subject to SDS-PAGE with serial dilutions of each homopolymer to determine the subunit proportion by using densitometry of Coomassie blue-stained subunit bands, and the molar ratio of the H to L chains was determined to be 1:6.8.
The rates of iron uptake by the three purified isoferritins were determined by using absorbance at 310 nm (Fig. 3). The initial rate of iron uptake by the heteropolymer was slower than that by the H homopolymer under standard conditions (pH 6.5 and an iron/ferritin molar ratio of 1,000:1). The rate of uptake by the heteropolymer was lower for the first 3 min, but it subsequently reached a peak and remained constant. By contrast, the rate of uptake by the L homopolymer was very low; the L homopolymer took 8 h before a sharp increase in the iron uptake rate was observed, and more than 12 h was required to reach the maximum iron content (data not shown). At a higher molar ratio of iron to ferritin (2,000:1), it took longer for both the H homopolymer and the heteropolymer to reach their maximum uptakes, requiring more than 6 and 9 min, respectively. In addition, the maximum iron contents of the H homopolymer and heteropolymer differed; 30% less iron was observed with the H homopolymer. At the same higher molar ratio, the initial rate of L homopolymer was still very low, whereas the maximum iron content at 12 h of incubation was more than 90% of that of the heteropolymer.
FIG. 3.
Iron uptake assay of homopolymeric and heteropolymeric apoferritins. Progressive plots of iron uptake monitored by absorbance at 310 nm with molar iron/ferritin ratios of 1,000:1 (open symbols) and 2,000:1 (solid symbols) are shown. The heteropolymer and H homopolymer are indicated by circles and triangles, respectively. The data for the L homopolymer are described in the text because the L homopolymer required prolonged incubation (>4 h) before a significant increase in iron uptake was observed. The data are the means from at least three independent experiments, for which there was less than 10% deviation.
Iron storage in recombinant yeast.
Iron incorporation and cell growth were measured for the recipient strain and transformants grown at high iron concentrations (Table 1). Transformant TYETFAG-1, which expressed a tadpole H homopolymer in a previous study, was used as a positive control. The iron content increased in the order heteropolymer, L homopolymer, and H homopolymer. Transformant TYFHLAG-1, expressing the heteropolymer, contained more than twice as much iron as transformants with H homopolymers. The L homopolymer had the second best iron storage; the iron uptake was increased by 50%. The iron incorporation in vivo increased significantly with the heterologous expression of ferritin, and the efficacy was further improved by expression of the heteropolymer.
TABLE 1.
Atomic absorption spectrometry of intracellular iron content
| Strain | Fe-citrate concn | Cells/mla (mean ± SD) | Iron content, ppmb (mean ± SD) |
|---|---|---|---|
| Recipient (2805-a7) | 0 | 3.0 × 108 ± 0.8 × 107 | 6.3 ± 1.2 |
| 20 | 1.0 × 108 ± 1.3 × 107 | 210 ± 12 | |
| Transformants | |||
| TYETFAG-1 | 0 | 3.2 × 108 ± 1.2 × 107 | 5.3 ± 0.9 |
| 20 | 1.5 × 108 ± 2.1 × 107c | 500 ± 51c | |
| TYEFHAG-1 | 0 | 3.3 × 108 ± 1.3 × 107 | 5.9 ± 0.9 |
| 20 | 1.5 × 108 ± 2.2 × 107c | 505 ± 62c | |
| TYEFLAG-1 | 0 | 3.2 × 108 ± 1.3 × 107 | 6.5 ± 0.9 |
| 20 | 1.4 × 108 ± 2.0 × 107c | 760 ± 81c | |
| TYFHLAG-1 | 0 | 3.2 × 108 ± 1.1 × 107 | 5.5 ± 0.9 |
| 20 | 1.4 × 108 ± 2.1 × 107c | 1,004 ± 61c |
Values were obtained from three replicates of each experiment repeated twice.
Parts per million of dry cell mass.
Value is significantly different from that for the recipient strain (P = 0.05).
DISCUSSION
Breeding a new expression host is desirable, since, in addition to targeted genetic traits, natural genetic shuffling can result in a broad genotype background from which a better-fit progeny can be obtained. We compared phytase expression in the bred strains and found that 10 progenies expressed as much as strain 2805, which is a well-known host strain for heterologous expression. In addition, the high expression levels of the bred strains were not restricted to phytase; other foreign genes (e.g., the glucose oxidase gene) were also expressed at high levels in our laboratory (Y. Y. Lim et al., unpublished). Of interest is that 10 of 19 had high expression levels (>13 IU/ml), while the rest had low expression levels (<5 IU/ml). Although further confirmation is necessary, the genetic data strongly suggest a single genetic determinant governing the high expression ability, which facilitates breeding a high-expression host with strain 2805 as the donor.
The molecular masses of the yeast-derived HuFH and HuFL were 27 and 24 kDa, respectively, which are larger than those of the native proteins. Eukaryotic ferritin subunits are processed into mature proteins by posttranslational modification (20), and the yeast-derived tadpole ferritin H chain was also larger than the native protein (24). These results suggest that the posttranslational modification that occurs in yeast differs from that in humans.
In order to express heteromers with different subunit ratios, a differential expression system is preferred to an equimolar expression system. This study used coexpression with two compatible vectors that have different copy numbers. This has several advantages over using a single vector with two target genes; it avoids possible intramolecular recombination, and relatively small pieces of DNA can be processed in a stepwise manner. In addition, using the same promoter for different target genes seems to be advantageous over using different promoters, because once the initial expression ratio is determined, the expression ratio would remain constant throughout the culture period, regardless of changes in the intracellular and extracellular environments.
A single monomeric band of the heteropolymer, with unvarying mobility, was observed in nondenaturing gels throughout the cultivation. If the expression levels of transformants with an identical integrative vector vary according to where the vector is integrated, these random integrative transformants can be used to obtain a broad spectrum of different combinations of H and L chains in vivo. Accordingly, it will be of interest to analyze other integrative transformants to determine the function-structure relationship of the isoferritin heteropolymers.
As shown in Fig. 2A, the heteropolymeric ferritin moved as a single monomeric band, and no homopolymeric bands were detected in nondenaturing gel electrophoresis. Therefore, the formation of heteropolymers is preferred in vivo. In addition, the single monomeric band indicated that the expressed subunits coassembled into a limited range out of the possible isoferritin spectrum, rather than forming a broad spectrum of isoferritins due to random interactions between the H and L chains. In an in vitro coassembly assay, the two denatured subunits coassembled into a heteropolymer of the predicted subunit composition, and the composition was dependent on the concentration of the denatured subunits in the renaturing mixture (22). If this is the case in vivo, the proportion of H chain in the heteropolymer (<13%) should reflect the ratio of gene expression of the H chain versus the L chain within the cell. Further studies are needed to determine how the apoferritin recognizes the intracellular concentration of each subunit and how it affects the subunit composition in ferritin complex. It will be of interest to compare the transcript levels of subunit genes in different tissues containing H-rich and L-rich ferritins.
The rates of iron uptake were similar for both the H homopolymer and the heteropolymer and differed markedly in the L homopolymer. With isoferritins coassembled from E. coli-expressed subunits, the iron uptake rate increased linearly up to an H-chain content of 35% and remained constant thereafter. Our results obtained with purified apoferritin are consistent with previous results obtained with in vitro-coassembled isoferritins: heteropolymer containing 10% H chain showed approximately 20% of the maximum rate in the first minute. Therefore, regardless of whether assembly is in vitro or in vivo, this suggests that the H-chain content determines the iron uptake rate.
Since the expression levels of isoferritin were similar for all three transformants, the improved iron incorporation of the transformed cell is due to expression of the heteropolymer rather than the homopolymer. The recommended iron consumption for an adult is 12 to 16 mg/day, which is much greater than that in recombinant yeast. Within these amounts, we do not know how much is required in bioavailable forms. It has been proved that oral administration of ferritin results in safe storage and that it is a good source of iron for the treatment of anemia (2). Accordingly, increasing the amount of iron-containing ferritin in animal feeds might reduce dietary iron deficiency and, perhaps, improve the dietary quality of livestock as an iron-fortified food for human consumption (7). Therefore, a further increase in the bioavailable iron content should effectively alleviate some of the problems related to iron deficiency in both humans and animals.
Acknowledgments
This work was supported by a grant from the Korean Ministry of Science and Technology and by Chollabukdo Province in support of regional research and development.
We thank the Institute for Molecular Biology and Genetics and the Center for University-Wide Research Facility at Chonbuk National University for providing facilities used in this research.
REFERENCES
- 1.Arosio, P., M. Yokota, and J. W. Drysdale. 1977. Characterization of serum ferritin in iron overload: possible identity to natural apoferritin. Br. J. Haematol. 36:199-207. [DOI] [PubMed] [Google Scholar]
- 2.Beard, J. L., J. W. Burton, and E. C. Theil. 1996. Purified ferritin and soybean meal can be source of iron for treating iron deficiency in rats. J. Nutr. 126:154-160. [DOI] [PubMed] [Google Scholar]
- 3.Bui, K., and P. Galzy. 1990. Food yeast, p. 241-265. In J. F. T. Spencer and D. M. Spencer (ed.), Yeast technology. Springer, Berlin, Germany.
- 4.Dix, D., J. Bridgham, M. Broderius, and D. Eide. 1997. Characterization of the FET4 protein of yeast. Evidence for a direct role in the transport of iron. J. Biol. Chem. 272:11770-11777. [DOI] [PubMed] [Google Scholar]
- 5.Drysdale, J. W., T. G. Adelman, P. Arosio, D. Casareale, P. Fitzpatrick, J. T. Harzard, and M. Yokota. 1977. Human isoferritins in normal and disease states. Semin. Hematol. 14:71-88. [PubMed] [Google Scholar]
- 6.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 30:527-534. [DOI] [PubMed] [Google Scholar]
- 7.Goto, F., T. Yoshihara, N. Shigemoto, S. Toki, and F. Takaiwa. 1999. Iron fortification of rice seed by the soybean ferritin gene. Nat. Biotechnol. 17:282-286. [DOI] [PubMed] [Google Scholar]
- 8.Hames, B. D. 1981. An introduction to polyacrylamide gel electrophoresis, p. 26-42. In B. D. Hames and D. Rickwood (ed.), Gel electrophoresis of proteins, a practical approach. IRL Press, Oxford, United Kingdom.
- 9.Harrison, P. M., and T. H. Lilley. 1989. Ferritin, p. 123-138. In T. M. Loehr (ed.), Iron carriers and iron proteins. VCH, New York, N.Y.
- 10.Ishitani, K., and I. Listowsky. 1975. Differences in subunit composition and iron content of isoferritins. J. Biol. Chem. 250:5446-5449. [PubMed] [Google Scholar]
- 11.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, Y. T., and K. S. Kim. 1994. Synthesis of active tadpole H-chain ferritin in Escherichia coli. Mol. Cell 4:125-129. [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Lee, C., A. Levin, and D. Branton. 1987. Copper staining: a five-minute protein stain for sodium dodecyl sulfate-polyacrylamide gels. Anal. Biochem. 166:308-312. [DOI] [PubMed] [Google Scholar]
- 15.Lim, Y. Y., E. H. Park, J. H. Kim, S. M. Park, H. S. Jang, Y. J. Park, S. W. Yoon, M. S. Yang, and D. H. Kim. 2001. Enhanced and targeted expression of fungal phytase in Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 11:915-921. [Google Scholar]
- 16.Mateos, F., C. Gonzalez, C. Dominguez, J. E. Losa, A. Jimemez, and J. L. Perez-Arellano. 1999. Elevated non-transferrin bound iron in the lungs of patients with Pneumocystis carinii pneumonia. J. Infect. 38:18-21. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell, D. A., L. Farh, T. K. Marshall, and R. J. Deschenes. 1994. A polybasic domain allows nonprenylated Ras proteins to function in Saccharomyces cerevisiae. J. Biol. Chem. 269:21540-21546. [PubMed] [Google Scholar]
- 18.Mortimer, R. K., and D. C. Hawthorne. 1969. The yeasts, vol. 1, Academic Press, New York, N.Y.
- 19.Park, E. H., Y. M. Shin, Y. Y. Lim, T. H. Kwon, D. H. Kim, and M. S. Yang. 2000. Expression of glucose oxidase by using recombinant yeast. J. Biotechnol. 81:35-44. [DOI] [PubMed] [Google Scholar]
- 20.Ragland, M., J. F. Briat, J. Gagnon, J. P. Laulhere, O. Massenet, and E. C. Theil. 1990. Evidence for a conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J. Biol. Chem. 265:18339-18344. [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Santambrogio, P., S. Levi, A. Cozzi, E. Rovida, A. Albertini, and P. Arosio. 1993. Production and characterization of recombinant heteropolymers of human ferritin H and L chains. J. Biol. Chem. 268:12744-12748. [PubMed] [Google Scholar]
- 23.SAS Institute, Inc. 1985. SAS/STAT: language guide for personal computers, version 6. SAS Institute, Inc., Cary, N.C.
- 24.Shin, Y. M., T. H. Kwon, K. S. Kim, K. S. Chae, D. H. Kim, and M. S. Yang. 2001. Enhanced iron uptake of Saccharomyces cerevisiae by heterologous expression of a tadpole ferritin gene. Appl. Environ. Microbiol. 67:1280-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theil, E. C. 1987. Ferritin: structure, gene regulation, and cellular function in animals, plants and microorganisms. Annu. Rev. Biochem. 56:289-315. [DOI] [PubMed] [Google Scholar]
- 26.Theil, E. C. 1990. Regulation of ferritin and transferrin receptor mRNAs. J. Biol. Chem. 265:4771-4774. [PubMed] [Google Scholar]
- 27.Theil, E. C. 1990. The ferritin family of iron storage proteins. Adv. Enzymol. 63:421-449. [DOI] [PubMed] [Google Scholar]
- 28.Theil, E. C., J. W. Burton, and J. L. Beard. 1997. A sustainable solution for dietary iron deficiency through plant biotechnology and breeding to increase seed ferritin control. Eur. J. Clin. Nutr. 51:S28-S31. [PubMed]
- 29.Valdivieso, M. H., K. Sugimoto, K. Y. Jahng, P. M. Fernandes, and C. Wittenberg. 1993. FAR1 is required for posttranscriptional regulation of CLN2 gene expression in response to mating pheromone. Mol. Cell. Biol. 13:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]