Abstract
Difficulties in efficiently propagating Kaposi's sarcoma-associated herpesvirus (KSHV) in culture have generated the impression that the virus displays a narrow host range. Here we show that, contrary to expectation, KSHV can establish latent infection in many adherent cell lines, including human and nonhuman cells of epithelial, endothelial, and mesenchymal origin. (Paradoxically, the only lines in which we have not observed successful latent infection are cultured lymphoma cell lines.) In most latently infected lines, spontaneous lytic replication is rare and (with only two exceptions) is not efficiently induced by phorbol ester treatment—a result that explains the failure of most earlier studies to observe efficient serial transfer of infection. However, ectopic expression of the KSHV lytic switch protein RTA from an adenoviral vector leads to the prompt induction of lytic replication in all latently infected lines, with the production of infectious KSHV virions. These results indicate (i) that the host cell receptor(s) and entry machinery for KSHV are widely distributed on cultured adherent cells, (ii) that latency is the default pathway of infection, and (iii) that blocks to lytic induction are frequent and largely reside at or upstream of the expression of KSHV RTA.
Kaposi's sarcoma-associated herpesvirus (KSHV; also called human herpesvirus 8) is a lymphotropic herpesvirus linked to several clinical disorders, including Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD) (5, 6, 27). In infected individuals, KSHV is detected primarily in B cells, and latent B-cell infection is also regularly observed with the KSHV-associated lymphoproliferative disorders PEL and MCD (10). In KS tumors, latent viral infection is evident in the endothelial (spindle) cells of the lesion, small subpopulations of which also support lytic replication (3, 28). KS, PEL, and MCD have not been observed in veterinary pathology, and KSHV has not been successfully transmitted experimentally to laboratory animals. For example, in experiments in which SCID/hu mice were exposed to KSHV, infection was largely limited to the B cells of the human explant, with no murine tissues displaying infection (9). Attempts to infect rhesus macaques have likewise not produced seroconversions or pathological lesions (R. Renne and D. Ganem, unpublished results). These observations suggest that the virus has a limited tissue tropism and narrow host range in vivo.
Attempts to propagate KSHV in vitro have encountered many obstacles, and these difficulties have often been assumed to reflect the restricted tropism inferred from KSHV biology in vivo. KSHV was initially cultivated from explanted PEL cells, in which latency was established in vivo; treatment of such cells with phorbol esters or sodium butyrate regularly results in induction of lytic replication and death of the culture (4, 5, 12, 14, 15, 19, 20, 21, 25, 30). While useful for viral stock preparation, these cells do not support serial transfer of infection. Two large studies have examined the infection of many other cell lines in culture. Blackbourn et al. examined 25 lines for infectibility by KSHV (as determined by PCR and reverse transcription [RT]-PCR-based detection of viral lytic transcripts) and found seven that would support KSHV entry and gene expression, but infectious virus could be recovered only from t-DMVECs (2). Renne et al. (23) examined 39 cell lines for infectibility by KSHV, using RT-PCR for a spliced late mRNA (encoding open reading frame 29 [ORF29] protein) as an indication that the line could support entry and lytic replication. They found that 11 of these 39 lines could support at least low-level spontaneous lytic replication—a surprisingly large number. None of these lines, however, appeared to support efficient serial passage. Most lines were tested in the absence of phorbols, but the few that were tested with this inducing agent also did not show efficient transfer of the ORF29 mRNA marker.
Apart from these large-scale surveys, most work on KSHV cultivability has involved the more detailed examination of individual cell lines. In 1997, Foreman et al. reported that HEK-293 cells exposed to material from KS biopsy specimens developed cytopathic effects (CPE) that could be serially transferred from one 293 monolayer to another (11). This suggested that such cells might support efficient de novo induction of lytic replication, much as is observed for herpes simplex viruses and cytomegalovirus (CMV) in culture. In fact, the ORF29 mRNA assay by Renne et al. (23) was set up with this in mind, and using this assay, these authors affirmed that 293 cells could indeed display expression of both latent and lytic markers. However, this and subsequent studies have come to divergent conclusions about serial transmission on 293 monolayers. When they examined cells for a lytic transcript, Renne et al. (23) found that 293 cells only inefficiently supported serial transfer of virus. More recent studies have employed recombinant viruses bearing green fluorescent protein (GFP) markers to monitor infection at the single-cell level. One (8) concluded, in agreement with Foreman et al. (11), that 293 cells could directly support entry into the lytic cycle; two others, however, reported that the default pathway after 293 infection is latency, from which induction to lytic replication was possible with tetradecanoyl phorbol acetate (TPA) (32, 33). Other recent studies have shown that primary (7) and immortalized (18, 22) human dermal microvascular endothelial cells also support both latent and lytic KSHV growth.
Much of the confusion that currently exists about KSHV host range in vitro stems from the fact that large-scale, systematic surveys for infectibility antedated the availability of good single-cell markers of latent and lytic infection. Moreover, the designs of early studies of transmission were predicated on direct entry into the lytic cycle upon de novo infection. We now know (18, 22, 32) that, as with Epstein-Barr virus (EBV), latency is likely to be the default pathway following de novo infection in vitro. Accordingly, we have reexamined the issue of KSHV host range in culture, using contemporary assays for detection of latent and lytic infection. Our data indicate that KSHV has a surprisingly broad host range in culture in that many cells can efficiently establish latency after de novo infection. However, most cells have blocks to lytic reactivation, at least when phorbols are used as the inducing agent. These blocks can be bypassed by provision of recombinant KSHV RTA protein in trans, indicating that the block is at or upstream of RTA expression. These findings rationalize many of the seemingly conflicting observations stemming from earlier work.
MATERIALS AND METHODS
Cells and media.
BCBL-1 cells are an EBV-negative PEL cell line and have been previously described (25). BCBL-1 cells were carried in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, glutamine, and β-mercaptoethanol. Telomerase-immortalized microvascular endothelial (TIME) cells are a dermal microvascular endothelial cell line immortalized with the telomerase reverse transcriptase subunit hTERT and have been described elsewhere (31). They were maintained in an EBM-2 medium bullet kit (Clonetics). Cells from the human foreskin fibroblast (HFF), 293 (human kidney epithelial cells), HeLa (cervical carcinoma cells), CV-1 (green monkey kidney cells), SLK (spindle cells), and 3T3 (murine fibroblasts) lines were maintained in Dulbecco modified Eagle medium (DMEM; Gibco) supplemented with 10% FBS, penicillin, and streptomycin. BJAB (EBV-negative B-cell lymphoma), Raji (EBV-positive B-cell lymphoma), Ramos (EBV-negative B-cell lymphoma), and Jurkat (T-cell line) cells were maintained in RPMI 1640 (Gibco) containing 10% FBS, penicillin, and streptomycin. CHO cells (Chinese hamster ovary cells) were grown in DMEM supplemented with 10% FBS, proline, penicillin, and streptomycin. QT-6 cells (quail fibroblasts) were grown in DMEM supplemented with 5% FBS, 1% chicken serum, 10% tryptone, penicillin, and streptomycin.
Viruses and infection.
Virus inoculum was obtained from induced BCBL-1 cells as previously described (18). Large volumes of induced supernatants were concentrated for 2 h at 13,000 rpm in a GSA rotor while smaller volumes were concentrated in an ultracentrifuge for 2 h at 15,000 rpm in an SW28 rotor. Virus was resuspended in media containing 2 μg of Polybrene/ml. Diluted virus stocks were used to inoculate cells for 2 h. The cells were then washed twice with complete media. For phosphonoformic acid (PFA) treatment, cells were kept in 0.5 mM PFA during and after KSHV infection, until virus was harvested. For induction by TPA, adenovirus expressing GFP (AdGFP), or adenovirus expressing RTA (AdRTA), the cells were treated immediately after infection or 48 h postinfection (48 hpi) with either media containing 20 ng of TPA/ml or 1,000 particles of AdGFP/cell or of AdRTA/cell and 0.5 μg of polylysine/ml (1 μg of polylysine/ml for HFFs) for 36 h. SLK cells were induced with sodium butyrate (1 mM; Calbiochem), 5-azacytidine (10 μM; Sigma), ionomycin (500 nM; Sigma), CoCl2 (100 μM; Sigma), tumor necrosis factor alpha (10 ng/ml; R&D Systems, Inc.), or interleukin-1 beta (3.5 ng/ml; Biosource International) 48 hpi for 36 h.
AdRTA construction.
AdRTA was generated by using the Adeno-X expression system (Clontech) according to the manufacturer's instructions. The ORF50 region was subcloned from pGem3-Flc50 (19) by ClaI digestion into a blunt site in pShuttle. The RTA expression cassette was then transferred to the Adeno-X viral DNA by using unique PI-SceI/I-CeuI sites. The recombinant adenovirus was amplified by infecting low-passage 293 cells at a multiplicity of infection (MOI) of 10 to 100 and allowing cells on the monolayer to round up, at which point the cells were collected. The cells were subjected to three freeze-thaw cycles, and then cell debris was pelleted by centrifugation. Virus was purified from the clarified supernatant by CsCl2 gradient centrifugation, and the concentration of virus was determined by measuring the optical density at 260 nm. The virus was diluted in 50% glycerol in 10 mM Tris-100 mM NaCl.
Transient transfections.
293 cells were transiently transfected with pCDNA3ORF50 (19) by using Fugene (Roche) according to the manufacturer's specifications 2 h after infection with KSHV.
Immunofluorescence.
Immunofluorescence was performed as previously described (19). The primary antibodies used were an anti-latency-associated nuclear antigen (LANA) peptide polyclonal antiserum from rabbits (provided by A. Polson) and mouse monoclonal antibodies that recognize ORF59 (Advanced Biotechnologies Inc.) and gpK8.1 (gift of L. Wu and B. Forghani) proteins. The primary antibodies were diluted 1:500, and secondary antibodies anti-rabbit fluorescein isothiocyanate and anti-mouse rhodamine (Santa Cruz) were diluted 1:300.
Gardella gel electrophoresis.
Uninduced and induced cells latently infected with KSHV were trypsinized, resuspended in complete media, and washed twice in phosphate-buffered saline. Cell pellets were frozen at −80°C and thawed prior to being loaded onto the gels. The lysis and separating gels were poured as previously described (13). The cell pellets (106 cells) were resuspended in loading buffer containing 5% Ficoll and 40 μg of RNase A/ml in Tris-borate-EDTA and then loaded onto the gels. The gels were run at 40 V for 2 h and then at 160 V for an additional 12 h in Tris-borate-EDTA. The gels were then blotted onto nylon (Hybond N+; Amersham) and probed with 32P-radiolabeled DNA corresponding to ORF73 and the K8.1 ORF.
RESULTS
Susceptibility to latent KSHV infection.
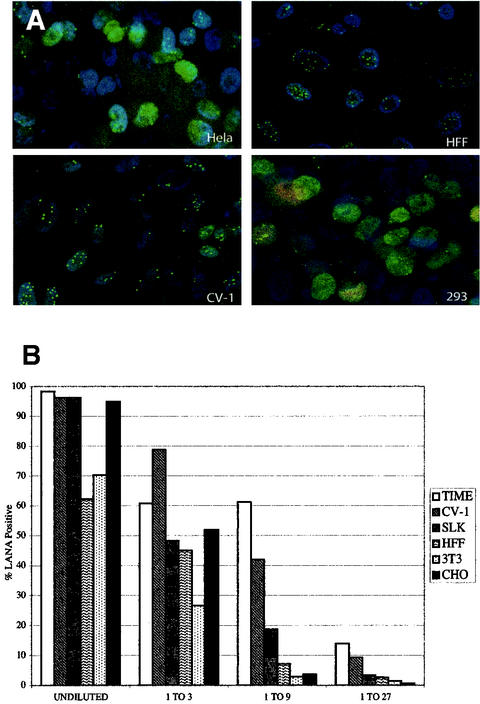
We examined 13 cell lines of different species and lineages to determine which were susceptible to KSHV infection. Concentrated supernatants of KSHV derived from induced BCBL-1 cells were used to infect the various cell lines. After adsorption for 2 h, the inoculum was removed and the cells were washed and incubated for 24 to 48 h. The cells were then examined for the expression of LANA, encoded by ORF73, by using an immunofluorescence assay (IFA). We found that all of the adherent cell lines examined, regardless of species or lineage, displayed LANA staining following exposure to the same undiluted viral stock (Table 1; IFA images representative of results for several lines are shown in Fig. 1A). LANA is a nuclear protein that exhibits a characteristic speckled staining pattern as a result of IFA. Most of the cell lines displayed numerous and prominent nuclear speckles in nearly every nucleus, suggesting a high level of infection. However, CHO (hamster fibroblasts) and QT-6 (quail fibroblasts) lines appeared to be less susceptible, since positive cells exhibited only a few speckles per nucleus (data not shown) and in the case of QT-6 cells fewer nuclei were positive.
TABLE 1.
Susceptibility of cells to latent KSHV infection
| Origin | Cell type | Susceptibilitya |
|---|---|---|
| Human | TIME | + |
| 293 | + | |
| HFF | + | |
| SLK | + | |
| HeLa | + | |
| Monkey | CV-1 | + |
| Rodent | 3T3 | + |
| CHO | +/− | |
| Quail | QT-6 | +/− |
| Human lymphoblastoid | BJAB | − |
| Raji | − | |
| Ramos | − | |
| Jurkat | − |
+, susceptible; −, not susceptible; +/−, partially susceptible.
FIG. 1.
KSHV infects a wide array of cell lines. (A) HeLa, HFF, CV-1, and 293 cells were infected with concentrated stocks of KSHV and then examined 36 hpi for expression of LANA (green), the KSHV latent antigen, and ORF59 protein (red), an early lytic protein, by IFA. The nuclei are stained with DAPI (blue). (B) TIME, CV-1, SLK, HFF, 3T3, and CHO cells were infected with threefold serial dilutions of a KSHV stock for 2 h. At 48 hpi, the percentage of LANA-positive nuclei was determined by IFA and plotted for each dilution and cell type.
To more quantitatively assess the susceptibility of cells to KSHV infection, we selected six representative cell lines for infection with serial dilutions of the same viral stock. Undiluted KSHV stocks infected between 60 and 100% of cells for all six cell lines tested, with TIME and CV1 cells being the most strongly positive with undiluted virus (Fig. 1B). (Similar findings were consistently observed with several independent stocks of virus; data not shown.) As shown in Fig. 1B, serial threefold dilutions of the stock resulted in diminished infection of all cell lines, with the falloff being the most rapid for HFFs and CHO and 3T3 cells. Our data suggest that TIME and CV-1 cells are almost equally susceptible to KSHV infection and that these two lines are modestly (5- to 10-fold) more susceptible than the remainder of the tested lines.
Lymphoid cell lines proved dramatically less susceptible to KSHV infection in vitro. Table 1 lists the B- and T-cell lines examined in this study and summarizes the results. We were unable to detect LANA staining in any of the four lymphoid cell lines tested after exposure to concentrated stocks of KSHV, under conditions in which 80 to 100% of TIME cells became LANA positive (Table 1). Several attempts were made to infect these cells under conditions other than those of our standard protocol of using cell-free virus derived from BCBL-1 cell supernatants. For example, lymphoid cells were cocultivated with either induced BCBL-1 cells (26) or lytically infected TIME cells, yet none of these conditions yielded LANA-positive nuclei in the recipient lymphoid cell lines.
To further characterize the latent infection of adherent cell lines, we examined the state of the viral genomes by Gardella gel analysis. KSHV viral DNA in latently infected cells is circular, while lytic replication is associated with the accumulation of linear viral DNA (24). These two forms can be clearly resolved by electrophoresis through Gardella gels (13). As shown in Fig. 2, the viral DNA in KSHV-infected, uninduced TIME, SLK, HFF, 3T3, CV-1, 293, and HeLa cells showed clear evidence of circular episomal forms; these comigrated with the authentic circular episomes of uninduced BCBL-1 cells. All these lines also displayed small amounts of linear DNA prior to induction. While in some cases (e.g., BCBL-1 and TIME cells) this is reflective of spontaneous lytic induction, in others it is likely the result of fragmentation of the circular forms during lysis and electrophoresis (see below) or the presence of residual linear virion DNA adherent to the cell surface.
FIG. 2.
KSHV DNA in latent and induced cell lines. Cells were latently infected with KSHV for 2 h and then either washed and maintained in complete media or infected with AdGFP or AdRTA. Cells were harvested 36 hpi and lysed, and the DNA was separated on a Gardella gel. Shown are the Southern blots of the Gardella gels probed for the viral K8.1 gene and ORF73. Linear, linear KSHV DNA; circular, circular episomal KSHV DNA.
Finally, we also examined these infected cells for the presence of ORF59 protein, a delayed-early gene product expressed only during the lytic cycle. We found that only a very small percentage of the infected cells expressed ORF59 protein, as determined by IFA, indicating that very few of the infected cells were undergoing spontaneous lytic replication (Fig. 1A). In general, for all the cell lines tested, less than 1% of infected cells were spontaneously entering the lytic cycle. Thus, by all three criteria—expression of latency-specific markers, establishment of circular viral DNA, and extinction of lytic markers—KSHV infection resulted in bona fide latent infection, indicating that, as previously reported for selected cell lines (18, 22, 33), latency is the default pathway following de novo infection in culture. Our findings with 293 cells agree with those of several other studies (32, 33), indicating that 293 cells are no exception to this rule.
TPA induction of KSHV-infected cells.
The ability of KSHV to establish a latent infection in different cell types led us to examine the ability of the virus to be lytically induced from latency in these cells. Since most previous studies have used phorbol esters for this purpose, we examined the effects of TPA treatment on the ability of the cells to support lytic replication. Latently infected cells were treated with TPA either immediately after infection or after 48 h and then examined by IFA for the delayed-early lytic ORF59 protein, a polymerase accessory factor, and the late envelope glycoprotein gpK8.1. As previously reported, treatment of latently infected BCBL-1 (25), TIME (18), and 293 (33) cells with TPA caused ∼20% of the infected cells to enter the lytic cycle and express ORF59 protein (data not shown). However, we found that TPA was unable to induce KSHV lytic replication in any other latently infected cell line (Table 2).
TABLE 2.
Responsiveness of latently infected cells to induction with TPA or overexpression of RTA
| Cell type | Induction of lytic replicationa by:
|
|
|---|---|---|
| TPA | RTA | |
| TIME | + | + |
| 293 | + | + |
| HFF | − | + |
| SLK | − | + |
| HeLa | − | ND |
| CV-1 | − | + |
ND, not determined; +, induced; −, not induced.
To see if this block to induction was specific to TPA, we selected one cell line (the endothelial line SLK) for more extensive analysis of other inducing stimuli. SLK cells were infected with KSHV and 48 h later were treated with various cytokines, DNA-damaging agents, and demethylating and hypoxia-inducing reagents and then examined by IFA for expression of lytic proteins. None of the stimuli used, alone or in combination, induced lytic replication in latently infected SLK cells (Table 3).
TABLE 3.
Various inducing agents used with latently infected SLK cellsa
| Inducing agentb | Description | Lytic replication |
|---|---|---|
| TPA | PKCc activator | − |
| Sodium butyrate | Histone deacetylase inhibitor | − |
| Ionomycin | Ionophore | − |
| 5-Azacytidine | DNA-damaging agent, demethylating agent | − |
| CoCl2 | Hypoxia-inducing agent | − |
| IL-1β | Cytokine | − |
| TNF-α | Cytokine | − |
All agents failed to induce lytic replication in SLK cells.
IL-1β, interleukin-1 beta; TNF-α, tumor necrosis factor alpha.
PKC, protein kinase C.
Induction of lytic replication by ectopic RTA expression.
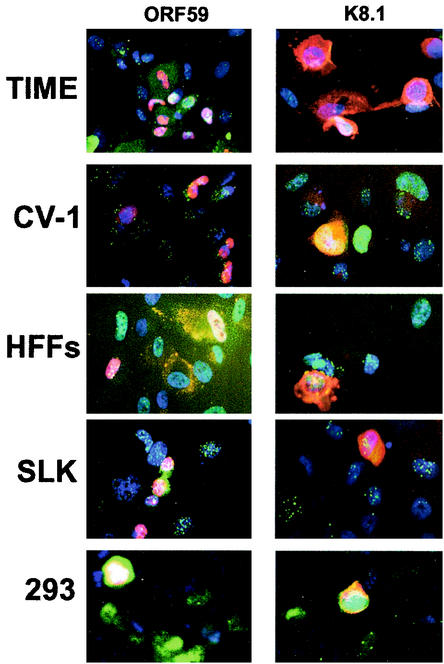
To better understand the nature of the block to lytic replication, we next asked if this block could be overcome by ectopic expression of the KSHV lytic switch protein RTA (14, 19, 30). For efficient delivery of RTA to latently infected cells, we employed a defective adenovirus (AdRTA) bearing the RTA coding region expressed from a CMV immediate-early promoter. Latently infected cells were superinfected with AdRTA and then analyzed by IFA for expression of ORF59 protein and gpK8.1. We detected expression of ORF59 protein and gpK8.1 in all KSHV-positive cell lines permissive of the adenovirus infection (Table 2). Figure 3 shows representative results for several cell lines bearing latent KSHV genomes. The cells in this experiment were stained for LANA (green) and ORF59 protein or gpK8.1 (red), and the nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole; blue). ORF59 protein is a nuclear protein and was detected in a significant number of TIME, CV-1, HFF, and SLK cells (Fig. 3, left panels). The percentage of cells expressing ORF59 protein or gpK8.1 varied from experiment to experiment depending upon the level of KSHV and AdRTA infections. Normally, 80 to 100% of TIME cells, 50 to 80% of HFFs, 40 to 100% of CV-1 cells, and 25 to 50% of SLK cells expressed ORF59 (data not shown). K8.1, a glycoprotein, was also detected in induced cells; typically, at any given time fewer cells stain for this late marker than for ORF59 protein (Fig. 3, right panels). Colocalization of the LANA stained with fluorescein isothiocyanate (green) and ORF59 protein or K8.1 stained with rhodamine (red) sometimes led to yellow staining of the nuclei. Plaques did not form as a result of AdRTA infection of cells latently infected with KSHV, but the cells were clearly dying and showed significant CPE by 36 h after infection with AdRTA.
FIG. 3.
Lytic markers of KSHV in infected cells. Cells latently infected with KSHV were induced by RTA overexpression. At 36 h postinduction, cells were stained for LANA (green) and the nuclei were stained with DAPI (blue). Cells were also stained for ORF59 protein (left panels) and K8.1 (right panels), shown in red.
It was not possible to use AdRTA for induction of several cell lines. For example, AdRTA infected CHO and 3T3 cells too inefficiently to allow for conclusions about the cell lines' inducibility. 293 cells could not be studied with this vector for a different reason—they express E1A and E1B, the two adenovirus proteins that would complement the defect of the vector and allow it to undergo lytic replication. Since HeLa cells are also reported to complement E1A-deficient adenovirus vectors (29), they too were not examined with AdRTA. But because of earlier controversies about the properties of 293 cells in supporting KSHV replication, we delivered RTA to them by transfection with an RTA expression vector and then analyzed the cells by IFA. We readily detected ORF59 protein and gpK8.1 in the transfected 293 cells, indicating that ectopic expression of RTA induced lytic replication (Fig. 3). The proportion of ORF59-positive 293 cells varied from 20 to 70% depending upon the percentage of the cells infected with KSHV and transfected with the RTA expression plasmid (data not shown). Although normally 293 cells are highly transfectable, the conditions for the infection with KSHV are detrimental to transfection and we routinely have a low transfection efficiency of infected cells. In general, 60% of those cells that were LANA positive and expressed RTA stained for ORF59 protein, indicating that, on a per cell basis, induction was fairly efficient in 293 cells.
The expression of late markers like gpK8.1 suggested that lytic viral DNA replication was occurring. To validate this finding, we examined infected cells postinduction by Gardella gel analysis. Latently infected TIME, HFF, and SLK cells were induced by superinfection with AdRTA. As a control, another flask of KSHV-infected cells was infected in parallel with a defective adenovirus expressing GFP (AdGFP). Induced cells were harvested at 36 hpi, and the DNA was then fractionated on a Gardella gel and detected by blot hybridization. We detected two bands in the induced lanes—a more slowly migrating form characteristic of circularized latent genomes and a faster-migrating form corresponding to linear monomers. The levels of linear DNA were significantly greater in cells induced with AdRTA than in AdGFP-infected cells, confirming that all the lines tested successfully induced lytic cycle DNA replication by overexpression of RTA.
Production of infectious KSHV from induced cells.
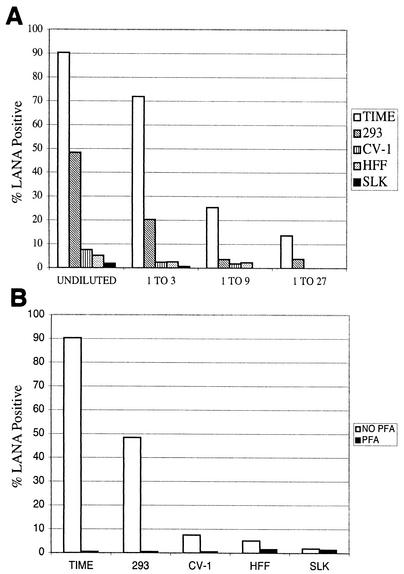
To determine if induced cells produced infectious KSHV virions, we examined the supernatants from induced cells for their ability to infect TIME cells. We infected TIME, CV-1, SLK, HFF, and 293 cells with BCBL-1-derived KSHV for 2 h and then immediately induced the cells by either superinfection of the cells with AdRTA or transfection of an RTA expression plasmid (for 293 cells). A sample of the cells was analyzed by IFA to determine the number of infected (LANA-positive) and lytically replicating (ORF59-positive) cells, while the remaining cells were incubated for 72 h. After 72 h, the supernatants were harvested, concentrated by ultracentrifugation, and used to infect new monolayers of TIME cells (as recipient cells). Infection was assayed by production of LANA in the recipient monolayer as judged by IFA. We detected LANA protein in TIME cells infected with the concentrated supernatants of induced TIME, 293, CV-1, HFF, and SLK cells, indicating that they all released infectious virus. To compare the amounts of virus released from the different induced lines, we determined the titers of the virus in the supernatants by examining the abilities of threefold serial dilutions of each supernatant to transfer LANA expression to recipient TIME cell monolayers. Figure 4A shows that TIME and 293 cell supernatants harbored the largest amount of infectious KSHV—perhaps 10 to 20 times as much as was generated by lines such as SLK, CV-1, and HFF. In all cases, production of infectious virus was blocked by PFA, an inhibitor of lytic KSHV DNA replication (Fig. 4B).
FIG. 4.
Production of infectious virus in induced cell lines. (A) Virus in the supernatants of induced KSHV-infected TIME, 293, CV-1, HFF, and SLK cells was concentrated by ultracentrifugation. Cells were induced by overexpression of RTA. Serial dilutions of the concentrated virus were then used to infect TIME cells. The infected TIME cells were stained for LANA at 48 hpi. The percentage of LANA-positive cells at each dilution for each cell type is shown. (B) Virus was harvested from the supernatants of induced TIME, 293, CV-1, HFF, and SLK cells infected in the presence or absence of 0.5 mM PFA. The percentage of LANA-positive cells for each cell type in the presence or absence of PFA is shown.
In such assays, the amount of virus produced by a given culture is a function of the number of cells initially latently infected by KSHV, the efficiency with which those cells are induced by Ad-RTA, and the burst size per infected cell. With these considerations in mind, the data presented in Fig. 4 allow us to make rough estimates of the relative efficiencies of virus production by the different lines. KSHV-positive TIME cell and HFF cultures reactivated the most efficiently, with 98 and 50% of the cells, respectively, expressing ORF59 protein. However, the virus titer from induced HFFs was about 20-fold less than that from TIME cells, suggesting that HFFs are poor virus producers. The SLK and CV-1 lines also appear to be relatively poor producers of virus; although 25 to 40% of these monolayers stained for lytic markers, the virus yields from the supernatants were again only around 5% of that from the TIME cell supernatant. 293 cells present a more complicated case. Because these cells had to be induced by transfection with RTA plasmids, only about 20% of these cells expressed RTA, of which about 60% (or 10 to 12% of the cells on the plate) stained for lytic markers. However, because 293 cells grow to a much higher saturation density than TIME cells, there were approximately 10 times as many 293 cells as TIME cells in the cultures whose supernatants were examined (data not shown). If one accounts for the 10-fold difference in cell number, there were approximately equal numbers of reactivated TIME and 293 cells in this experiment. If so, production of infectious KSHV is only about twofold less efficient in 293 cells than in TIME cells on a per cell basis.
DISCUSSION
These results firmly establish a number of conclusions about the host range of KSHV in culture. First, it is clear that most adherent cell lines, irrespective of species of origin or tissue lineage, are permissive for viral entry and the establishment of latency. This indicates that receptors and other entry machinery for KSHV must be very broadly distributed. Recently, Akula et al. identified integrin α3β 1 as a receptor for KSHV (1); the wide distribution of integrins on cells (1; reviewed in reference 17) accords well with our findings. Nonetheless, there clearly are cultured cells that lack susceptibility to KSHV infection—most notably (both in our study and those of others [2, 23]) established lines of lymphoid origin. This is paradoxical, since, as a gammaherpesvirus, KSHV's natural reservoir is expected to be in lymphoid cells and experimental studies show that the B cell is the principal infected cell type in the peripheral blood of KSHV-positive patients. In fact, primary B cells can be infected in vitro, although the efficiency of this process has not been well quantified and may be low (16, 20). We do not know the reason for the lack of susceptibility of established B-cell lines, since at least one of the lines tested, BJAB, expresses α3β 1 (J. T. Bechtel, unpublished data). Perhaps these cells have lost essential coreceptors or other postentry factors that are ubiquitously expressed in most other cells; alternatively, they may have acquired dominantly acting inhibitors of entry or infection. Clearly, further studies will be needed to clarify this issue.
The ability to infect primate and rodent cells in vitro, while somewhat counter to prevailing (albeit relatively unexamined) beliefs, was in fact foreshadowed by earlier work (23). In the previous study, cell lines were exposed to KSHV and examined directly for the spliced late mRNA of ORF29 protein by RT-PCR. In that assay, which detects cells that have undergone spontaneous lytic replication, two nonhuman lines scored positive for KSHV infection: BHK (baby hamster kidney) and OMK (owl monkey kidney) cells. Given this evidence of broad host range, why have attempts to infect mice (9) and higher primates (Renne and Ganem, unpublished) generally not succeeded? Several possible explanations can be entertained. First, of course, cultured cells may not accurately reflect the properties of the cells and tissues from which they were derived. More likely, though, nonhuman hosts may indeed allow entry of virus into a small number of host cells in an experimental infection, but absolute or relative blocks to spontaneous lytic reactivation (of the type we characterized most extensively for SLK) may prevent sufficient spread of infection to allow seroconversion.
Our findings help to rationalize many of the seemingly conflicting impressions about KSHV growth derived from earlier studies. A few influential early studies (2, 23) looked for evidence of KSHV propagation by serial passage in the absence of phorbols or other inducing agents, much as one might do for herpes simplex virus type 1 or CMV. An earlier study of this type (23) showed that many cells could support trace levels of lytic replication (as judged by RT-PCR for detection of ORF29 protein) but that this level was insufficient to sustain rounds of serial passage. Our present survey, and the work of others in selected individual lines (7, 18, 22, 32, 33), makes it clear that such a design is fated to show a lack of serial transmission because the default pathway for the virus is latency, from which spontaneous reactivation is very infrequent. If even after infection with a high MOI less than 1% of cells enter the lytic phase, measurable serial infection from supernatants of such monolayers will be expected to rapidly disappear. Similarly, our finding that phorbols do not induce lytic replication in most cells explains many of the earlier failures to detect serial passage of KSHV in the presence of this agent (2, 23).
We are less able to understand the development and passage of CPE in the original studies of Foreman et al (11). In the absence of deliberate lytic induction, we do not typically observe CPE in monolayers of 293 cells exposed to KSHV under conditions sufficient to infect 50 to 80% of the cells in the culture. However, we find it very unlikely that Foreman et al. could have successfully identified 293 cells as a useful line for KSHV propagation had such CPE not been occurring. One observation that might help resolve this paradox is that we have noted an increased frequency of spontaneous lytic reactivation when very high MOIs are used for infection (M. Lagunoff, J. T. Bechtel, and B. Glaunsinger, unpublished data). If the initial inocula used by Foreman et al. happened to have extremely high titers of infectious material, it is conceivable that this might have generated transmissible CPE in the absence of phorbols (11). We also note that in studies by Ciufo et al. with primary endothelial cells, spontaneous lytic induction and latent infection occurred side by side at rather high levels—so, clearly, there are in vitro conditions in which CPE can arise without the deliberate addition of inducers (7). A last possibility is that strains of virus derived from KS may differ from those induced from laboratory-passaged PEL cells.
If our results in culture are representative of events in vivo, then it seems likely that the inefficiency of spontaneous lytic reactivation is the main force behind the narrow host range of KSHV in nature. Our results indicate that, whatever the biochemical basis for this block may be, it can be overcome by ectopic expression of KSHV RTA. That is, there are no absolute blocks to virus production downstream of RTA—though, as noted above, cells do differ in the relative efficiency with which they can support virion production in the presence of RTA (Fig. 4). The more significant barriers to KSHV reactivation appear to be occurring upstream of RTA, suggesting that the control of RTA expression may be the pivotal determinant of KSHV spread in vivo.
Acknowledgments
We thank Craig McCormick for the gift of AdGFP. We thank Britt Glaunsinger for preparation of AdRTA stocks, Adam Grundhoff for technical assistance, and Britt Glaunsinger, Chris Sullivan, and Craig McCormick for critical reading of the manuscript.
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 2.Blackbourn, D. J., E. Lennette, B. Klencke, A. Moses, B. Chandran, M. Weinstein, R. G. Glogau, M. H. Witte, D. L. Way, T. Kutzkey, B. Herndier, and J. A. Levy. 2000. The restricted cellular host range of human herpesvirus 8. AIDS 14:1123-1133. [DOI] [PubMed] [Google Scholar]
- 3.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 4.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delecluse, H. J., M. Kost, R. Feederle, L. Wilson, and W. Hammerschmidt. 2001. Spontaneous activation of the lytic cycle in cells infected with a recombinant Kaposi's sarcoma-associated virus. J. Virol. 75:2921-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dittmer, D., C. Stoddart, R. Renne, V. Linquist-Stepps, M. E. Moreno, C. Bare, J. M. McCune, and D. Ganem. 1999. Experimental transmission of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J. Exp. Med. 190:1857-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foreman, K. E., J. Friborg, Jr., W. P. Kong, C. Woffendin, P. J. Polverini, B. J. Nickoloff, and G. J. Nabel. 1997. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336:163-171. [DOI] [PubMed] [Google Scholar]
- 12.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 13.Gardella, T., P. Medveczky, T. Sairenji, and C. Mulder. 1984. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J. Virol. 50:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 16.Kliche, S., E. Kremmer, W. Hammerschmidt, U. Koszinowski, and J. Haas. 1998. Persistent infection of Epstein-Barr virus-positive B lymphocytes by human herpesvirus 8. J. Virol. 72:8143-8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreidberg, J. A. 2000. Functions of alpha3beta1 integrin. Curr. Opin. Cell Biol. 12:548-553. [DOI] [PubMed] [Google Scholar]
- 18.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 20.Mesri, E. A., E. Cesarman, L. Arvanitakis, S. Rafii, M. A. Moore, D. N. Posnett, D. M. Knowles, and A. S. Asch. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J. Exp. Med. 183:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, G., M. O. Rigsby, L. Heston, E. Grogan, R. Sun, C. Metroka, J. A. Levy, S. J. Gao, Y. Chang, and P. Moore. 1996. Antibodies to butyrate-inducible antigens of Kaposi's sarcoma-associated herpesvirus in patients with HIV-1 infection. N. Engl. J. Med. 334:1292-1297. [DOI] [PubMed] [Google Scholar]
- 22.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renne, R., M. Lagunoff, W. Zhong, and D. Ganem. 1996. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J. Virol. 70:8151-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 26.Sakurada, S., H. Katano, T. Sata, H. Ohkuni, T. Watanabe, and S. Mori. 2001. Effective human herpesvirus 8 infection of human umbilical vein endothelial cells by cell-mediated transmission. J. Virol. 75:7717-7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 28.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinwaerder, D. S., C. A. Carlson, and A. Lieber. 2001. Human papilloma virus E6 and E7 proteins support DNA replication of adenoviruses deleted for the E1A and E1B genes. Mol. Ther. 4:211-216. [DOI] [PubMed] [Google Scholar]
- 30.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venetsanakos, E., A. Mirza, C. Fanton, S. R. Romanov, T. Tlsty, and M. McMahon. 2002. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp. Cell Res. 273:21-33. [DOI] [PubMed] [Google Scholar]
- 32.Vieira, J., M. L. Huang, D. M. Koelle, and L. Corey. 1997. Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J. Virol. 71:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]