Abstract
Odors that distinguish one individual from another member of the species and are determined by polymorphic genes are called odortypes. Odortypes and their considerable societal significance have been studied intimately only in mice and mainly with respect to the genes of the major histocompatibility complex. Further understanding and the matter of human relevance have been hampered by the apparent restriction of odortype expression to urine. The present finding that odorants comprising prerenal odortypes are already present in blood, albeit in masked form, affords the basis of a comprehensive view of odortypes. Accordingly, major histocompatibility complex and other polymorphic genes of antiquity are seen inter alia as agents of normal variation, which entails quantitative variation in output of odorant metabolites. Relatively few such normal variations should suffice for a vast range of compound odors whose specificity is determined by combinative assortment of the same set of individual volatile compounds.
Keywords: major histocompatibility complex, chemosensation, olfaction, behavior
The prediction that major histocompatibility complex (MHC) genes serve to confer personal olfactory identity has been well substantiated by us (1–3) and subsequently by others (4, 5). MHC odortypes have been documented in mice (6–9), rats (4, 10, 11), and humans (12, 13), most potently as constituents of urine, and constitute a salient nonimmunological function of the MHC. That odortype information permeates voided urine has led to the question of whether bacteria might be involved in odorant specification (14), but it has been shown that urine drawn directly from the bladder and urine of germ-free mice are adequate sources of MHC odortype. Clearly, bacteria are not essential (15).
The presence of MHC odortypes in bladder urine suggests that they also might be expressed in prerenal fluids, notably plasma. However, in previous studies (16) such odortypes were not detected in serum. It remained possible, however, that odortypes are represented in serum but either in amounts too minute to be detected in standard behavioral assays or bound to carrier molecules and, thus, undetectable.
Therefore, we sought to determine whether MHC odorants are present cryptically in serum and bound to other circulating molecules, possibly MHC molecules themselves. To this end, mice were trained, using our standard methods (17), to discriminate dilute urine collected from MHC-congenic mice. We then evaluated whether they would recognize MHC-distinctive odors that were produced in serum, either untreated or treated with protease to liberate bound volatile compounds.
The study described here occupied a period of 14 months and involved more than 7,000 training and testing trials with urine, and 214 blind testing trials with serum, in the standard Y maze system.
MATERIALS AND METHODS
Mice.
All mice used in this study were males bred in mouse rooms at the Monell Center. Odor-donor mice were of the inbred strain C57BL/6J (B6, MHC type H-2b) and its congenic partner strain C57BL/6J-H-2k (B6-H-2k). This pair of congenic strains is genetically identical except for the chromosomal segment containing the MHC region. Of the six mice used as odor sensors, three were B6-H-2k and three were B6.
Urine Collection and Preparation.
Voided mouse urine, obtained by gentle abdominal pressure, was collected directly into a sterile tube. A panel of 40–50 male donors provided the urine for the training trials. After each collection, urine samples were frozen at −20°C until needed. For testing, pairs of samples (each 0.3–0.4 ml) were defrosted and placed at room temperature in two 3.5-cm-diameter Petri dishes.
Serum Collection and Preparation.
Serum donors, 25 B6 and 20 B6-H-2k males, were lightly anesthetized with ether and bled from the tail. Blood from each group was collected in a glass test tube and allowed to clot for 2 hr at ambient temperature. The serum then was spun at 4,000 rpm at 5°C to remove any remaining blood cells. The serum was separated into two aliquots. One was treated with Pronase, a proteolytic enzyme, as follows: proteins and peptides were degraded with a high concentration (2 mg/ml) of Pronase (Sigma Pronase type XIV: bacterial from Streptomyces griseus) for 2 hr at ambient temperature. As a control for the possible effects of endogenous degradation, the untreated serum was also held at room temperature for 2 hr. Both the untreated serum and the Pronase-treated serum samples then were stored at –20°C until testing.
Y Maze.
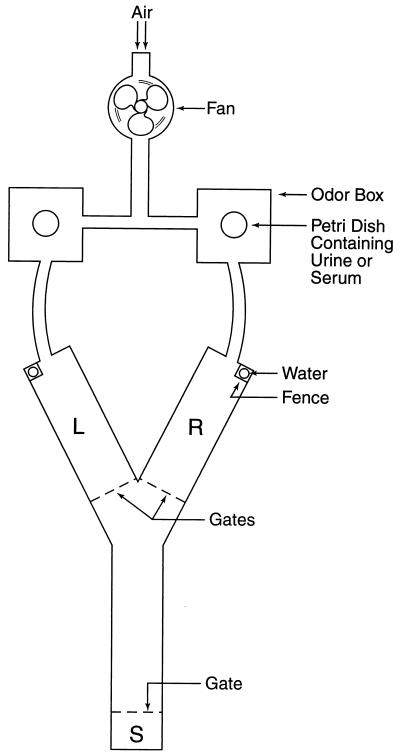
The design and operation of the Y maze used in studying odortypes are detailed elsewhere (18) and outlined in the legend for Fig. 1. In short, the two arms of the maze are scented by air currents conducted through chambers containing odor-source materials (urine or serum) in Petri dishes.
Figure 1.
Y maze. Air drawn by a fan through a tube whose inlet is near the input vent supplying the laboratory is conducted through the left and right odor boxes. Each odor box has a hinged lid to admit a Petri dish containing urine or serum, the odor sources. The air currents then pass to the left (L) and right (R) arms of the maze, which have hinged, transparent lids. Each arm of the maze is fitted with a plastic tube perforated at the bottom to make one drop of water available. Each water tube is guarded by a fence that is raised only if the mouse enters the arm scented by the odor concordant with its training. Each arm of the maze is fitted with a gate that is lowered once the mouse has entered. If the choice is discordant, the fence is not raised, and the mouse is returned to the starting compartment (S). If the choice is concordant, the fence is raised to give access to the drop of water. The time interval in the starting compartment is set at 30 sec to allow for changing the Petri dishes in the odor boxes and for replacing the drop of water (if indicated); after this, on a timed signal, all three gates are raised to commence the next trial. Left–right placing is decided by a series of random numbers. The time taken for the trained mouse to make a choice is 2 or 3 sec; the choice is made without pause, or after sniffing at the entrance to the arms, or sometimes with brief retracing from one arm to the other.
Training and Testing Procedures.
The six trained mice, all males, are identified in Table 1. For training and testing in the Y maze, gates were raised and lowered in timed sequence of up to 48 consecutive trials, the paired urine samples being changed for each trial. Reward for the correct response was a drop of water; the trainee mouse had been deprived of water for 23 hr. Each mouse first was trained to discriminate between urine donors of two unrelated strains B6 (H-2b) and AKR (H-2k), then between the H-2 congenic strains B6 (H-2b) versus congenic B6-H-2k. After successful training (>80% concordance), interspersed unrewarded trials (see below) at an average frequency of one in four were included to accustom the mice to occasional absence of reward after a correct response. The mice performed with comparable accuracy in these trials. The mice then were trained to discriminate dilute urine (urine/water = 1:4) because it was assumed that serum signals would not be as potent as urinary signals. During the long periods between tests, the mice were given routine dilute urine training sessions once or twice every 2 weeks to maintain proficiency.
Table 1.
Percentage concordance (no. of trials) of trained mice to urine in training trials and serum in test trials
| Mouse | Dilute
voided urine
|
Untreated serum, generalization‡ | Protease-treated serum generalization | |
|---|---|---|---|---|
| Rewarded | Unrewarded† | |||
| 1 | 87 (179) | 91 (33) | 44 (18) | 53 (15) |
| 2 | 85 (219) | 81 (42) | 61 (23) | 58 (19) |
| 3 | 91 (208) | 95 (43) | 50 (24) | 83 (18)* |
| 4 | 88 (224) | 86 (42) | 68 (22) | 84 (19)* |
| 5 | 84 (201) | 73 (37) | 45 (22) | 87 (15)* |
| 6 | 86 (109) | 90 (21) | 60 (10) | 67 (9) |
| Means | 87 ± 1 | 86 ± 4 | 55 ± 4† | 73 ± 7*† |
Mouse 1 and 5 were reinforced for B6-H-2k; all others were reinforced for B6 (H-2b). Mouse number 6 died before the last tests could be completed.
P < 0.005 (null hypothesis = 50%), binomial test (note: only significant serum trials are indicated for individual animals; all individual urine trials are also significant at this level).
P < 0.05 (treated > untreated), Wilcoxon test for matched pairs.
For an explanation of the terms “generalization” and “unrewarded,” see Materials and Methods.
Generalization.
This procedure is described fully elsewhere (18). Its purpose is to test serum samples without reward and thus obviate the possibility that incidental or genetically unrelated cues are being learned and responded to. The principle is that if there is no reward there can be no learning. Also, the generalization procedure lends itself to blind testing of coded samples, because the operators of the maze are not required to supply reward for concordant choices. To maintain reinforcement (concordant response to the learned scent), the unrewarded serum samples to be tested were interspersed uniformly with concurrent continued testing of the familiar urine sources accompanied by reward for concordant choice. In short, the trained mice were never rewarded for concordant choices during serum trials that were interspersed within the rewarded urine sample presentations.
Testing Schedule.
Testing of the six animals listed in Table 1 was conducted in three to five sessions, with treated serum alternating with untreated serum and 2 to 5 days separating each session. Serum generalization trials were interspersed among 40–50 training trials with dilute urine (1 part urine, 4 parts water). This procedure was conducted twice with approximately 7 months between the two groups of test sessions. This long period between groups of test sessions was instituted to minimize adventitious learning of non-MHC-relevant signals (i.e., the difference between the odors of urine and serum independent of MHC odortype similarities).
RESULTS
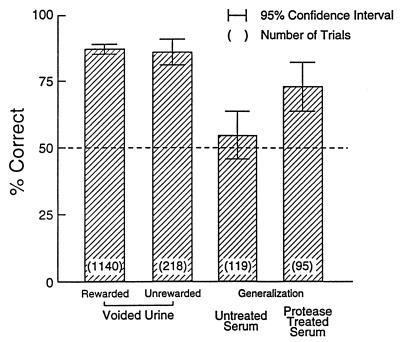
Previous unpublished studies failed to show significant discrimination of untreated serum by mice trained to distinguish urine of H-2 congenic mice (see also ref. 16), and Table 1 shows a similar lack of generalization from dilute urine to untreated serum. Only three of the six mice tested exhibited greater than 50% concordance (not significant), and for no mouse was the generalization score statistically significant. In contrast (see Table 1 and Fig. 2), in generalization trials with Pronase-treated serum, which was intended to liberate bound odorants, all six test mice responded with greater than 50% concordance (P < 0.02), and three individual mice exhibited significant generalization (P < 0.005 for each).
Figure 2.
Percentage of responses concordant with training in response to urine and serum samples. See text for details.
DISCUSSION
To the untrained human nose, the odors of dilute mouse urine and Protease-treated serum were substantially different, the odor of the urine being much stronger and of a “mousy” character, whereas the serum seemed almost odorless. Nevertheless, the trained mice discriminated the treated serum samples according to MHC type, favoring the arm of the maze representing the treated serum concordant with their learned response to urine. Clearly, the Protease-treated serum contains odorants qualitatively and quantitatively representing those in urine that distinguish mice according to their MHC types.
What are these odorants? Until recently, extensive use of conventional physicochemical methods such as gas chromatography and mass spectroscopy have not revealed significant characteristics distinguishing urinary constituents of MHC-congenic mice. Main technical difficulties are the great array and diversity of volatile compounds in urine and the likelihood that the vast range of specific odortypes arises from genetically determined quantitative variations among sets of the same odorants. Under this latter assumption it is the distinctive pattern that is characteristic, and disturbance of this pattern of compound odorants through typical chemical separation procedures likely would destroy odortype information (19).
We recently have reported (20) evidence for distinctive patterns of volatiles according to MHC type. In a behaviorally active dimethyl ether extract of acidified urine a series of carboxylic acids has been found that distinguishes male mice differing only at the MHC. Behavioral tests suggest that most or all of the signal from H-2 resides in this active fraction although this fraction does not, itself, smell “mouse-like” (unpublished observations). Although mass spectrometry indicates the presence of neutral compounds as well as the acids, these have not yet been implicated in the chemical differences between samples of urine from congenic mice. Because these volatile acids are abundant (≈1 mg/ml) and strongly odorous in mouse urine, it seems probable that they play a critical part in the olfactory discrimination of MHC-congenic mice.
Because the pattern of odorants characterizing the MHC-determined odortype is sufficiently similar in serum and urine, it follows that the odorant pattern is established prerenally; one proven source is the hemopoietic system (21). A likely mechanism for odor-type specification may be that soluble MHC gene products themselves bind circulating odorants selectively, presumably after they have lost their bound peptide, and then release them mainly during the course of renal processing and excretion.
Although there is then a need to account for the several independent non-MHC-odortype loci identified throughout the rest of the mouse genome (22), including both sex chromosomes (23), there is no evidence that any of these exhibit the extensive diversity that renders the MHC unique.
Studies with H-2 mutant mice (24, 25) and class 1 knockout mice (26) prove that MHC genes themselves, and not adjacent odorant-coding genes, are responsible, at least in large part, for MHC odortypes.
Odortype specification and communication are by no means alone as nonimmunological functions of the MHC, and, indeed, these may represent primordial functions of far greater antiquity than acquired immunity (27–31). Moreover, there is reason to believe that MHC genes specify odortypes in species other than mice, including humans, as indicated above. Such odortypes often may serve similar purposes in different species. For example, human mating choices can be influenced by MHC genes although there is no direct proof that body odors mediate this effect (ref. 32, but see also ref. 33). We have found that paternal MHC type can be recognized in the scent of pregnant mice (34), and other studies implicate a similar phenomenon in humans (35).
It is not necessary to invoke natural selection to account for the presence of MHC-regulated odorants in body fluids; they may be natural by-products of normal MHC gene variation. Organisms as diverse as marine invertebrates and mice and humans may have seized these serendipitously available volatile signals of individual identity to identify appropriate mates, thereby avoiding inbreeding, or to recognize siblings, parents, or offspring.
Finally, economy of hypothesis requires a comprehensive account of odortypes in the simplest terms, and this is proposed here, as follows.
Odortypes are secondary, not primary, genetic traits, and so are ubiquitous, e.g., among mammals, regardless of particular members’ ability to sense them; it is a question of olfactory ability; thus, rats distinguish the odortypes of mice with the same exquisite precision as mice themselves (36). Visual identification among human individuals is no doubt the polymorphic anatomical parallel, quite likely involving some of the same variable genes such as the MHC.
Lewis Thomas, in 1974 (37), founded the study of odortypes by asking whether dogs might “sniff out our histocompatibility types for us.” Whether humans can sniff out dogs’ histocompatibility types for them is a matter of interest.
Acknowledgments
We thank Maryanne Curran for excellent technical assistance. This research was supported in part by National Science Foundation Grant IBN-9728787.
ABBREVIATION
- MHC
major histocompatibility complex
References
- 1.Beauchamp G K, Yamazaki K, Boyse E A. Sci Am. 1985;253:86–92. doi: 10.1038/scientificamerican0785-86. [DOI] [PubMed] [Google Scholar]
- 2.Boyse E A, Beauchamp G K, Yamazaki K. Trends Genet. 1987;3:97–102. [Google Scholar]
- 3.Yamazaki K, Beauchamp G K, Imai Y, Bard J, Thomas L, Boyse E A. In: Chemical Signals in Vertebrates 6. Doty R L, Müller-Schwarze D, editors. New York: Plenum; 1992. pp. 189–196. [Google Scholar]
- 4.Singh P B, Brown R E, Roser B. Nature (London) 1987;327:161–164. doi: 10.1038/327161a0. [DOI] [PubMed] [Google Scholar]
- 5.Potts W K, Manning C J, Wakeland E K. Nature (London) 1991;352:619–621. doi: 10.1038/352619a0. [DOI] [PubMed] [Google Scholar]
- 6.Beauchamp G K, Gilbert A N, Yamazaki K, Boyse E A. In: Chemical Signals in Vertebrates 4. Duvall D, Müller-Schwarze D, Silverstein R M, editors. New York: Plenum; 1986. pp. 413–422. [Google Scholar]
- 7.Boyse E A, Beauchamp G K, Bard J, Yamazaki K. In: Psychoneuroimmunology II. Ader R, Felter D L, Cohen N, editors. New York: Academic; 1991. pp. 831–846. [Google Scholar]
- 8.Brown J L, Eklund A. Am Nat. 1994;143:435–461. [Google Scholar]
- 9.Yamazaki K, Beauchamp G K, Bard J, Thomas L, Boyse E A. In: Chemical Senses: Genetics of Perception and Communication. Wysocki C J, Kare M R, editors. New York: Dekker; 1991. pp. 211–225. [Google Scholar]
- 10.Brown R E, Singh P B, Roser B. Physiol Behav. 1987;40:65–73. doi: 10.1016/0031-9384(87)90186-7. [DOI] [PubMed] [Google Scholar]
- 11.Singh P B, Brown R E, Roser B. J Exp Med. 1988;168:195–211. doi: 10.1084/jem.168.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferstl R, Eggert F, Westphal E, Zavazava N, Muller-Ruchholtz W. In: Chemical Signals in Vertebrates 6. Doty R L, Müller-Schwarze D, editors. New York: Plenum; 1992. pp. 205–211. [Google Scholar]
- 13.Wedekind C, Seebeck T, Bettens F, Paepke A J. Proc R Soc London Ser B. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- 14.Singh P B, Herbert J, Roser B, Arnott L, Tucker D K, Brown R E. J Chem Ecol. 1990;16:1667–1682. doi: 10.1007/BF01014099. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki K, Beauchamp G K, Imai Y, Bard J, Phelan S P, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1990;87:8413–8416. doi: 10.1073/pnas.87.21.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown R E, Roser P, Singh P B. Behav Genet. 1987;19:659–674. doi: 10.1007/BF01066029. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki K, Beauchamp G K, Bard J, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1982;79:7828–7831. doi: 10.1073/pnas.79.24.7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi M, Yamazaki K, Beauchamp G K, Bard J, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1981;78:5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyse E A, Beauchamp G K, Yamazaki K, Bard J. In: Kin Recognition. Hepper P G, editor. New York: Cambridge Univ. Press; 1991. pp. 148–161. [Google Scholar]
- 20.Singer A G, Beauchamp G K, Yamazaki K. Proc Natl Acad Sci USA. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki K, Beauchamp G K, Thomas L, Boyse E A. J Exp Med. 1985;162:1377–1380. doi: 10.1084/jem.162.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beauchamp G K, Yamazaki K, Duncan H, Bard J, Boyse E A. In: Chemical Signals in Vertebrates 5. MacDonald D W, Müller-Schwarze D, Natynczuk S E, editors. Oxford Univ. Press; 1990. pp. 244–254. [Google Scholar]
- 23.Yamazaki K, Beauchamp G K, Matsuzaki O, Bard J, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1986;83:4438–4440. doi: 10.1073/pnas.83.12.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki K, Beauchamp G K, Egorov I K, Bard J, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1983;80:5685–5688. doi: 10.1073/pnas.80.18.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamazaki K, Beauchamp G K, Shen F W, Bard J, Boyse E A. Immunogenetics. 1991;34:129–131. doi: 10.1007/BF00211425. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki K, Beauchamp G K, Bard J, Boyse E A. Behav Genet. 1998;28:486. [Google Scholar]
- 27.Thomas L. In: Fourth International Congress of Immunology. Neter E, Milgrom F, editors. Basel: Karger; 1975. p. 2. [Google Scholar]
- 28.Grossberg R K, Quinn J F. Nature (London) 1986;322:456–459. [Google Scholar]
- 29.Gill T J. Am J Reprod Immunol. 1996;35:211–215. doi: 10.1111/j.1600-0897.1996.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 30.Apanius V, Penn D, Slev P R, Ruff L R, Potts W K. Crit Rev Immunol. 1997;17:179–224. doi: 10.1615/critrevimmunol.v17.i2.40. [DOI] [PubMed] [Google Scholar]
- 31.Corriveau R A, Huh G S, Shatz C I. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 32.Ober C, Weikamp L R, Cox N, Dytch H, Kostyu D, Elias S. Am J Hum Genet. 1997;61:497–504. doi: 10.1086/515511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedrick P W, Black F L. Am J Hum Genet. 1997;61:505–511. doi: 10.1086/515519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beauchamp G K, Yamazaki K, Curran M, Bard J, Boyse E A. Immunogenetics. 1994;39:109–113. doi: 10.1007/BF00188613. [DOI] [PubMed] [Google Scholar]
- 35.Beauchamp G K, Katahira K, Yamazaki K, Mennella J A, Bard J, Boyse E A. Proc Natl Acad Sci USA. 1995;92:2617–2621. doi: 10.1073/pnas.92.7.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauchamp G K, Yamazaki K, Wysocki C J, Slotnick B M, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1985;82:4186–4188. doi: 10.1073/pnas.82.12.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas L. The Lives of a Cell. New York: Viking; 1974. pp. 16–19. [Google Scholar]