Abstract
We have previously shown that human breast cancer cells that overexpress erbB-2 are growth factor-independent. In order to test the contribution of erbB-2 to this and other transformed phenotypes without the genetic instability of cancer cells, erbB-2 was overexpressed in human mammary epithelial (HME) cells. ErbB-2-overexpressing HME cells exhibit several transformed phenotypes including cell surface α4 integrin downregulation and invasiveness. We formulated a model for invasiveness that depends on a cell's ability to downregulate α4 integrin. As small G-proteins play a role in cytoskeleton remodeling and as this is a likely route for α4 integrin trafficking, we investigated the role of small G-proteins and their downstream signals in mediating α4 integrin downregulation and invasiveness using Rac 1. Dominant-negative Rac 1 blocked erbB-2-mediated invasion and reversed erbB-2-mediated α4 integrin downregulation. In addition, constitutively active Rac 1 induced Ó4 integrin downregulation and invasiveness. In erbB-2-overexpressing and in constitutively active Rac 1-expressing cells, a p38MAP kinase (p38MAPK) inhibitor blocked invasiveness and reversed α4 integrin downregulation. These data suggest a model in which erbB-2 signaling activates Rac 1, which, in turn, activates p38MAPK, leading to the downregulation of α4 integrin. These data strengthen the model where loss of α4 integrin at the cell surface, leading to reduced α4 integrin binding to plasma fibronectin, plays a role in erbB-2-mediated invasiveness.
Keywords: Rac 1, p38MAPK, α4 integrin, invasion, erbB-2
Introduction
With breast cancer, as with other cancers, mortality is more commonly due to metastatic lesions than to primary tumors [1]. Therefore, understanding the molecular changes that generate the metastatic behavior of primary cancer cells is a major area that needs to be addressed in breast cancer. We have chosen erbB-2-overexpressing human mammary epithelial (HME) cells to study the changes associated with invasiveness. ErbB-2 overexpression occurs in about 30% of human breast cancers (HBC) and correlates with a poor prognosis [2,3]. We have shown that HME cells that overexpress erbB-2 exhibit transformed phenotypes similar to HBC cells [4–6]. These phenotypes included invasiveness [5,6].
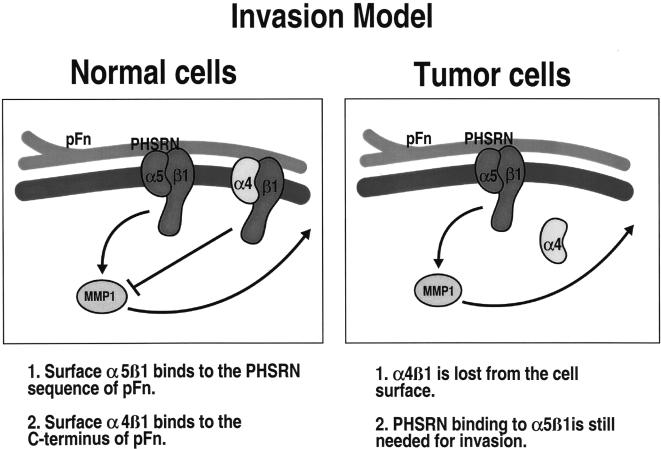
Using a novel assay [7] to study the invasive capacity of human cancer cells, we have described how specific interactions between plasma fibronectin (pFn) and Fn receptors act to influence the invasive capacity of normal and neoplastic cells. We have found that normal HME cells, which express both the α4β1 and α5β1 Fn receptors, are not able to invade naturally occurring basement membranes [sea urchin embryo extracellular matrix (SU-ECM) membranes] [5]. However, normal cells can be stimulated to invade SUECM membranes by ligand binding to α5β1 integrin in the absence of α4β1 binding [8]. This can be accomplished by incubating cells with a peptide fragment of Fn, which contains the α5β1 binding motif, but not the α4β1 binding site, or by using serum or full-length pFn in the presence of α4-blocking antibodies [8]. These findings suggest that signaling from α4β1 integrin acts to block invasion-stimulatory signals that result from α5β1 integrin activation. Consistent with this model, we have found that HBC and HME cells transformed by the overexpression of erbB-2 are also invasive in the SUECM assay, and that these cells express very low levels of α4β1 integrin on the cell surface [5]. Interestingly, these transformed cells express equivalent levels of α4 integrin as normal cells, but α4β1 is not present at the cell surface [5]. These observations suggest that the signaling pathways operative in HBC cells result in the altered trafficking of α4β1 integrins in a manner that directly influences invasive capacity (Figure 1).
Figure 1.
Model for invasion. Normal HME cells express the Fn receptors α4β1 and α5β1 integrin on the cell surface and are not able to invade basement membrane substrates. α4β1 and α5β1 integrin bind plasma Fn. α5 integrin can bind the PHSRN sequence in module 9 of plasma Fn, whereas α4 recognizes plasma Fn C-terminal to the α5 recognition sites. We have shown that HME cells that are binding PHSRN-containing fragments without α4 binding to Fn are able to invade. HBC cells and erbB-2-overexpressing HME cells have lost the expression of α4 integrin on the cell surface and are able to invade basement membranes. Therefore, the interaction of plasma Fn with α5β1 integrin is essential for invasion, and the presence of α4β1 binding to plasma Fn blocks the Fn-induced invasion signals. Data we have published elsewhere indicate that α5β1 signaling goes through MMP-1 to mediate invasion.
We investigated the signaling pathways activated in HBC cells and erbB-2-transformed HME cells that result in the downregulation of α4β1 from the cell surface and in the acquisition of invasive capacity. We found that, while inhibitors of phosphatidylinositol 3′ kinase (PI3K) activity could block invasion, they did not result in the reexpression of cell surface α4β1 integrin. However, we did find that a constitutively active form of Rac 1 could induce both α4β1 cell surface downregulation and invasive capacity in HME cells. Furthermore, a dominant negative form of Rac 1 could block the invasive capacity of transformed HME cells and induce the reappearance of α4β1 on the cell surface. Finally, p38MAP kinase (p38MAPK), which can be activated as a result of Rac 1 signaling [9,10], was found to be the proximate mediator of α4β1 cell surface downregulation. Thus, the results implicate Rac 1 and p38MAPK signaling in the cell surface downregulation of α4β1 integrin, which plays a direct role in the acquisition of the invasive phenotype of HBC cells.
Materials and Methods
Cell Culture
The base medium for H16N2-erbB-2, H16N2-Rac V12, H16N2erbB-2/Rac N17, and H16N2-PTP (vector-only) cells was Ham's F12 medium supplemented with 0.1% bovine serum albumin, 0.5 µg/ml fungizone, 5 µg/ml gentamicin, 5 mM ethanolamine, 10 mM HEPES, 5 µg/ml transferrin, 10 µM T3, 50 µM selenium, and 1 µg/ml hydrocortisone. H16N2-pTP cell medium was further supplemented with 10 ng/ml epidermal growth factor. Cells infected with retroviral expression vectors were selected in 100 µg/ml geneticin (G418) or with 1 µg/ml puromycin for 2 weeks. All cell culture reagents were obtained from Sigma (St. Louis, MO).
Retroviruses and Infection
pTPerbB-2 retrovirus construction was previously described [4]. The dominant negative Rac 1 (Rac N17) and constitutively active Rac 1 (Rac V12) constructs were generous gifts from Dr. Lilli Petruzelli. These Rac cDNA are contained in the pRET60 retroviral vector. The Pak virus constructs were a generous gift from Dr. Surenganie Dharmawadhane [11]. These cDNA are in pREvTRE retroviral vector. The PTEN gene is contained in the pTP2000 bicistronic retroviral vector that we have used previously. Viruses were packaged as previously described [4] and incubated with cells in the presence of polybrene overnight. Antibiotic selection was initiated 24 hours after the end of the infection process, yielding stably expressing HME cells.
Invasion
Cells were suspended in 0.23% trypsin/EDTA (Catalog no. 15050-057; Gibco Life Technologies, Grand Island, NY) and placed on SU-ECM basement membranes with or without fetal calf serum (FCS), according to established methods [7], for 4 hours at 37°C, the time required to observe maximal invasion percentages for normal and metastatic cells (Ref. [7] and data not shown). The percentages of spread and adherent cells were evaluated in each assay to check viability prior to fixation in 2% formaldehyde and scored at x400 magnification using phase contrast optics. Viability ranged from 90% to 98% in all assays. Mean invasion percentages resulted from at least two independent determinations involving the scoring of all cells in contact with the invasion substrates. Each determination was performed in duplicate. LY294002 (Catalog no. L-9908; Sigma) stock solution was 25 mM in 100% ethanol. LY294002 was added to fresh cell media to a final concentration of 25 M for 24 hours prior to the start of the assay. A 10 mM stock solution of PD98059 (Catalog no. 513000; Calbiochem, San Diego, CA) was made in 100% ethanol. PD98059 was added to a final concentration of 10 µM for 24 hours prior to the start of the assay. A 10 mM stock of SRC inhibitor (PD0173952; a gift from Pfizer, Ann Arbor, MI) was made in 100% ethanol. The SRC inhibitor was added to a final concentration of 1 µM for 24 hours prior to the start of the assay. Ten-millimolar stock solutions of SB202190, SB203580, or p38MAPK inhibitor (Catalog nos. 559388, 559389, and 506126; Calbiochem) were made in 100% ethanol. Ten-micromolar SB202190, SB203580, or p38MAPK inhibitor was added for 24 hours prior to the start of the assay. CI-1033 (a gift from Pfizer) in ethanol was added at 1 µM for 24 hours prior to the start of an assay; 10 µl of 100% ethanol was added to an identical plate as a control.
Fluorescence-Activated Cell Sorting (FACS) Analysis
Cells were grown on tissue culture plastic for at least 2 days. Cells were then washed twice in phosphate-buffered saline (PBS), removed from the dish with 10 mM EDTA, pH 8.0, in 1 x Hank's balanced salt solution, then incubated with FITC-conjugated anti-integrin α4 integrin antibody (Catalog no. MAB1382F; Chemicon International, Temecula, CA) for 1 hour at 37°C. After washing twice in PBS, the cells were fixed in 70% EtOH and subjected to FACS. Each cell type was analyzed in at least two other experiments.
Protein Blots
Cells were lysed in a buffer containing 20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 1% NP-40, 10% glycerol, 1 mM Na3VO4, 1 mM PMSF, 1% aprotinin, and 20 µg/ml leupeptin. Protein concentrations were equalized using the Lowry method. For whole cell lysates, Laemmli sample buffer was added and the samples were boiled. Equal amounts of protein were separated by SDS-PAGE. The proteins were blotted to PVDF membranes and probed with α-PTEN antibody (Catalog no. sc-9145; Santa Cruz Biotechnology, Santa Cruz, CA) or α-Rac 1 antibody (Catalog no. R56220; Transduction Laboratories, Lexington, KY). Each protein blot was repeated in two independent experiments.
Results
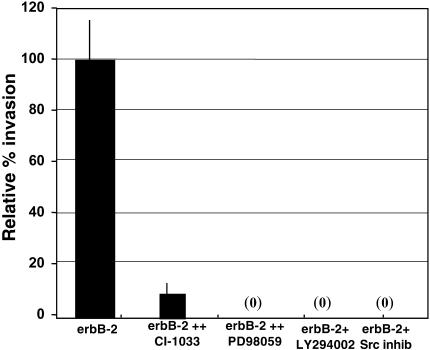
We have demonstrated that the downregulation of cell surface α4 integrin is required for erbB-2-transformed HME cells and HBC cells to invade naturally occurring basement membranes [5,8]; however, we did not determine the erbB-2-triggered signal transduction pathway that mediates this phenomenon. In order to elucidate the signal transduction events that lead to α4 integrin downregulation, cell surface α4 integrin expression was assessed using nonpermeabilized cells that were incubated with a FITClabeled anti-α4 integrin antibody and analyzed by FACS. Using this procedure in combination with a 24-hour incubation with CI-1033, an erbB-2 kinase inhibitor [12,13], we found that the α4 integrin downregulation from the cel surface in erbB-2-overexpressing cells was fully reversible and dependent on erbB-2 kinase activity (Table 1). Using the same incubation times, we also found that erbB-2-mediated invasion was inhibited by CI-1033 by subjecting the cells to Su-ECM invasion assays (Figure 2).
Table 1.
Cell Surface α4 Integrin Expression.*
| Cell Line | α4 Expression† |
| PTP | 22.3 |
| erbB-2 | 9.8 |
| erbB-2+EtOH | 8.7 |
| erbB-2+CI-1033 | 25.5 |
| CI-1033 is an EGFR family kinase inhibitor | |
| erbB-2+LY294002 | 11.0 |
| LY294002 is a PI3K inhibitor | |
| erbB-2/PTEN WT | 12.0 |
| erbB-2+PD98059 | 9.4 |
| PD98059 is a MEK inhibitor | |
| erbB-2+PD173952 | 5.0 |
| PD173952 is a SRC inhibitor |
As determined by FACS analysis.
Percent positive cells.
Figure 2.
Invasion induced by erbB-2 is inhibited by a variety of pathways. ErbB-2-overexpressing cells were incubated for 24 hours with 1 µM of the pan-erbB inhibitor CI-1033, 10 µM of the MEK inhibitor PD98059, 25 µM of the PI3K inhibitor LY294002, or 1 µM of the Src inhibitor PD173952, then subjected to SU-ECM invasion assays. Invasion percentages are relative to the invasion capacity of untreated erbB-2 cells. Each assay was performed in duplicate and repeated at least twice.
As erbB-2-mediated invasion was dependent on PI3K [5], we investigated whether or not PI3K or its downstream effector molecules could reverse α4 integrin downregulation. The data in Figure 2 and Table 1 show that inhibitors of PI3K (LY294002 and in Ref. [5]) blocked invasion but failed to result in the reappearance of α4 integrin on the cell surface. Therefore, we investigated other molecules that have been shown to be involved in erbB-2 signaling for their role in α4 integrin trafficking. Table 1 shows that inhibitors of MEK (PD98059) or Src (PD173952) also did not result in the reappearance of α4 integrin on the cell surface, but were able to block invasion (Figure 2). Based on these observations, we concluded that trafficking of α4 integrin from the cell surface is not mediated by PI3K-, MAPK-, or Src-activated pathways.
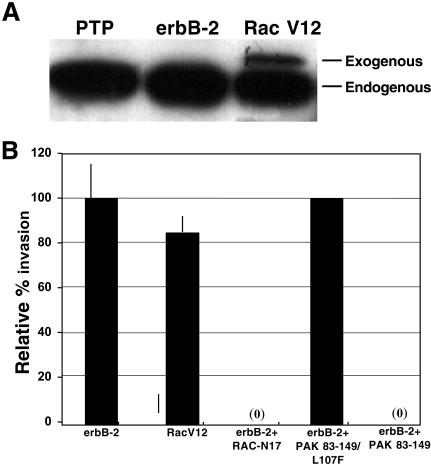
As actin reorganization can be facilitated by small G-proteins [14,15] and actin filaments have been linked to integrins [16,17], we hypothesized that small G-proteins could mediate the trafficking of α4 integrin from the cell surface and affect invasion. As a first step in the analysis of the role of small G-proteins in the invasive phenotype of HBC cells and oncogene-transformed HME cells, we examined the ability of constitutively active Rac 1 to induce an invasive phenotype in immortalized HME cells, and the ability of dominant negative Rac 1 to block invasiveness in erbB-2-overexpressing HME cells. We stably expressed either Rac V12 (constitutively active Rac 1) or Rac N17 (dominant negative Rac 1) in HME cells using retroviral expression vectors. Figure 3A shows the expression of the exogenous Rac V12 in the transduced cells. The data in Figure 3B demonstrate that small G-proteins play a central role in the invasive phenotype of oncogene-transformed HME cells. Not only was Rac V12 able to confer full invasive capacity on the HME cells, but erbB-2-overexpressing cells lost invasive capacity after transduction with Rac N17 (Figure 3B).
Figure 3.
Rac 1 facilitates erbB-2-mediated invasion. H16N2 cells were infected with retroviral expression vectors containing Rac and PAK constructs. Proteins from whole cell lysates were separated on 10% SDS-PAGE and blotted to PVDF membrane. (A) To validate expression of Rac V12, immunoblots were prepared and probed with an anti-Rac 1 antibody. Endogenous and exogenous Rac 1 are indicated. (B) Constitutively active Rac 1 (Rac V12)-expressing cells, dominant negative Rac 1 (Rac N17)-expressing erbB-2 cells, or erbB-2-overexpressing cells containing the dominantly acting PAK construct (PAK 83–149) or a control construct (PAK 83–149/L107F) were subjected to SU-ECM invasion assays. Invasion percentages are relative to the invasion capacity of untreated erbB-2 cells. Each assay was performed in duplicate and repeated at least twice.
Relative levels of cell surface α4 integrin were measured in cells expressing erbB-2 or Rac 1 mutants using FACS. Consistent with the observation that Rac 1 can induce invasion in HME cells, Table 2 shows that expression of Rac V12 induced cell surface α4 integrin downregulation. Furthermore, expression of Rac N17 in erbB-2-overexpressing cells elevated the levels of α4 integrin on the cell surface, reversing erbB-2-mediated α4 downregulation (Table 2). Thus, these results not only implicate activation of small G-proteins in the invasive phenotype exhibited by erbB-2-overexpressing HME cells, they indicate that small G-protein-mediated pathways are involved in the loss of cell surface α4 integrin, which is essential for the invasive phenotype.
Table 2.
Cell Surface α4 Integrin Expression.*
| Cell Line | α4 Expression† |
| PTP | 22.3 |
| erbB-2 | 9.8 |
| erbB-2+EtOH | 8.7 |
| erbB-2+SB202190 | 21.2 |
| SB202190 is a p38 MAPK inhibitor | |
| Rac V12 | 11.4 |
| Rac V12 is a constitutively active form of Rac 1 | |
| Rac V12 +SB202190 | 22.0 |
| SB202190 is a p38MAPK inhibitor | |
| Rac N17 | 21.0 |
| Rac N17 is a dominant negative form of Rac 1 |
As determined by FACS analysis.
Percent positive cells.
To further explore whether Rac 1 signaling plays a role in α4 integrin downregulation and invasion, we utilized two PAK constructs. PAK is a serine /threonine kinase that binds to activated Rac 1 [9]. One PAK construct contained the PAK autoinhibitory domain that interacts in trans with the catalytic domain to specifically block PAK phosphorylation of exogenous substrates (PAK 83–149). The second construct has a mutation in the same domain so that it no longer binds to PAK (PAK 83-149/L107F) [11,18,19]. We expressed these constructs independently in erbB-2-overexpressing cells, and tested them for invasive capacity. Figure 3B shows that only the PAK 83-149 construct could block erbB-2-mediated invasion, whereas the PAK mutant domain was without effect. These results indicate that PAK is involved in erbB-2-mediated invasion.
These results demonstrate that activation of small G-proteins can play an important role in the acquisition of invasive capacity in transformed HME cells, and suggest that Rac 1 is the downstream G-protein responsible for α4 integrin downregulation in erbB-2-overexpressing cells. However, these results do not rule out the involvement of other small G-proteins such as CDC 42 that also bind to and activate PAK.
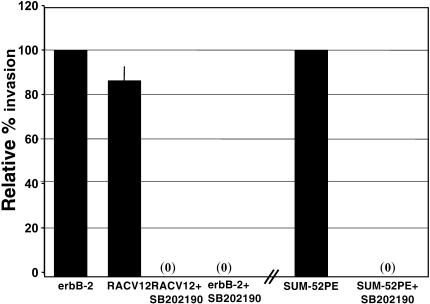
Activation of p38MAPK leads to cytoskeleton reorganization [20,21] and is downstream of Rac 1 [9,10]. p38MAPK has also been shown to play a role in upregulating matrix metalloproteinase (MMP) production, which regulates invasion [22,23]. Therefore, we utilized the p38MAPK inhibitor, SB202190, to determine if p38MAPK played a role in α4 integrin downregulation and invasion. ErbB-2-overexpressing and Rac V12-expressing HME cells were incubated with 10 µM SB202190 for 24 hours, and then assayed for cell surface α4 integrin and for invasiveness. SB202190 exposure induced the reexpression of α4 integrin on the cell surface of erbB-2-overexpressing or Rac V12-expressing cells to levels seen in vector control cells (Table 2) without changing the total amount of α4 in the cells (data not shown). In addition, Figure 4 shows that SB202190 blocked the invasive capacity of erbB-2-overexpressing cells, Rac V12-expressing cells, and the SUM-52 HBC cell line. Similar results were obtained with 10 µM of two other p38MAPK inhibitors, SB203580, and a separate p38MAPK inhibitor available from Calbiochem (data not shown). These results link Rac 1 signaling pathways, involving the activation of p38MAPK, to the trafficking of α4 integrin away from the cell surface and, subsequently, to invasion.
Figure 4.
Invasiveness is dependent on p38MAPK. SUM-52PE human breast cancer cells, ErbB-2-overexpressing HME cells, and Rac V12-expressing cells were exposed for 24 hours to 10 µM SB202190, then were subjected to SU-ECM invasion assays. Invasion percentages are relative to the invasion capacity of untreated erbB-2 cells. Each assay was performed in duplicate and repeated at least twice.
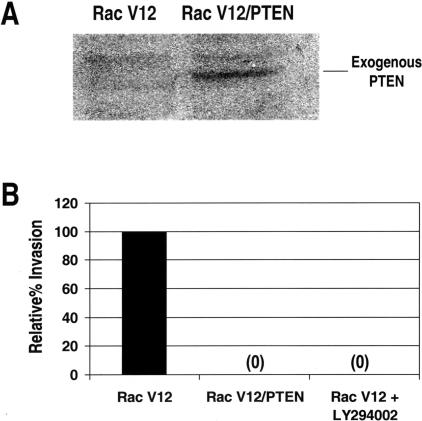
As we have shown that erbB-2-mediated invasion is dependent on PI3K and as there is evidence that PI3K and Rac 1 can activate each other [24–30], we wanted to determine if PI3K signaling was required for invasion in Rac V12-expressing cells. Figure 5 shows that cells expressing constitutively active Rac do not invade SU-ECM substrates in the presence of LY294002. Rac V12-expressing cells also do not invade if they are infected with a retroviral vector containing wild-type PTEN (Figure 5). These findings indicate that Rac 1 is not downstream of PI3K in the signaling required for invasion. It is possible that Rac V12 itself activates PI3K, which, when blocked, also inhibits invasion. It is also likely that Rac 1 and PI3K, once activated, stimulate separate pathways that are both required for invasion. Thus, it is likely that multiple pathways are required for invasion and there is likely to be significant cross-talk between these pathways.
Figure 5.
Rac 1-mediated invasiveness is dependent on PI3K. (A) To confirm expression of exogenous PTEN in transduced cells, immunoblots were prepared and probed with anti-PTEN antibody. (B) SU-ECM invasion assay of Rac V12-expressing cells, Rac V12-expressing cells exposed to 25 µM LY294002, or Rac V12-expressing cells containing exogenous PTEN. Invasion percentages are relative to the invasion capacity of untreated Rac V12 cells. Each assay was performed in duplicate and repeated at least twice.
In summary, the results presented here implicate Rac 1 signalling, through PAK and p38MAPK, in α4 integrin downregulation and suggest that α4 integrin downregulation plays an important role in the invasive phenotype mediated by the overexpression of erbB-2.
Discussion
The correlation between erbB-2 overexpression and a poor outcome in HBC has been known for some time [2,3]. However, the phenotypes mediated by the increase in erbB-2 activity have been difficult to determine due to the inherent genomic instability of cancer cells. To circumvent the problems associated with the genetic changes in HBC cells, we have overexpressed erbB-2 in HME cells to levels seen in breast cancers that demonstrate amplification and overexpression of erbB-2 [4]. Due to the overexpression of erbB-2, these cells are growth factor-independent, anchorage-independent, motile, and invasive [4–6]. To determine how erbB-2 mediates these phenotypes, we investigated erbB-2-induced signaling pathways and their roles in mediating these phenotypes.
We have developed a model for the acquisition of invasive capacity by HBC cells [5,8] (Figure 1). Normal HME cells bind pFn with both α4β1 and α5β1 integrins. Normal cells can be induced to invade using only the α5β1 binding site in pFN, as long as there is no binding to α4β1 by pFn. This indicates that cell surface α4β1 integrin, when it is bound to pFn, blocks the invasion signal initiated by the binding of α5β1 to pFn. We previously found that HBC cells, as well as erbB-2-overexpressing HME cells, had a decrease in cell surface α4 integrin but not a decrease in the overall level of α4 integrin [5]. These data are suggestive of a trafficking phenomenon in tumor and transformed cells that correlate with invasiveness. According to our model, loss of α4 integrin from the cell surface facilitates the invasive phenotype by alleviating the block of invasion induced by α4β1 integrin binding to pFn. This hypothesis was tested in several ways and on different cancer and normal cell types and was found to be a common mechanism by which cells regulate invasiveness [7,8,31]. Supporting this model, Qian et al. [32] found that the overexpression of α4 integrin in melanoma cells inhibited invasion.
We have previously shown that erbB-2-mediated invasiveness is dependent on PI3K [5]. Here we show that PI3K signaling pathways do not play a role in cell surface α4 integrin downregulation (Table 1). These data implicate another erbB-2-induced signaling pathway in α4 integrin downregulation. As small G-proteins are involved in rearrangement of the actin cytoskeleton [14,15] and the cytoskeleton is linked to integrins [16,17], we hypothesized that erbB-2 mediates α4 integrin downregulation by activating small G-proteins and their signaling pathways.
Constitutively active and dominant negative mutants of Rac 1 were used to test this hypothesis. The data obtained with inhibitors of downstream effector targets of Rac 1 and with the constitutively active Rac 1 mutant correlated with the data obtained using the same inhibitors and a dominant negative Rac 1 mutant in erbB-2-overexpressing cells (Figure 3 and Table 2). The findings suggest that Rac 1 is in the erbB-2 pathway and that it mediates α4 integrin downregulation and, subsequently, invasion.
The findings also indicate that erbB-2, signaling through Rac 1 to p38MAPK, causes α4 integrin trafficking away from the cell surface (Figure 4 and Table 2). Interestingly, we did not find that the overall levels of p38MAPK enzymatic activity were significantly elevated in erbB-2- or Rac-expressing cells compared to control cells (not shown). However, whereas control cells did require the presence of exogenous growth factors to maintain p38MAPK activity, this enzyme was active in a growth factor-independent manner in erbB-2- and Rac V12-expressing cells. These findings suggest qualitative, rather than quantitative, alterations in p38MAPK signaling in the downregulation of α4 integrin that takes place in transformed cells and not in normal HME cells.
Given that our model for invasion implicates signals that cause α4 integrin retrafficking in invasion, the finding that a protein that plays a role in cytoskeletal reorganization causes α4 integrin to be trafficked away from the cell surface is not surprising. What is perhaps surprising is that Rac 1-mediated invasion was also dependent on PI3K signaling (Figure 5) because the main mechanism by which Rac 1 mediates invasion in erbB-2-overexpressing cells is by α4 integrin downregulation, and we demonstrated that PI3K does not play a role in this phenotype (Table 1). These observations suggest that Rac 1 is capable of altering integrin trafficking as well as activating PI3K signaling, which results in full invasive capacity. There is evidence to indicate that, under certain conditions, Rac 1 can activate PI3K activity and signaling [26–30]. However, it is also clear that, regardless of their mechanisms of activation, Rac 1 and PI3K activate separate pathways, both of which are required for invasion. Rac 1-stimulated p38MAPK activation is required for α4 integrin downregulation, and this is not modulated by PI3K inhibitors. Similarly, evidence to be published elsewhere demonstrates that PI3K signaling activates specific protein kinase C isoforms that are important in invasive capacity, and this signaling does not directly involve Rac 1.
In summary, our data indicate that erbB-2 overexpression in HME cells results in activation of Rac 1 (or another Rac family member), resulting in the activation of p38MAPK and the subsequent downregulation of α4 integrin. Invasive capacity also requires signaling from PI3K, which does not itself influence integrin trafficking, but activates other pathways required for invasion.
Acknowledgements
We acknowledge Lillie Petruzelli for the gift of the Rac V12 and Rac N17 viruses, Jack Dixon for the gift of the PTEN gene, and Suranganie Dharmawardhane for the gift of the PAK constructs.
Footnotes
This work was supported by NIH grant no. CA 70354-05.
References
- 1.Sporn MB. The war against cancer. Lancet. 1997;347:1377–1381. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]
- 2.Berger MS, Locher GW, Saurer S, Gullick WJ, Waterfield MD, Groner B, Hynes NE. Correlation of c-erbB-2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading #132. Cancer Res. 1988;48:1238–1243. [PubMed] [Google Scholar]
- 3.Guerin M, Gabillot M, Mathieu MC, Travagli JP, Spielmann NA, Riou G. Structure and expression of c-erbB-2 and EGF receptor genes inflammatory and non-inflammatory breast cancer: prognostic significance. Int J Cancer. 1989;43:201–208. doi: 10.1002/ijc.2910430205. [DOI] [PubMed] [Google Scholar]
- 4.Woods Ignatoski KM, LaPointe AJ, Radany EH, Ethier SP. ErbB-2 overexpression in human mammary epithelial cells confers growth factor independence. Endocrinology. 1999;140:3615–3622. doi: 10.1210/endo.140.8.6939. [DOI] [PubMed] [Google Scholar]
- 5.Woods Ignatoski KM, Maehama T, Markwart SM, Dixon JE, Livant DL, Ethier SP. ERBB-2 overexpression confers PI 3′-kinase-dependent invasion capacity on human mammary epithelial cells. Br J Cancer. 2000;82:666–674. doi: 10.1054/bjoc.1999.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods Ignatoski KM, Livant DL, Markwart S, Grewal NK, Ethier SP. The role of PI3′ kinase and its downstream signals in erbB-2-mediated transformation. Cell Growth Differ (submitted for publication) 2002 [PubMed] [Google Scholar]
- 7.Livant DL, Linn S, Markwart S, Shuster J. Invasion of selectively permeable sea urchin embryo basement membranes by metastatic tumor cells, but not by their normal counterparts. Cancer Res. 1995;55:5085–5093. [PubMed] [Google Scholar]
- 8.Jia Y, Markwart SM, Gransden LA, Woods Ignatoski KM, Ethier SP, Livant DL. Integrin receptors regulate MMP1 dependent invasion by breast cancer cells and by mammary epithelial cells. Neoplasia (submitted for publication) 2002 [Google Scholar]
- 9.Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, Bokoch GM. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 10.Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 11.Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000;11:3341–3352. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao GS, Murray S, Ethier SP. Radiosensitization of human breast cancer cells by a novel erbB family receptor tyrosine kinase inhibitor. Int J Radiat Oncol Biol Phys. 2000;48:1519–1528. doi: 10.1016/s0360-3016(00)01358-4. [DOI] [PubMed] [Google Scholar]
- 13.Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, Hook KE, Keller PR, Leopold WR, Loo JA, McNamara DJ, Nelson JM, Sherwood V, Smaill JB, Trumpp-Kallmeyer S, Dobrusin EM. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci USA. 1998;95:12022–12027. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 15.Zigmond SH. Signal transduction and actin filament organization. Curr Opin Cell Biol. 1996;8:66–73. doi: 10.1016/s0955-0674(96)80050-0. [DOI] [PubMed] [Google Scholar]
- 16.Maher PA, Singer SJ. An integral membrane protein antigen associated with the membrane attachment sites of actin microfilaments is identified as an integrin beta-chain. Mol Cell Biol. 1998;8:564–570. doi: 10.1128/mcb.8.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller SC, Hasegawa T, Yamada SS, Yamada KM, Chen WT. Transmembrane orientation of the fibronectin receptor complex (integrin) demonstrated directly by a combination of immunocytochemical approaches. J Histochem Cytochem. 1988;36:297–306. doi: 10.1177/36.3.2449491. [DOI] [PubMed] [Google Scholar]
- 18.Zenke FT, King CC, Bohl BP, Bokoch GM. Identification of a central phosphorylation site in p12-activated kinase regulating autoinhibition and kinase activity. J Biol Chem. 1999;274:32565–32573. doi: 10.1074/jbc.274.46.32565. [DOI] [PubMed] [Google Scholar]
- 19.Zhao ZS, Manser E, Chen ZQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in aPAK inhibition of PAK kinases reveals their morphological roles downstream of Cdc 42 and Rac 1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem Cell Biol. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- 21.Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 22.Reunanen N, Li SP, Ahonen M, Focshi M, Han J, Kahari VM. Activation of p38a mitogen-activated protein kinase enhances collagenase-1 (MMP-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J Biol Chem. 2002;277:32360–32368. doi: 10.1074/jbc.M204296200. [DOI] [PubMed] [Google Scholar]
- 23.Park MJ, Park IC, Hur JH, Kim MS, Lee HC, Woo SH, Lee KH, Rhee CH, Hong SI, Lee SH. Modulation of phorbol ester-induced regulation of matrix metalloproteinases by SB 203580, a specific inhibitor of p38 mitogen-activated protein kinase. J Neurosurg. 2002;97:112–118. doi: 10.3171/jns.2002.97.1.0112. [DOI] [PubMed] [Google Scholar]
- 24.Akasaki T, Koga H, Sumimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J Biol Chem. 1999;274:18055–18059. doi: 10.1074/jbc.274.25.18055. [DOI] [PubMed] [Google Scholar]
- 25.Ma AD, Abrams CS. Pleckstrin homology domains and phospholipid-induced cytoskeletal reorganization. Thromb Haemost. 1999;82:399–406. [PubMed] [Google Scholar]
- 26.Zhang J, King WG, Dillon S, Hall A, Feig L, Rittenhouse SE. Activation of platelet phosphatidylinositide 3-kinase requires the small GTP-binding protein Rho. J Biol Chem. 1993;268:22251–22254. [PubMed] [Google Scholar]
- 27.Stephens L, Hawkins PT, Eguinoa A, Cooke F. A heterotrimeric GTPase-regulated isoform of PI3K and the regulation of its potential effectors. Philos Trans R Soc Lond B Biol Sci. 1996;351:211–215. doi: 10.1098/rstb.1996.0018. [DOI] [PubMed] [Google Scholar]
- 28.Bokoch GM, Vlahos CJ, Wang Y, Knaus UG, Traynor-Kaplan AE. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J. 1996;315:775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter CL, Tolias KF, Couvillon AC, Hartwig JH. Signal transduction pathways involving the small G proteins rac and Cdc42 and phosphoinositide kinases. Adv Enzyme Regul. 1997;37:377–390. doi: 10.1016/s0065-2571(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 30.Ren XD, Schwartz MA. Regulation of inositol lipid kinases by Rho and Rac. Curr Opin Genet Dev. 1998;8:63–67. doi: 10.1016/s0959-437x(98)80063-4. [DOI] [PubMed] [Google Scholar]
- 31.Livant DL, Brabec RK, Pienta KJ, Allen DL, Kurachi K, Markwart S, Upadhyaya A. Anti-invasive, antitumorigenic, and antimetastatic activities of the PHSCN sequence in prostate carcinoma. Cancer Res. 2000;60:309–320. [PubMed] [Google Scholar]
- 32.Qian F, Vaux DL, Weissman IL. Expression of the integrin alpha 4 beta 1 on melanoma cells can inhibit the invasive stage of metastasis formation. Cell. 1994;77:335–347. doi: 10.1016/0092-8674(94)90149-x. [DOI] [PubMed] [Google Scholar]