Abstract
The malfunctioning of the endoplasmic reticulum (ER) of cells in hosts ranging from yeast to mammals can trigger an unfolded protein response (UPR). Such malfunctioning can result from a variety of ER stresses, including the inhibition of protein glycosylation and calcium imbalance. To cope with ER stresses, cells may rely on the UPR to send a signal(s) from the ER to the nucleus to stimulate appropriate cellular responses, including induction of chaperone expression. During Japanese encephalitis virus (JEV) infection, the lumen of the ER rapidly accumulates substantial amounts of viral proteins for virus progeny production. In the present study, we demonstrate that as evidenced by certain chaperone inductions, JEV infection triggers the UPR in fibroblast BHK-21 cells and in neuronal N18 and NT-2 cells, in which JEV results in apoptotic cell death. By contrast, no UPR was observed in apoptosis-resistant K562 cells infected by JEV. JEV infection also activates expression of CHOP/GADD153, a distinctive transcription factor often induced by the UPR, and appears to trigger activation of p38 mitogen-activated protein kinase, a posttranslational activator of CHOP. Ectopic enforcement of CHOP expression enhanced JEV-induced apoptosis, whereas treatment with a p38-specific inhibitor, SB203580, partially blocked JEV-induced apoptosis. Interestingly, bcl-2 overexpression and treatment with a pancaspase inhibitor, z-VAD-fmk, inhibited CHOP induction and diminished JEV-induced apoptosis, suggesting that Bcl-2 and caspases could be the upstream regulators of CHOP. Our results thus suggest that virus-induced ER stress may participate, via p38-dependent and CHOP-mediated pathways, in the apoptotic process triggered by JEV infection.
Infection by Japanese encephalitis virus (JEV), a member of the family Flaviviridae, may cause acute encephalitis with a high mortality rate in humans and induce severe cytopathic effects in various types of cultured cells (56). JEV is thought to replicate primarily in the cytoplasm and to mature on the intracellular membranes of infected cells. Employing the intrinsic secretory pathways in cells, JEV buds from the membranes of the endoplasmic reticulum (ER) and Golgi apparatus to release mature virions (46). One of the major morphological changes in JEV-infected cells, as well as in cells infected by other flaviviruses, is proliferation and hypertrophy of the rough endoplasmic reticular (rER) membranes, where virus particles accumulate (20, 36). Replication of JEV appears to trigger apoptosis in infected culture cells, and an enforced expression of the human proto-oncogene bcl-2 in BHK-21 cells, although it does not influence virus production, appears to delay the process of JEV-induced apoptosis (31). Even though prominent alterations in ER membrane proliferation can be readily observed in flavivirus-infected cells, little is known about the role that the ER plays in virus-induced cytopathicity.
All eukaryotic-cell ER consist of an extensive membranous network that provides a unique compartment for the posttranslational modification, folding, and oligomerization of newly synthesized proteins. The ER is also considered to be the major signal-transducing organelle within the cell, and one of its action modes is to release calcium from the ER reservoir when the cell responds to environmental cues (12, 25, 42). The ER is extremely sensitive to alterations in homeostasis, and in response to a variety of stimuli, certain signals are transduced from the ER to both the cytoplasm and the nucleus, resulting in adaptation for survival or induction of apoptosis (12, 25, 42). Several endogenous imbalances in cells often contribute to malfunction of the ER (also called ER stress), including massive protein production, loss of Ca2+ homeostasis, inhibition of N-linked glycosylation, and accumulation of mutant proteins (25).
Recent studies have shown that the signals originating from ER stress are evolutionarily conserved from yeast to mammalian cells and may be involved in the pathogenesis of several human diseases (12, 25, 42). One such ER response is the unfolded protein response (UPR), which triggers a unique signaling cascade from the ER to the nucleus of a cell. The UPR can be characterized by the enhanced expression of several chaperones, such as glucose-regulated proteins (GRPs), BiP/GRP78, and GRP94 (26), and of other ER-resident proteins, such as calreticulin and protein disulfide isomerase (PDI). In addition, the UPR triggers a cellular response to globally inhibit translation at the initiation step, thereby preventing further accumulation of unfolded protein in the ER. Similar to double-stranded-RNA-activated protein kinase (PKR), pancreatic eIF-2α kinase (50)/PKR-like ER kinase (PERK) (19), when activated by the UPR, phosphorylates eIF-2α, the α subunit of eukaryotic translation initiation factor 2, thus leading to repression of protein synthesis (19, 44). Recently, a new UPR pathway involving another ER transmembrane kinase/RNase, IRE1, which suppresses protein synthesis via cleavage of rRNA, was reported (24). Conceivably, both chaperone induction and translational repression are normal cellular responses that relieve ER stress in cells.
Under attack by certain harsh ER stresses, some cells are unable to adjust themselves in time and may send signals from the ER to induce apoptosis. In fact, in response to ER stress, a death-related transcription factor, CHOP (C/EBP homologous protein), is robustly expressed (6, 27, 59). CHOP is also known as GADD153 (growth arrest- and DNA damage-inducible gene 153), which was originally identified by treatment of cells with a variety of DNA-damaging agents (17). In cultured cells, overexpression of CHOP triggers not only cell cycle arrest but also apoptosis in several types of cells (3, 33, 67). Mouse embryonic fibroblasts derived from chop−/− animals displayed less apoptosis when treated with ER stress inducers; moreover, compared to the wild-type and heterologous animals, chop−/− mice treated with tunicamycin, a glycosylation inhibitor, exhibited a more attenuated apoptotic appearance in the renal epithelium cells of the kidney (68). The role of CHOP in the apoptotic process was further revealed in a recent report, which demonstrated that elevated CHOP expression gave rise to the down-regulation of Bcl-2 and the production of reactive oxygen species (34). These results suggest that death signals stemming from the ER can be sent to the nucleus and that CHOP is likely one of the downstream components in the UPR-mediated apoptotic pathway.
The transcription activity of CHOP can also be enhanced by a stress-inducible p38 mitogen-activated protein kinase (MAPK) (59). The MAPK signaling pathway is one of the major signal transduction systems for eukaryotic cells to manage extracellular stimuli (8). Among the MAPK superfamily, p38 MAPKs play essential roles in inflammatory responses, cell differentiation, cell growth, and cell death (41). The p38 MAPKs activated by proinflammatory cytokines participate in the recruitment of leukocytes to the inflammation sites (21). Treatment by a specific p38 MAPK inhibitor, SB203580 (SB), appears to attenuate both p38 activation and the degree of disease severity in inflammation, arthritis and other joint diseases, septic shock, and myocardial injury (28). In addition, ample evidence suggests a correlation between p38 MAPK activation and the apoptotic pathway (40). Infections by several viruses, including DNA viruses (51, 65), RNA viruses (22, 23, 35, 39), and retroviruses, have been shown to activate p38 MAPKs (15, 43, 49). However, the exact roles of p38 MAPKs in virus replication and virus-induced cytopathic effect (CPE) remain largely unidentified.
The ER is the central factory where JEV performs its protein synthesis, virus assembly, and final maturation (46). JEV infection has been shown to cause marked cellular hypertrophy and extensive proliferation of the secretory apparatus, including the rER and Golgi complexes, in PC12 (rat pheochromocytoma) (20) and BHK-21 (baby hamster kidney) cells (57, 58). Previously, we also showed that the four small hydrophobic JEV nonstructural proteins, which are primarily ER membrane associated, possess the ability to permeabilize the membranes (9). In the present study, we investigated ER stress induced by JEV infection and found that JEV triggers the UPR in infected cells. This ER-mediated signaling cascade appears not only to induce CHOP expression but also to involve p38 MAPK activation. Bcl-2 and caspases appear to function upstream to regulate CHOP activation. Our results suggest that the UPR triggered by JEV infection may play a crucial role in the JEV-induced apoptotic process.
MATERIALS AND METHODS
Viruses and cell lines.
A neurovirulent JEV strain, RP-9, was employed throughout this study (13). The propagation of virus was carried out in BHK-21 cells utilizing RPMI-1640 medium containing 2% fetal calf serum (FCS; GIBCO). Virus titers were determined by a plaque-forming assay on BHK-21 cells as previously described (31). N18, a mouse neuroblastoma cell line (2) (kindly provided by D. E. Griffin, Johns Hopkins University, Baltimore, Md.), and K562 (ATCC CCL-243) were grown in RPMI-1640 medium containing 10% FCS. NT-2 (ATCC CRL-1973), a human neuronal precursor cell line, was cultured in Opti-MEM (GIBCO) supplemented with 10% FCS.
Electron microscopy.
The electron microscopy technique used in this article was previously described (57). Briefly, N18 cells infected with JEV (multiplicity of infection [MOI] = 1) were collected as cell pellets at 36 h postinfection, fixed in 2.5% glutaraldehyde containing 0.1 M cacodylate buffer (pH 7.4) for 1 h, and then washed overnight in PBS. The pellets were dehydrated in a graded series of ethyl alcohols and then embedded in LR White acrylic resin. Thin sections (100 nm thick) were cut by glass knives and collected on 270-mesh nickel or gold grids. Samples were stained by floating them on a fresh 50% aqueous saturated solution of uranyl acetate for 12 min, after which they were rinsed twice with distilled water and then stained immediately with lead citrate. Ultrastructural micrographs of cells were taken under a Zeiss 900 electron microscope.
Drug treatment and antibodies.
Tunicamycin (Sigma) and Ca2+ ionophore A23187 (Sigma) were used as positive controls for the induction of the UPR. Pharmacological blocking of p38 MAPK and inhibition of caspase activation were performed with the inhibitors SB (Calbiochem) and z-VAD-fmk (Bachem), respectively. Mouse monoclonal anti-GRP94, rabbit polyclonal anti-BiP/GRP78, anti-PDI, and anti-calnexin antibodies were from Affinity BioReagents. Rabbit anti-p38 MAPK and CHOP/GADD153 polyclonal antibodies were from Santa Cruz. Specific anti-phosphorylated p38 MAPK antibodies were from New England BioLabs. Internal control of immunoblotting was performed with anti-actin antibody (Upstate Biotechnology).
BiP promoter driving CAT ELISA.
BHK-21 and N18 cells were transfected with 1 μg of a BiP/GRP78 promoter driving a chloramphenicol acetyltransferase (CAT) expression plasmid (10), kindly provided by C. C.-K. Chao (Chang Gung Medical College, Taoyuan, Taiwan), with Lipofectamine (GIBCO-BRL). Twenty-four hours after transfection, the cells were infected with JEV at an MOI of 5 and incubated further for 12, 24, or 36 h. The cell lysates were harvested, and the levels of CAT expression were measured by a CAT enzyme-linked immunosorbent assay (ELISA; Boehringer Mannheim) according to the manufacturer's instructions.
Flow cytometry analysis.
Apoptotic assays were carried out by surface staining with Annexin-V-Alexa 568 (Boehringer Mannheim), a Ca2+-dependent phospholipid-binding protein with high affinity for phosphatidylserine, following the manufacturer's suggestions. The stained cells were analyzed by FACScaliber (Becton Dickinson), and the collected data were analyzed using Modfit 2.0 (Becton Dickinson) and WinMDI version 2.8 (Scripp Institute) software. Samples were gated, according to a two-parameter dot plot showing forward scatter and side scatter, to exclude any cell debris that was not within the normal cell size. In the experiment to assess the effect of CHOP on JEV-induced apoptosis, the cells were transfected with 0.4 μg of pEGFP (Clontech) plus 1.2 (1:3) or 2 (1:5) μg of a plasmid expressing hamster CHOP protein (18), kindly provided by A. J. Fornace, Jr. (National Cancer Institute, National Institutes of Health, Bethesda, Md.). The total amounts of DNA were made equal by adding pcDNA3 to each transfection sample. Twelve hours posttransfection, the cells were mock infected or infected with JEV (MOI = 1) for 32 h before being harvested for staining with Annexin-V and then analyzed by flow cytometry.
Functional assay of p38 MAPK.
Functional assays for JEV-induced p38 MAPK activation were performed with the GAL4-CHOP PathDetect system (Stratagene). N18 and NT-2 cells were cotransfected with 0.1 μg of the reporter pFR-Luc plasmid and 0.4 μg of the transactivator pFA-CHOP plasmid for 16 h. The cells were mock infected or infected with JEV (MOI = 5) for various periods, and the cell lysates were harvested for luciferase assay (Promega) in a 96-well black plate with a Top-Count luminometer (Biocounter). Activated p38 MAPK can enhance the transcriptional activity of CHOP and consequently drive luciferase reporter expression. The data shown are the means of three independent wells with standard errors.
Western immunoblot analysis.
The cells were disrupted with lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1 mM EDTA) containing a cocktail of protease inhibitors (Boehringer Mannheim). For detection of phospho-p38 MAPK, 100 mM NaF and 17.5 mM β-glycerophosphate were also added to the lysis buffer. The cell lysates were mixed with an equal volume of sample buffer (2% sodium dodecyl sulfate, 50 mM Tris-HCl [pH 6.8], 0.1% bromophenol blue, 10% glycerol, 5% β-mercaptoethanol), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane (Hybond-C Super; Amersham). The nonspecific antibody-binding sites were blocked with 5% skim milk in phosphate-buffered saline and then reacted with the primary antibody. The blots were then treated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Cappel) or goat anti-rabbit antibody (Amersham) and finally developed with an ECL system (Amersham).
RESULTS
JEV infection triggers proliferation of ER membranes.
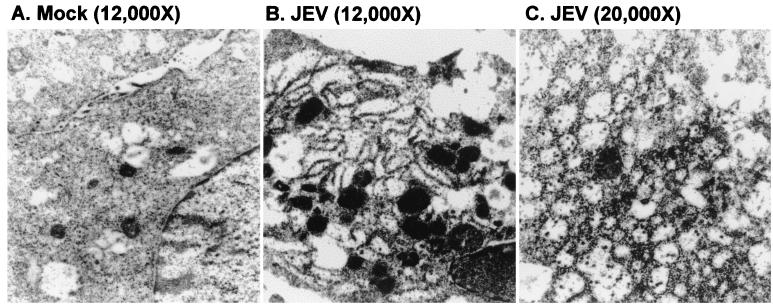
JEV infection often induces severe CPEs in cultured cells, including baby hamster kidney BHK-21 cells, murine neuroblastoma N18 cells, and human neuronal progenitor NT-2 cells (31). Apoptosis, revealed by nuclear condensation and DNA ladder formation, has been identified in these JEV-infected cells (31). JEV infection in PC12 and BHK-21 cells has been shown to cause extensive proliferation of the rER and Golgi complexes (20, 57, 58). In the present study, we first characterized the detailed cytopathology of JEV-infected neuronal N18 cells by electron microscopic examination. The appearance of dilated and hypertrophic membranous lamellae was evident in JEV-infected cells (Fig. 1B) but not in mock-infected cells (Fig. 1A). Numerous scattered virus-like particles were present in the diluted cisternae of the rER (Fig. 1C). This observation confirms that JEV readily stimulates excessive alterations of the membranous structure of the rER in the infected cells. We next investigated the role of this virus-induced ER stress in JEV-mediated CPE.
FIG. 1.
JEV infection induces hypertrophy of ER membranes. N18 cells were mock infected (A) or infected with JEV at an MOI of 1 (B and C) and were examined by electron microscopy 36 h postinfection. Magnifications are in parentheses.
JEV infection activates the expression of chaperone proteins.
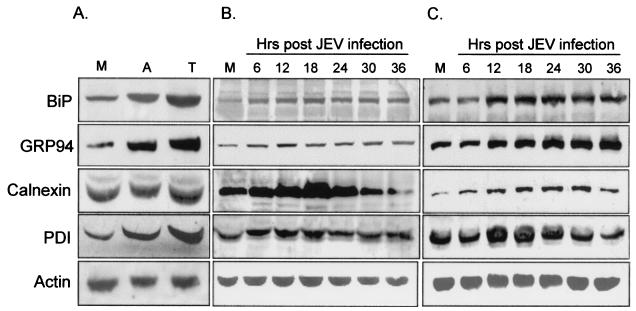
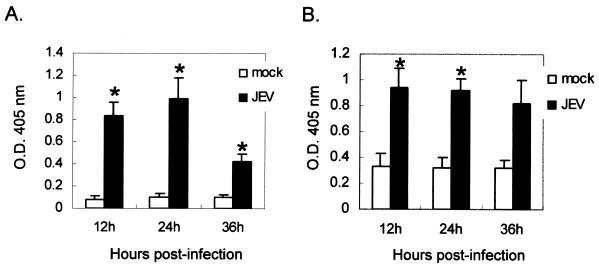
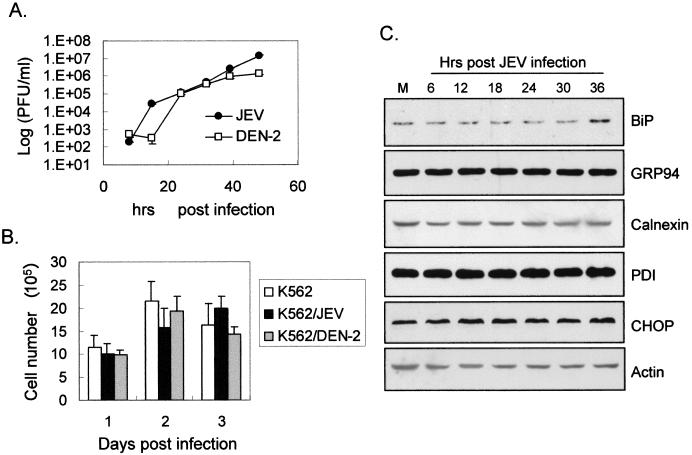
The UPR often leads cells to increase their expression of ER-resident proteins such as chaperones (12, 25). We first investigated whether BHK-21 cells were susceptible to ER stress inducers, such as tunicamycin and A23187, to activate chaperone expression. Compared to an untreated control, treatment of BHK-21 cells with tunicamycin or A23187 resulted in augmented expression of the chaperones BiP/GRP78, GRP94, and PDI (Fig. 2A). Calnexin, another ER membrane-bound chaperone, showed unchanged expression profiles after drug treatment, as was previously demonstrated (25). In the experimental group (Fig. 2B and C), BHK-21 cells infected by JEV indeed produced a higher expression of all the ER-resident proteins tested compared to mock-infected cells (Fig. 2B). Notably, the expression of calnexin increased gradually from 6 h postinfection, peaking at 18 h and waning steadily thereafter (Fig. 2B). Calnexin is not well known to be regulated by ER stress, whereas it is highly induced in JEV-infected cells, probably due to the large amount of viral glycoproteins accumulated. In the neuronal N18 cells infected by JEV (Fig. 2C), we also found substantial induction of BiP/GRP78, GRP94, calnexin, and PDI from 6 to 36 h postinfection. To further clarify whether the mechanism of chaperone induction occurs at the transcriptional level, we transfected BHK-21 (Fig. 3A) and N18 (Fig. 3B) cells with a plasmid carrying a CAT reporter gene driven by the BiP/GRP78 promoter and examined the effect of JEV infection on reporter activity. As shown in Fig. 3, CAT expression was significantly elevated in BHK-21 and N18 cells infected with JEV but not in mock-infected cells. Taken together, these results establish that JEV infection is a potent ER stress inducer in BHK-21 and N18 cells, and they also imply that the virus may trigger the UPR by induction of ER chaperones, probably via up-regulation at the transcriptional level.
FIG. 2.
JEV infection enhances the expression of chaperones. (A) BHK-21 cells treated with 2 μg of A23187/ml (lane A), 2 μg of tunicamycin/ml (lane T), or left untreated (lane M) were harvested 12 h later for Western immunoblotting using antibodies specific for BiP/GRP78, GRP94, calnexin, PDI, and actin as indicated at the left. (B and C) BHK-21 (B) and N18 (C) cells were mock infected (lanes M) or infected with JEV at an MOI of 1, and the cell lysates at the indicated time points (shown above the lanes) were prepared and determined for the expression of chaperone proteins by immunoblotting as described for panel A.
FIG. 3.
Transcriptional activity of BiP/GRP78 promoter is enhanced by JEV infection. The BiP/GRP78/CAT plasmid, which carries the full-length promoter of BiP/GRP78 to drive the CAT reporter gene, was transfected into BHK-21 (A) or N18 (B) cells. Twelve hours posttransfection, the cells were infected with JEV at an MOI of 1, and the cell lysates were collected for CAT assay at the indicated time points postinfection using a CAT-ELISA kit. The results shown are representative data from two independent experiments. The asterisks indicate significant differences between mock- and JEV-infected cells by Student's t test (P < 0.05).
JEV-induced UPR provokes CHOP expression.
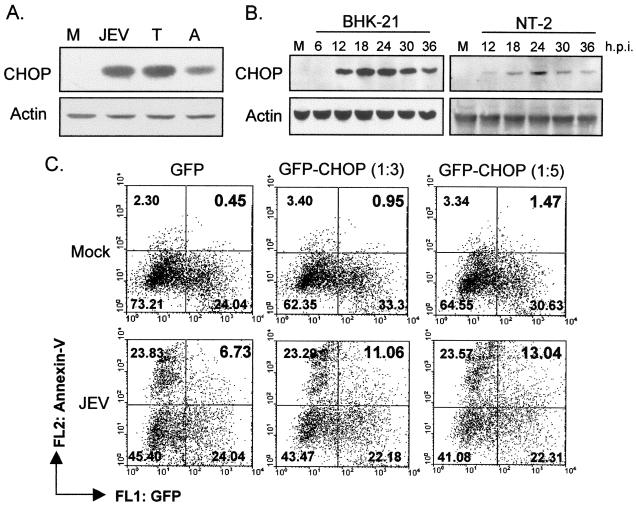
Transcription factor CHOP/GADD153, a member of the C/EBP family, can be activated in cells suffering from ER stress (62), and CHOP activation appears to contribute to subsequent cell growth arrest and apoptosis (68). We found that JEV infection activated CHOP expression in BHK-21 cells to a level comparable to that activated by the ER stress inducers tunicamycin and A23187 (Fig. 4A). In addition, upon JEV infection, CHOP began to be expressed at approximately 12 h and peaked at around 18 to 24 h postinfection in BHK-21 and in human neuronal NT-2 cells (Fig. 4B); the times and the levels of CHOP induction seemed to match well with the time course and severity of apoptosis induced by JEV infection (data not shown). To further investigate the relationship of CHOP induction and apoptosis in JEV-infected cells, we examined whether overexpression of CHOP could influence the course of JEV-induced apoptotic cell death. We cotransfected BHK-21 cells with either a CHOP-expressing plasmid (18) or a vector control (pcDNA3) plus a green fluorescent protein-expressing plasmid in different ratios. These transfected cells were subsequently challenged with JEV infection, and the level of apoptotic response was analyzed by Alexa 568-conjugated Annexin-V staining as revealed by flow cytometry analysis. As shown in Fig. 4C, the population of double-positive cells, representing the transfected and apoptotic cells, was increased in a CHOP dose-dependent manner as the cells were infected by JEV. These results imply that CHOP may be involved in JEV-induced UPR signaling and the subsequent apoptosis pathway.
FIG. 4.
JEV infection triggers CHOP induction. (A) BHK-21 cells were treated with 2 μg of A23187/ml (lane A) or 2 μg of tunicamycin/ml (lane T) or were infected with JEV (lane JEV) or left untreated as a control (lane M). After a 12-h incubation, CHOP expression in the cell lysates was analyzed by Western immunoblotting, and the amounts of actin were used as internal controls for sample loading. (B) Kinetics of CHOP expression in JEV-infected BHK-21 and NT-2 cells. The cells were either mock infected (lanes M) or infected with JEV for the indicated periods (as shown above the lanes), and the cell lysates were harvested for Western immunoblotting using anti-CHOP or anti-actin antibodies. p.i., postinfection. (C) Overexpression of CHOP enhanced JEV-induced apoptosis. The hamster CHOP gene was transfected into BHK-21 cells in a 3-to-1 or 5-to-1 ratio with a green fluorescent protein (GFP) plasmid (Clontech). After 12 h of transfection, the cells were infected with JEV at an MOI of 1 for 32 h. The cells were then stained with Alexa 568-conjugated Annexin-V (Boehringer Mannheim) to determine the apoptotic ratio. The numbers shown in the right upper corner in each plot are the percentages of double-positive cells, representing the ratio of transfected apoptotic cells in each cell population.
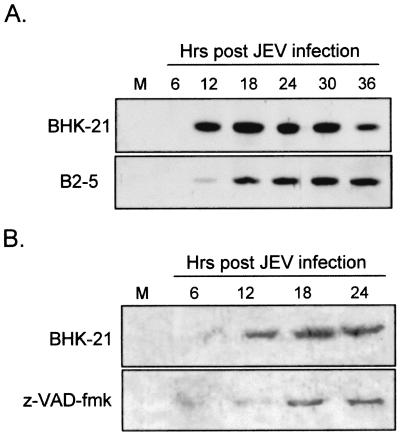
CHOP induction by JEV can be down-regulated by Bcl-2 overexpression and treatment with caspase inhibitor.
Overexpression of antiapoptotic Bcl-2 delays JEV-induced apoptosis in BHK-21 cells (30, 31). The protective effects of Bcl-2 primarily involve stabilization of mitochondrial membrane potential and prevention of the release of cytochrome c and other apoptosis-inducing factors from mitochondria to the cytosol (1, 11). Bcl-2, which is also located on the ER membrane and the outer nuclear envelope, appears to slow down CHOP-mediated apoptosis (33, 34). To investigate whether Bcl-2 could influence the JEV-induced UPR, the levels of CHOP induction in BHK-21 cells were compared to those in a bcl-2-overexpressing BHK-21 cell line, B2-5 (31). As the results in Fig. 5A show, compared to the parental cells, not only was the kinetics of CHOP induction delayed, but CHOP levels were also reduced in JEV-infected B2-5 cells. To explore whether CHOP induction by JEV involves caspase activities, 50 μM z-VAD-fmk, a cell-permeable pancaspase inhibitor, was used to treat infected cells. We found that after JEV infection, z-VAD-fmk treatment diminished CHOP induction compared to that in untreated cells (Fig. 5B). This observation suggests that the CHOP-mediated apoptotic pathway triggered by JEV is a Bcl-2-inhibitable and caspase-dependent process. Since in the experiments described above we employed cells that were susceptible to JEV-induced apoptosis, we next examined whether UPR and CHOP activation could also be seen in cells resistant to apoptosis triggered by JEV infection. Similar to our previous dengue virus study (32), we found that human erythroleukemia K562 cells, even though they support productive JEV replication (Fig. 6A) to a level similar to that in BHK-21 cells (13), were completely resistant to JEV-induced CPE as measured by trypan blue exclusion (Fig. 6B). Notably, as shown in Fig. 6C, following JEV infection, K562 cells showed unchanged patterns of both chaperone induction and CHOP activation over time, indicating that JEV failed to elicit the UPR in these cells. These results suggest a correlation between the ability of JEV infection to induce the UPR and CPE in target cells.
FIG. 5.
Bcl-2 overexpression and pancaspase inhibitor attenuate CHOP expression induced by JEV infection. (A) Kinetics of CHOP induction followed by JEV infection in parental BHK-21 or in B2-5, a bcl-2-overexpressing BHK-21 cell line, were determined by Western immunoblotting as described in the legend to Fig. 4. (B) JEV-mediated CHOP induction was delayed by treatment of BHK-21 cells with a pancaspase inhibitor, z-VAD-fmk. JEV-infected BHK-21 cells were treated with either dimethyl sulfoxide (upper panel) or 50 μM z-VAD-fmk (lower panel) for the indicated periods postinfection. M, mock- infected cells.
FIG. 6.
Productive infection with JEV did not cause cytotoxicity and UPR in K562 cells. (A) One-step growth curve of JEV and dengue virus serotype 2 (DEN-2) on K562 cells. K562 cells with JEV or DEN-2 (MOI = 5) adsorbed were incubated for various times before their culture supernatants were harvested for plaque-forming assays on BHK-21 cells to determine the virus titers (PFU per milliliter). (B) K562 cell viability after JEV or DEN-2 infection. The numbers of viable mock-, JEV-, or DEN-2-infected K562 cells were determined by trypan blue exclusion 1, 2, or 3 days postinfection. The error bars indicate standard errors. (C) Expression profiles of chaperones and CHOP in JEV-infected K562 cells. K562 cells were mock infected (lane M) or infected with JEV for the indicated periods, and the lysates were harvested for Western immunoblotting assays. The antibodies used in each assay are shown at the right.
p38 MAPK is involved in JEV-induced apoptosis.
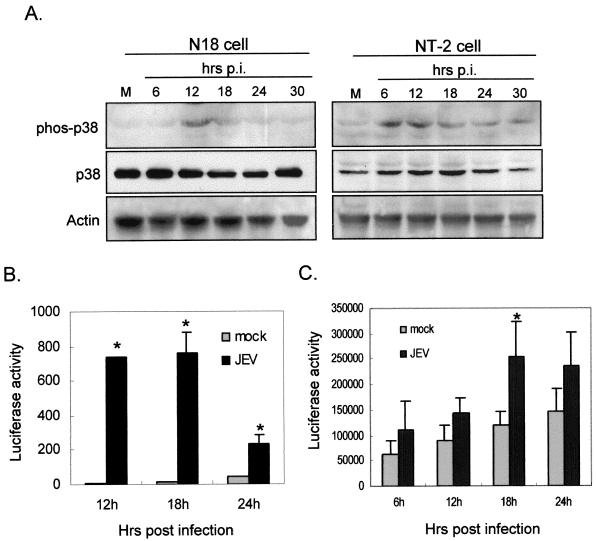
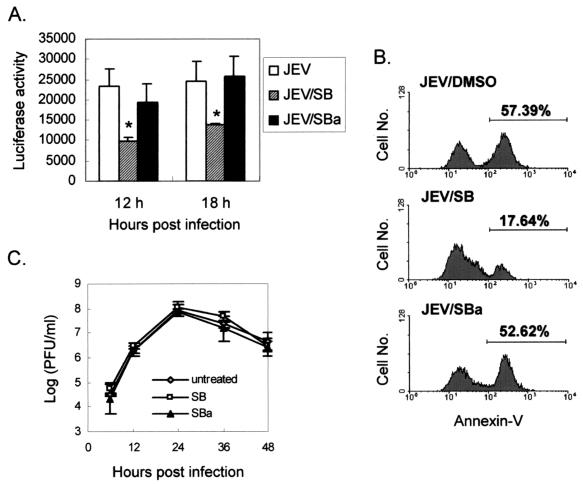
Infection by viruses from several families activates p38 MAPK, which could stimulate CHOP induction at the posttranslational level (59). In Fig. 7A, we show that the phosphorylated form of p38 MAPK was inducibly elevated in JEV-infected N18 and NT-2 cells but not in mock-infected cells; the total amounts of p38 protein remained steady during these periods (Fig. 7A). To determine whether activated p38 MAPK in JEV-infected cells stimulates downstream CHOP activation, we employed a GAL4-CHOP reporter system for the p38 functional assay. In this assay, only if the cytoplasmic GAL4-CHOP fusion protein is phosphorylated by the upstream p38 MAPK can it translocate into the nucleus and trigger the gal4 promoter-driven reporter system. The data in Fig. 7B show that, compared to that in mock-infected cells, luciferase activity was dramatically increased in JEV-infected N18 cells; similarly, JEV infection induced a significant elevation of luciferase activity in NT-2 cells at 18 h postinfection (Fig. 7C). This activation was suppressed by treatment of the cells with a specific p38 inhibitor, SB, but not by its ineffective analog, SB202474 (SBa) (Fig. 8A), indicating the specific role of p38 MAPK in this assay system.
FIG. 7.
JEV infection triggers p38 MAPK activation. (A) The lysates of JEV-infected N18 and NT-2 cells were tested for p38 activation by Western immunoblotting using antibodies specific for the phosphorylated form of p38 (phos-p38) and total p38 proteins (p38). p.i., postinfection. (B and C) Functional assays of JEV-induced p38 activation in N18 (B) and NT-2 (C) cells were performed using the GAL4-CHOP PathDetect system as described in Materials and Methods. The cells were first cotransfected with the reporter pFR-Luc and the transactivator pFA-CHOP, and the resulting cells were mock infected or infected with JEV for the indicated periods. The cell lysates were then prepared for luciferase assay. The data shown here are the means of three independent experiments with standard errors. The asterisks indicate significant differences between mock- and JEV-infected cells by Student's t test (P < 0.05).
FIG. 8.
Inhibition of p38 MAPK activity alleviates JEV-induced apoptosis. (A) SB (5 μM) inhibited JEV-induced p38 activation. The specific p38 inhibitor SB and its ineffective analog, SBa, were added to the medium of JEV-infected N18 cells after adsorption. Activity of p38 MAPK was measured as described in the legend to Fig. 6. The resulting data are the means of three independent experiments with standard errors. The asterisks indicate significant differences between the mock- and the JEV-infected cells by Student's t test (P < 0.05). (B) Flow cytometry analysis of JEV-induced apoptosis by annexin-V staining in infected (MOI = 5) N18 cells treated with dimethyl sulfoxide (DMSO), SB, or SBa 24 h postinfection. (C) Effects of p38 inhibitor on JEV production in N18 cells. The virus titers from the culture supernatants of JEV-infected N18 cells treated with 10 μM SB or SBa or left untreated were determined by plaque-forming assays. The error bars indicate standard errors.
We next examined the role of p38 MAPK in JEV-induced apoptosis. Compared to solvent- or SBa-treated N18 cells, the p38-inhibitor SB significantly diminished the amount of apoptotic population induced by JEV infection at 24 h postinfection (Fig. 8B). SB also appeared to blunt JEV-induced CPE, as measured by lactate dehydrogenase release, in a dose-dependent manner (data not shown). This inhibitory effect of SB on JEV-induced apoptosis did not appear to involve blocking virus yields, since treatment with 10 μM SB did not interfere with JEV production in N18 cells (Fig. 8C). Together, these results suggest that JEV infection activates the p38 MAPK, which in turn phosphorylates and thereby activates its downstream substrate, CHOP, to transmit the death signal to the nuclei of stressed cells.
DISCUSSION
In this study, we demonstrated that JEV infection initiates the UPR by inducing expression of chaperones in cells doomed to die (Fig. 2) but not in cells naturally resistant to JEV-induced cell death (Fig. 6). We also found that the level of CHOP induced seems to correlate with how cells will respond to the viral assault. Obvious CHOP induction in JEV-infected BHK-21 cells appeared to cause apoptosis; delay of CHOP stimulation by either overexpression of Bcl-2 or a broad-spectrum caspase inhibitor, z-VAD-fmk, reduced the apoptotic process, whereas in the absence of CHOP induction, K562 cells appeared to exhibit no apoptosis at all following JEV infection (Fig. 5 and 6). In response to JEV infection, the generation of the UPR, therefore, correlates well with the extent of CHOP induction in the apoptotic process. Although it is not clear exactly how the UPR triggers CHOP induction, our results clearly show that JEV is an ER stress inducer capable of provoking the UPR that either copes with the stress or leads the stressed cells to undergo apoptotic death.
We have also shown that JEV infection activates p38 MAPK, which in turn activates the CHOP pathway by triggering translocation of phosphorylated CHOP into the nucleus to activate gene expression (Fig. 7). In Fig. 8, we show that blockage of p38 MAPK activity by its specific inhibitor, SB (29), attenuated the extent of CHOP induction and subsequent cell death by JEV infection, strongly suggesting a crucial role for CHOP in JEV-induced apoptosis. CHOP is a transcription factor with a leucine zipper motif, whose induction has been closely linked to the perturbation of homeostasis in the ER (62) and the development of apoptosis (68). A set of genes termed DOCs (for downstream of CHOP) has recently been shown to mediate the CHOP-dependent signaling pathway (61). However, the hierarchical relationship between the UPR and p38 MAPK activation by JEV infection remains unclear and needs to be further studied. Conceivably, JEV may first induce the UPR, which then sends a signal to activate p38 MAPK and the downstream CHOP induction. Alternatively, p38 MAPK may be activated first by JEV infection, which subsequently renders the cells more sensitive to ER stresses as the virus replicates so that the UPR results. It is also possible that JEV simultaneously stimulates the UPR and p38 MAPK in a parallel manner and that both of them activate the downstream CHOP pathway. In any case, it is interesting to study further the exact role of p38 MAPK in the JEV-induced UPR.
We also found that the antiapoptotic Bcl-2 protein (1) suppressed CHOP induction upon JEV challenge (Fig. 5). A previous study utilizing intracellular cytostaining revealed that the subcellular distribution of Bcl-2 is on the membrane of not only mitochondria but also the ER, as well as on the outer membrane of the nuclear envelope (14). Bcl-2 is a multifunctional protein which directly or indirectly prevents the release of cytochrome c from mitochondria and subsequent procaspase-9 activation (45). A recent report linked CHOP-mediated apoptosis induced by ER stress to a mechanism that involves down-regulation of Bcl-2 expression, depletion of intracellular glutathione, and a burst of free radicals (34). Conceivably, Bcl-2 overexpressed on the ER membrane might attenuate the UPR induced by JEV infection, thereby lessening the virus-triggered apoptosis (31), which might contribute to the establishment of persistent JEV infection in bcl-2-overexpressing cells (30). Alternatively, since activation of p38 MAPK has been shown to result in Bcl-2 protein degradation (66), the ability of enforced Bcl-2 expression to protect JEV-infected cells from apoptosis (31) might be due to its adequate compensation for Bcl-2 lost through the p38-mediated apoptotic pathway.
The activation of PERK, as revealed by a mobility shift assay for PERK phosphorylation, has been conveniently used as an early marker for ER stress since the discovery of the gene (19, 50). Employing a similar approach, we have tried to determine the activation status of PERK in JEV-infected BHK-21 cells by using an anti-PERK antibody (kindly provided by David Ron, New York University Medical School). Nonetheless, for JEV infection, and even for positive control using the ER stress inducers thapsigargin and A23187, we failed to see any PERK signals in BHK-21 cells. This observation could be because the endogenous level of PERK in BHK-21 cells was too low to be detected. Thus, the potential role of PERK activation in the JEV-induced UPR is unclear from our present study. Furthermore, Ire1p and Hac1p of Saccharomyces cerevisiae are two of the major players found in the yeast UPR (25, 48); after being activated by ER stress, Ire1p, an ER-resident protein kinase as well as an endonuclease, triggers production of Hac1p, a transcription factor that binds to the UPR element present in promoters of certain genes regulated by the UPR. The mammalian Ire1p homologues have been identified as IRE1α (52) and IRE1β (60), which have been proven to play roles in the mammalian UPR, although in a much more complex manner than in yeast (37, 53). Mammalian IRE1s have been shown to activate c-Jun N-terminal kinases (54) and an alternative ER stress-signaling pathway mediated by the transcription factor ATF6 (64). These results illustrate that the UPR is a novel cellular signaling pathway involving transduction of ER-derived signals into the nucleus. The possible roles of IRE1 and PERK in the JEV-induced UPR is of interest and should be further characterized to clarify whether they are the initial kinases responsible for transducing the signal out of the ER.
Dramatic proliferation of intracellular ER membranous structures is one of the hallmarks of flavivirus infection (36, 46). In fact, many positive-strand RNA viruses need to modify intracellular membranes of their host cells in order to create a compartment suitable for virus replication (5, 47, 55, 63). Although this phenomenon has been well documented, little is known about how viruses induce intracellular membrane proliferation. ER proliferation can be achieved when the cells coordinately augment the biosynthesis of ER-resident proteins and lipid components for the membrane. These two pathways are intimately connected (16) and can be regulated at the transcriptional level in the signaling pathway (12). Our data here show that JEV infection, similar to other ER stimuli (25), can trigger not only intracellular-membrane proliferation but also ER chaperone induction (Fig. 2), probably through transcriptional regulation (Fig. 3). These results imply that JEV-induced ER membrane proliferation may be closely associated with the virus-induced UPR. How does JEV infection induce the UPR? In JEV-infected cells, three viral proteins are glycosylated and accumulated in the ER lumen, namely, the precursor of membrane protein (prM), the envelope protein (E), and the nonstructural protein NS1. Accumulation of viral glycoproteins misfolded in the ER may contribute to UPR induction. In addition, several small hydrophobic nonstructural proteins of JEV located on the ER membrane have the ability to modify membrane permeability (9). These viroporins (7) may cause homeostasis imbalance of calcium and other ions in the ER, thereby triggering the UPR. Moreover, during virus maturation, virions budding out from the ER appear to consume the constituents of phospholipid and sterol of the ER membrane, which may not only activate the UPR but also induce ER proliferation.
The pancaspase inhibitor z-VAD-fmk delayed the onset of JEV-triggered CHOP induction (Fig. 5B). Still, it remains unclear which caspases may function upstream and be responsible for regulation of such CHOP induction. Recently, a new apoptotic pathway identified from stressed ER was found to execute its death signal through the activation of ER-resident caspase-12 (38). Caspase-12 activation is a somewhat late event in response to accumulation of misfolded proteins in the ER, which can be inhibited by the inhibitor z-VAD-fmk (38). Infection by respiratory syncytial virus activates caspase-12, and inhibition of caspase-12 by antisense oligonucleotides markedly attenuates respiratory syncytial virus-induced apoptosis (4). These observations raise the possibility that the UPR utilizes caspase-12 and/or other caspases to transduce the apoptotic signal from the ER. Although a direct association between CHOP-induced apoptosis and caspase activation has not yet been established, our data (Fig. 5B) suggest a possible connection between the two. It will be interesting to determine whether Bcl-2 overexpression can also blunt the activation of ER-resident caspase-12 during the occurrence of the UPR.
In conclusion, this study demonstrates that as an ER stress inducer, JEV infection is capable of triggering a characteristic UPR, which is essential for stressed cells to consequently undergo the apoptotic process. This signal pathway appears to involve p38 MAPK activation and CHOP induction. Thus, our results here strongly suggest an important role for the ER in JEV-induced apoptosis, which together with the mitochondrial pathway ensures that infected cells commence their suicidal process if they are not able to calmly coexist with the virus.
Acknowledgments
We thank C. C.-K. Chao for the BiP/CAT plasmid, A. J. Fornace, Jr., for the CHOP/gadd153 cDNA clone, David Ron for anti-PERK antibody, and Douglas Platt for editorial correction of the manuscript.
Y.-L.L. was supported by grants from the National Health Research Institute (NHRI-CN-CL8903P) and the National Science Council (NSC-89-2318-B-001-021-M51), Republic of China. C.-L.L. was supported by grants from NSC (NSC 89-2320-B-016-089) and from the Department of Defense, Republic of China (DOD-90-43).
REFERENCES
- 1.Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322-1326. [DOI] [PubMed] [Google Scholar]
- 2.Amano, T., E. Richelson, and M. Nirenberg. 1972. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc. Natl. Acad. Sci. USA 69:258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barone, M. V., A. Crozat, L. Tabaee, L. Philipson, and D. Ron. 1994. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 8:453-464. [DOI] [PubMed] [Google Scholar]
- 4.Bitko, V., and S. Barik. 2001. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell Biochem. 80:441-454. [DOI] [PubMed] [Google Scholar]
- 5.Carette, J. E., M. Stuiver, J. Van Lent, J. Wellink, and A. Van Kammen. 2000. Cowpea mosaic virus infection induces a massive proliferation of endoplasmic reticulum but not Golgi membranes and is dependent on de novo membrane synthesis. J. Virol. 74:6556-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson, S. G., T. W. Fawcett, J. D. Bartlett, M. Bernier, and N. J. Holbrook. 1993. Regulation of the C/EBP-related gene gadd153 by glucose deprivation. Mol. Cell. Biol. 13:4736-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrasco, L. 1995. Modification of membrane permeability by animal viruses. Adv. Virus Res. 45:61-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y. S., C. L. Liao, C. H. Tsao, M. C. Chen, C. I. Liu, L. K. Chen, and Y. L. Lin. 1999. Membrane permeabilization by small hydrophobic nonstructural proteins of Japanese encephalitis virus. J. Virol. 73:6257-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao, C. C., W. C. Yam, L. K. Chen, and S. Lin-Chao. 1992. Cloning of a functional Burkitt's lymphoma polypeptide-binding protein/78 kDa glucose-regulated protein (BiP/GRP78) gene promoter by the polymerase chain reaction, and its interaction with inducible cellular factors. Biochem. J. 286:555-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao, D. T., and S. J. Korsmeyer. 1998. BCL-2 family: regulators of cell death. Annu. Rev. Immunol. 16:395-419. [DOI] [PubMed] [Google Scholar]
- 12.Chapman, R., C. Sidrauski, and P. Walter. 1998. Intracellular signaling from the endoplasmic reticulum to the nucleus. Annu. Rev. Cell Dev. Biol. 14:459-485. [DOI] [PubMed] [Google Scholar]
- 13.Chen, L. K., Y. L. Lin, C. L. Liao, C. G. Lin, Y. L. Huang, C. T. Yeh, S. C. Lai, J. T. Jan, and C. Chin. 1996. Generation and characterization of organ-tropism mutants of Japanese encephalitis virus in vivo and in vitro. Virology 223:79-88. [DOI] [PubMed] [Google Scholar]
- 14.Chen-Levy, Z., and M. L. Cleary. 1990. Membrane topology of the Bcl-2 proto-oncogene protein demonstrated in vitro. J. Biol. Chem. 265:4929-4933. [PubMed] [Google Scholar]
- 15.Cohen, P. S., H. Schmidtmayerova, J. Dennis, L. Dubrovsky, B. Sherry, H. Wang, M. Bukrinsky, and K. J. Tracey. 1997. The critical role of p38 MAP kinase in T cell HIV-1 replication. Mol. Med. 3:339-346. [PMC free article] [PubMed] [Google Scholar]
- 16.Cox, J. S., R. E. Chapman, and P. Walter. 1997. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol. Biol. Cell 8:1805-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fornace, A. J., Jr., I. Alamo, Jr., and M. C. Hollander. 1988. DNA damage-inducible transcripts in mammalian cells. Proc. Natl. Acad. Sci. USA 85:8800-8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornace, A. J., D. W. Nebert, M. C. Hollander, J. D. Luethy, M. Papathanasiou, J. Fargnoli, and N. J. Holbrook. 1989. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol. Cell. Biol 9:4196-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271-274. [DOI] [PubMed] [Google Scholar]
- 20.Hase, T., P. L. Summers, P. Ray, and E. Asafo-Adjei. 1992. Cytopathology of PC12 cells infected with Japanese encephalitis virus. Virchows Arch. B 63:25-36. [DOI] [PubMed] [Google Scholar]
- 21.Herlaar, E., and Z. Brown. 1999. p38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today 5:439-447. [DOI] [PubMed] [Google Scholar]
- 22.Huttunen, P., T. Hyypia, P. Vihinen, L. Nissinen, and J. Heino. 1998. Echovirus 1 infection induces both stress- and growth-activated mitogen-activated protein kinase pathways and regulates the transcription of cellular immediate-early genes. Virology 250:85-93. [DOI] [PubMed] [Google Scholar]
- 23.Iordanov, M. S., J. M. Paranjape, A. Zhou, J. Wong, B. R. Williams, E. F. Meurs, R. H. Silverman, and B. E. Magun. 2000. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol. Cell. Biol. 20:617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwawaki, T., A. Hosoda, T. Okuda, Y. Kamigori, C. Nomura-Furuwatari, Y. Kimata, A. Tsuru, and K. Kohno. 2001. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat. Cell Biol. 3:158-164. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman, R. J. 1999. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13:1211-1233. [DOI] [PubMed] [Google Scholar]
- 26.Kozutsumi, Y., M. Segal, K. Normington, M. J. Gething, and J. Sambrook. 1988. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332:462-464. [DOI] [PubMed] [Google Scholar]
- 27.Lawson, B., J. W. Brewer, and L. M. Hendershot. 1998. Geldanamycin, an hsp90/GRP94-binding drug, induces increased transcription of endoplasmic reticulum (ER) chaperones via the ER stress pathway. J. Cell Physiol. 174:170-178. [DOI] [PubMed] [Google Scholar]
- 28.Lee, J. C., S. Kumar, D. E. Griswold, D. C. Underwood, B. J. Votta, and J. L. Adams. 2000. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology 47:185-201. [DOI] [PubMed] [Google Scholar]
- 29.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 30.Liao, C. L., Y. L. Lin, S. C. Shen, J. Y. Shen, H. L. Su, Y. L. Huang, S. H. Ma, Y. C. Sun, K. P. Chen, and L. K. Chen. 1998. Antiapoptotic but not antiviral function of human bcl-2 assists establishment of Japanese encephalitis virus persistence in cultured cells. J. Virol. 72:9844-9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao, C. L., Y. L. Lin, J. J. Wang, Y. L. Huang, C. T. Yeh, S. H. Ma, and L. K. Chen. 1997. Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J. Virol. 71:5963-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, Y. L., C. L. Liao, L. K. Chen, C. T. Yeh, C. I. Liu, S. H. Ma, Y. Y. Huang, Y. L. Huang, C. L. Kao, and C. C. King. 1998. Study of Dengue virus infection in SCID mice engrafted with human K562 cells. J. Virol. 72:9729-9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto, M., M. Minami, K. Takeda, Y. Sakao, and S. Akira. 1996. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 395:143-147. [DOI] [PubMed] [Google Scholar]
- 34.McCullough, K. D., J. L. Martindale, L. O. Klotz, T. Y. Aw, and N. J. Holbrook. 2001. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21:1249-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGilvray, I. D., Z. Lu, A. C. Wei, A. P. Dackiw, J. C. Marshall, A. Kapus, G. Levy, and O. D. Rotstein. 1998. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J. Biol. Chem. 273:32222-32229. [DOI] [PubMed] [Google Scholar]
- 36.Monath, T., and F. Heinz. 1996. Flaviviruses: Japanese encephalitis virus, p. 984-989. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 37.Mori, K. 2000. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101:451-454. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa, T., H. Zhu, N. Morishima, E. Li, J. Xu, B. A. Yankner, and J. Yuan. 2000. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98-103. [DOI] [PubMed] [Google Scholar]
- 39.Nakatsue, T., I. Katoh, S. Nakamura, Y. Takahashi, Y. Ikawa, and Y. Yoshinaka. 1998. Acute infection of Sindbis virus induces phosphorylation and intracellular translocation of small heat shock protein HSP27 and activation of p38 MAP kinase signaling pathway. Biochem. Biophys. Res. Commun. 253:59-64. [DOI] [PubMed] [Google Scholar]
- 40.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 41.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell Signal 12:1-13. [DOI] [PubMed] [Google Scholar]
- 42.Pahl, H. L. 1999. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol. Rev. 79:683-701. [DOI] [PubMed] [Google Scholar]
- 43.Popik, W., and P. M. Pitha. 1998. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology 252:210-217. [DOI] [PubMed] [Google Scholar]
- 44.Prostko, C. R., J. N. Dholakia, M. A. Brostrom, and C. O. Brostrom. 1995. Activation of the double-stranded RNA-regulated protein kinase by depletion of endoplasmic reticular calcium stores. J. Biol. Chem. 270:6211-6215. [DOI] [PubMed] [Google Scholar]
- 45.Reed, J. C. 1997. Double identity for proteins of the Bcl-2 family. Nature 387:773-776. [DOI] [PubMed] [Google Scholar]
- 46.Rice, C. M. 1996. Flaviviridae: the viruses and their replication, p. 931-959. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 47.Schlegel, A., T. H. Giddings, M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 70:6576-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shamu, C. E. 1998. Splicing: HACking into the unfolded-protein response. Curr. Biol. 8:R121-R123. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro, L., K. A. Heidenreich, M. K. Meintzer, and C. A. Dinarello. 1998. Role of p38 mitogen-activated protein kinase in HIV type 1 production in vitro. Proc. Natl. Acad. Sci. USA 95:7422-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi, Y., K. M. Vattem, R. Sood, J. An, J. Liang, L. Stramm, and R. C. Wek. 1998. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18:7499-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suomalainen, M., M. Y. Nakano, K. Boucke, S. Keller, and U. F. Greber. 2001. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 20:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tirasophon, W., A. A. Welihinda, and R. J. Kaufman. 1998. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12:1812-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urano, F., A. Bertolotti, and D. Ron. 2000. IRE1 and efferent signaling from the endoplasmic reticulum. J. Cell Sci. 113:3697-3702. [DOI] [PubMed] [Google Scholar]
- 54.Urano, F., X. Wang, A. Bertolotti, Y. Zhang, P. Chung, H. P. Harding, and D. Ron. 2000. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664-666. [DOI] [PubMed] [Google Scholar]
- 55.van der Meer, Y., E. J. Snijder, J. C. Dobbe, S. Schleich, M. R. Denison, W. J. Spaan, and J. K. Locker. 1999. Localization of mouse hepatitis virus nonstructural proteins and RNA synthesis indicates a role for late endosomes in viral replication. J. Virol. 73:7641-7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaughn, D. W., and C. H. Hoke, Jr. 1992. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol. Rev. 14:197-221. [DOI] [PubMed] [Google Scholar]
- 57.Wang, J. J., C. L. Liao, Y. W. Chiou, C. T. Chiou, Y. L. Huang, and L. K. Chen. 1997. Ultrastructure and localization of E proteins in cultured neuron cells infected with Japanese encephalitis virus. Virology 238:30-39. [DOI] [PubMed] [Google Scholar]
- 58.Wang, J. J., C. L. Liao, C. I. Yang, Y. L. Lin, C. T. Chiou, and L. K. Chen. 1998. Localizations of NS3 and E proteins in mouse brain infected with mutant strain of Japanese encephalitis virus. Arch. Virol. 143:2353-2369. [DOI] [PubMed] [Google Scholar]
- 59.Wang, X. Z., and D. Ron. 1996. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272:1347-1349. [DOI] [PubMed] [Google Scholar]
- 60.Wang, X. Z., H. P. Harding, Y. Zhang, E. M. Jolicoeur, M. Kuroda, and D. Ron. 1998. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 17:5708-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, X. Z., M. Kuroda, J. Sok, N. Batchvarova, R. Kimmel, P. Chung, H. Zinszner, and D. Ron. 1998. Identification of novel stress-induced genes downstream of chop. EMBO J. 17:3619-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, X. Z., B. Lawson, J. W. Brewer, H. Zinszner, A. Sanjay, L. J. Mi, R. Boorstein, G. Kreibich, L. M. Hendershot, and D. Ron. 1996. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol. 16:4273-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Westaway, E. G., J. M. Mackenzie, M. T. Kenney, M. K. Jones, and A. A. Khromykh. 1997. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 71:6650-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshida, H., T. Okada, K. Haze, H. Yanagi, T. Yura, M. Negishi, and K. Mori. 2000. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20:6755-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zachos, G., B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097-5103. [DOI] [PubMed] [Google Scholar]
- 66.Zachos, G., M. Koffa, C. M. Preston, J. B. Clements, and J. Conner. 2001. Herpes simplex virus type 1 blocks the apoptotic host cell defense mechanisms that target Bcl-2 and manipulates activation of p38 mitogen-activated protein kinase to improve viral replication. J. Virol. 75:2710-2728. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Zhan, Q., K. A. Lord, I. Alamo, Jr., M. C. Hollander, F. Carrier, D. Ron, K. W. Kohn, B. Hoffman, D. A. Liebermann, and A. J. Fornace, Jr. 1994. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol. 14:2361-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zinszner, H., M. Kuroda, X. Wang, N. Batchvarova, R. T. Lightfoot, H. Remotti, J. L. Stevens, and D. Ron. 1998. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12:982-995. [DOI] [PMC free article] [PubMed] [Google Scholar]