Abstract
The magnitude and breadth of cytotoxic-T-lymphocyte (CTL) responses induced by human immunodeficiency virus type 1 (HIV-1) envelope protein from which the hypervariable V3 loop had been deleted (ΔV3) were evaluated in the HLA-A2/Kb transgenic mice. It was demonstrated that vaccines expressing the ΔV3 mutant of either HIV-1IIIB or HIV-189.6 envelope glycoprotein induced broader CD8+ T-cell activities than those elicited by the wild-type (WT) counterparts. Specifically, the differences were associated with higher responses to conserved HLA-A2-restricted CTL epitopes of the envelope glycoprotein and could be correlated with an increased cell surface occupancy by the epitope-HLA-A2 complexes in target cells expressing the ΔV3 mutant. Using recombinant vaccinia virus expressing heterologous gp160 of primary HIV-1 isolates in a murine challenge system, we observed that the extent of resistance to viral transmission was higher in animals immunized with the ΔV3 than the WT envelope vaccine. The protection was linked to the presence of envelope-specific CD8+ T cells, since depletion of these cells by anti-CD8 antibody treatment at the time of challenge abolished the vaccine-induced protection. The results from our studies provide insights into approaches for boosting the breadth of envelope-specific CTL responses.
The current challenge for the design of an effective human immunodeficiency virus type 1 (HIV-1) vaccine is to develop immunization strategies to elicit both stronger and broader immune responses against diverse viral species. Such vaccines include ones in which cellular immunity is stimulated by live, recombinant viruses or DNA-based vectors (4, 25). The envelope glycoprotein is an important component of an efficacious vaccine for AIDS because it represents a target for cell-mediated immunity and neutralizing antibody responses (7, 32, 39, 42, 45, 47, 57). However, to persist as a chronic infection, HIV-1 has evolved ways to escape from cytotoxic-T-lymphocyte (CTL) recognition and to limit the generation of neutralizing antibodies. For example, the unequivocal demonstration of CTL escape mutations arising specifically within regions of the viral genome encoding immunodominant CTL epitopes has been suggested to contribute to viral spread and the inability of anti-HIV-1 immunity to prevent the onset of AIDS (24, 31). There is unusually extensive shielding of the conserved regions of gp120 by nonimmunogenic carbohydrates (47). The CD4-binding site is recessed (32, 57) and the escape mutants can be generated in a relatively facile way, even to antibodies against the CD4-binding site (32, 57). Additionally, the variable loops of gp120 hide the coreceptor-binding site until the interaction with CD4 occurs, thereby minimizing the time and space available for antibodies to intervene against this stage of the fusion process (32, 39, 57). All these factors have an impact on the design of vaccines for inducing broadly reactive immune responses capable of recognizing the genetically diverse viral quasispecies (39, 51).
Although the correlates of immune protection against HIV-1 infection have not yet been clearly defined, several lines of evidence suggest that CTL might represent an important component of protective immunity. For example, the clearance of HIV-1 from plasma during the primary infection occurs prior to the appearance of neutralizing antibodies in newly infected individuals (40). Similarly, vaccinated macaques were able to efficiently control the virus challenge in the absence of detectable neutralizing antibodies, particularly those animals that were immunized with live, attenuated-virus vaccines (8, 15). Recent studies have demonstrated that polyvalent envelope glycoprotein vaccine elicited a broader neutralizing antibody response but it was unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques (11). Despite the absence of any detectable neutralizing antibody, animals in the polyvalent vaccine group, but not those immunized with the monovalent vaccine, exhibited markedly lower levels of virus in plasma than monkeys in the control group, suggesting a superior cell-mediated immunity responses induced by the polyvalent vaccine (11). Therefore, it appears that cellular responses capable of recognizing highly conserved epitopes within the envelope glycoprotein, with the concomitant ability to recognize these epitopes on more than one strain of HIV-1, might provide protection against heterologous viral challenge.
The HIV-1 envelope glycoprotein contains five highly variable regions, designated V1 through V5, the first four of which form loops through intramolecular disulfide bonds. These variable regions may likely cover a significant portion of the exposed surface on the trimeric gp120 complex, as suggested from antigenic probing with monoclonal antibodies (41) and crystallographic data of the envelope core (33). Among the variable regions of gp120, the V3 loop induces both humoral and cellular responses against HIV-1 (29), possibly explaining the antigenic variation associated with this region. To broaden gp160-specific immune responses, vaccine strategies including dampening of the immunodominant epitopes through addition of N-linked carbohydrate to the V3 loop (22) or using gp160 proteins that lack V3 and/or V1 and V2 regions have been developed (35, 49). However, their abilities to enhance cross-reactive immune CTL responses have not yet been systematically explored.
In this study, we examined the magnitude and antigenic repertoire of envelope-specific CTL responses induced in HLA-A2/Kb transgenic mice by vaccines consisting of wild-type (WT) envelope or envelope glycoprotein from which the V3 loop had been deleted (ΔV3). Using HIV-1 gp160-specific peptides with the HLA-A2-binding motif, we showed that immunization of the transgenic mice with the ΔV3 env vector enhanced CD8+ T-cell responses to conserved epitopes of gp160 and broadened CTL responses to heterologous env gene products. The latter was associated with higher protection against challenge with recombinant vaccinia virus (rVV) expressing heterologous envelope glycoproteins in mice immunized with the ΔV3 mutant.
MATERIALS AND METHODS
Vector construction.
The env gene from the HIV-1IIIB isolate provided as a pIIIB plasmid DNA by B. Cullen (Howard Hughes Medical Institute, Duke University Medical Center, Durham, N.C.) was used for construction of the gp160 segment with a vpu deletion by a two-step PCR protocol (29). In the first step, two fragments were synthesized with an overlap. One fragment was generated with primer A containing the SalI site and Kozak sequence annealed to the rev start codon (5′-AAGGTCGACGCCGCCACCATGGCAGGAAGAAGCGGA-3′) together with primer B (5′-GTTATCACTACATTACATGTATTACTTACTG-3′) with translation termination codons after the vpu start codon. Another fragment was generated with primer C (5′-GTAATGTAGTGATAACAAATAGCAATAGTAGCATTAG-3′) in conjunction with primer D (5′-ACAGGTACCCCATAATAGTAGACTGTG-3′) containing the KpnI restriction site. The nucleotide sequences for the restriction enzyme cleavage sites are underlined. These two fragments were then used together in a second reaction along with primers A and D to generate the env fragment with a vpu deletion. The final product was digested with SalI and KpnI and exchanged with the analogous fragment derived from the pSVIII-env plasmid expressing either the WT or the mutant with residues 297 to 329 deleted (Δ297-329) (10, 58) provided by J. Sodroski (Dana-Farber Cancer Institute, Boston, Mass.). The entire fragment between the SalI and XhoI restriction sites was cloned into the XhoI restriction site of the pcDNA3.1(−) expression vector containing the cytomegalovirus (CMV) promoter (Invitrogen, Carlsbad, Calif.). After DNA sequence verification, the expression of the env gene products was confirmed by immunoprecipitation of the envelope glycoprotein from radiolabeled 3T3 cells transfected with the envelope-pcDNA3.1 plasmid. For the immunization studies, the pcDNA3.1 vector expressing either the WT or the ΔV3 mutant was digested with NotI and PmeI, and the env genes were cloned into NotI and SmaI sites of the pCI vector to generate plasmids pCI-ΔV3 and pCI-WT.
The rVVs expressing the WT and the ΔV3 mutant were generated using the pPEenv 15 DNA plasmid expressing the 2.5-kb fragment of gp160 (a generous gift from P. Earl, Laboratories of Viral Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, Md.) in which the two termination signals for vaccinia virus early gene expression have been altered in the env sequence by site-directed mutagenesis (18). Briefly, the PvuII and AocI fragment of gp160 containing the V3 deletion was isolated from the pCI-ΔV3 vector and exchanged with the analogous fragment derived from the pPEenv15 plasmid. The env genes encoding WT or ΔV3 protein were then subcloned into the SalI and NotI sites of the pSC11 vector (a gift from L. Eisenlohr, Thomas Jefferson University, Philadelphia, Pa.) to generate plasmids pSC-ΔV3 and pSC-WT. Plasmids pSC-ΔV3 and pSC-WT were used to generate recombinant vΔV3 and vWT clones by homologous recombination as described previously (17, 18).
The adenovirus (Ad) recombinants with E1/E3 deletions expressing the WT and the ΔV3 mutant of gp160 were generated using the pShuttle CMV plasmid (53). The env genes derived from plasmids pCI-WT and pCI-ΔV3 were cloned into the pShuttle CMV vector utilizing NotI and SalI sites. The Ad-WT and Ad-ΔV3 recombinants with E1/E3 deletions were generated in the Vector Core Facility at University of North Carolina (Chapel Hill, N.C.).
PCR was used to clone DNA encoding the gp140 segment of the HIV-189.6 env gene. First, a gp120-gp41 cleavage site mutant of HIV-189.6 gp140 was generated by replacing the furin cleavage motif with a hexameric Leu-Arg motif as described (7). We used primer a (5′-TTGCGCCTTCGACTCCGGCTAAGACTGAGGGGAATAGGAGCTGTGTTC-3′) and primer b (5′-CCGGAGTCGAAGGCGCAAACGTAATGCCCTGGTGGGTGCTAC-3′) in combination with the flanking upstream primer c (5′-AAAGGCGCCAGTGCTGCAAAAGAAAAAACG-3′) and downstream primer d (5′-TATCAAATGCGGCCGCCTATATATACCACAGCCAGTTTGT-3′) containing the NarI and NotI restriction enzyme cleavage sequences, respectively. The final PCR product was purified and cloned into NarI and NotI sites of the pNGVL-7 vector (University of Michigan, Ann Arbor) by fusing the env open reading frame with tissue plasminogen activator secretory signal sequence (tPA) under the control of the human CMV immediate early promoter. Rev was not included in the gp140-expressing plasmids, as expression of envelope with the tPA leader in pNGVL-7 allows envelope expression without Rev (35). The integrity of the plasmid was verified by restriction enzyme cleavage and DNA sequence analysis.
The ΔV3 envelope mutant of the HIV-189.6 isolate was generated by utilizing a two-step PCR protocol. In the first step, two fragments were synthesized with a set of overlapping primers. One fragment was generated with primer e containing the NarI site (5′-AAAGGCGCCAGTGCTGCAAAAGAAAAAACG-3′) together with primer f (5′-ACAATGGCCGGCACCTGTACAATTAATTAC-3′). The second fragment was generated with primer g (5′-GGTGCCGGCCATTGTAACATTAGTAGAGCA-3′) in conjunction with primer h containing the Bsu36I restriction site (5′-CCTCCTGAGGATTGATTAAAGGCTATTGTT-3′), using the pNGVL-7 plasmid with the WT gp140 gene as a template. These two fragments were then used together in a second reaction along with primers e and h to generate the ΔV3 env fragment. The final product was exchanged with the corresponding segment of the WT 89.6 gp140 protein fused in frame with the tPA secretory signal sequence in the pNGVL-7 plasmid. After sequence verification, the expression of the env gene products was confirmed by immunoprecipitation from radiolabeled HeLa cells transfected with the pNGVL-7-env construct.
Synthetic peptides.
Two envelope-specific peptides that map in the C1 region of gp120 and the C-terminal regions of gp41 (peptide D1, amino acids [aa] 121 to 129 [KLTPLCVTL], and peptide D2, aa 813 to 822 [SLLNATAIAV], respectively), and three peptides in the V3 loop of gp120 (peptide 29, aa 291 to 307 [SVEINCTRPNNNTRKRI]; peptide 35/36, aa 308 to 322 [RIQRGPGRAFVTIGK]; and peptide I10, aa 311 to 320 [RGPGRAFVTI]) have been previously described (16, 28, 50). The Gag-specific peptide with HLA-A2-binding motif (peptide p17, aa 77 to 85 [SLYNTVATL]) was used as a control. Peptides D1 and D2 match the optimally active synthetic peptides recognized by epitope-specific CTLs in seropositive, asymptomatic HLA-A2+ individuals and conform to the A2 consensus motif (16), whereas the I10 peptide lacks the A2 anchor residues but possesses structural features that confer promiscuous A2 binding (2). The specificity of peptides 29 and 35/36 has been defined through recognition by polyclonal human CTL in association with the HLA-A2 molecules (12, 30). All peptides were synthesized using 9-fluorenylmethoxy carbonyl methodology, purified by reverse-phase high-performance liquid chromatography, and characterized by amino acid analysis and laser desorption mass spectroscopy at The Wistar Institute (Philadelphia, Pa.).
Pulse-chase and immunoprecipitation.
Separate cultures of 106 HeLa cells were infected with vWT and vΔV3 at a multiplicity of infection of 5. After 2 h of infection, cells were washed and subsequently incubated with methionine-free RPMI 1640 medium for 20 min. The cells were then pulsed with 150 μCi of [35S]methionine (DuPont-New England Nuclear, Boston, Mass.) for 10 min, washed in RPMI 1640 medium containing 10% fetal calf serum (FCS) and chased for 15, 60, 120, and 240 min. At the end of each chase time, the cells were pelleted and lysed using Nonidet P-40 buffer (0.5% Nonidet P-40, 0.5 M NaCl, and 10 mM Tris-HCl [pH 7.5]). Equal amounts of radiolabeled cell lysates and supernatants, based on the protein concentration, were immunoprecipitated with a mixture of sera from HIV-1-infected individuals followed by polyclonal rabbit anti-human immunoglobulin (Ig) (Organon Teknika, West Chester, Pa.) and protein A-Sepharose CL-4B (Pharmacia Biotech, Piscataway, N.J.). Immunoprecipitates were separated by sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis, and the radioactivity in gels was quantified with a PhosphorImager (STORM 840; Amershan Pharmacia Biotech, Piscataway, N.J.).
Vaccinia viruses.
The rVV expressing the full-length gp160 of HIV-1IIIB (vPE16) (19), the HIV-1IIIB gag-pol genes (vVK1) (26), and the WR strain of nonrecombinant vaccinia virus (vac) (37) were provided by B. Moss (Laboratories of Viral Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, Md.). The rVV expressing the envelope glycoprotein of the primary VI-06 (vV1) (46) and 89.6 (vBD3) (14) HIV-1 isolates were provided by K. Luzuriaga (University of Massachusetts Medical Center, Worcester) and R. Collman (University of Pennsylvania, Philadelphia), respectively. VI-06 is a “transmitted” primary isolate derived from an infant with perinatal HIV-1 infection (46) that exhibits 8 to 10% sequence variation compared with the envelope glycoprotein of the HIV-1IIIB isolate. The second envelope glycoprotein derives from a dualtropic HIV-189.6 primary isolate which uses CXCR4 and CCR5, as well as a number of other chemokine coreceptors for entry (14). The HIV-189.6 isolate exhibits 82% identity with IIIB gp160 sequence with the highest (92%) and the lowest (64%) sequence homology in the C1 region and the V3 loop, respectively.
Mice and immunization.
The HLA-A2/Kb transgenic mice, expressing hybrid molecules bearing the α1 and α2 domains of HLA-A2.1 fused to the α3 domain of H-2Kb, were generated by microinjecting the HLA-A2/Kb chimeric gene into fertilized eggs obtained by crossing (C57BL/6 × DBA/2)F1 mice. The transgenic founder mice were backcrossed to C57BL/6 (H-2b) mice (34, 52). The transgenic mice were provided by L. Sherman (The Scripps Research Institute, San Diego, Calif.). Mice received intramuscular injections in the quadriceps with 100 μg of DNA plasmid encoding WT (pCI-WT) or ΔV3 mutant (pCI-ΔV3) on days 0, 14, and 28. Control mice received the plasmid DNA. To augment the level of envelope-specific immune responses in the HLA-A2/Kb transgenic mice, the pED plasmid DNA (27), which encodes murine interleukin 12p40 (IL-12p40) (10 μg) and IL-12p35 (10 μg), was coadministered with the envelope-specific vaccine. In some experiments, mice immunized with the WT or ΔV3 envelope-specific DNA vaccine received intraperitoneal (i.p.) injections with rVV (107 PFU) expressing the respective env gene products. The booster immunization with rVV was carried out 2 weeks after DNA vaccine priming. Control mice were immunized with vac.
ELISPOT assay.
The number of gamma interferon (IFN-γ)-secreting T cells specific for HIV-1 envelope-specific peptides or the env gene products in splenocytes of the HLA-A2/Kb transgenic mice was determined by the enzyme-linked immunospot (ELISPOT) method (23) 3 weeks after the last immunization. Briefly, 96-well nitrocellulose plates (multiscreen-MAIP; Millipore, Bedford, Mass.) were coated with rat anti-mouse monoclonal antibody (MAb) (15 μg/ml) directed to IFN-γ (MAB785, R&D Systems Inc., Minneapolis, Minn.) in 0.05 M carbonate-bicarbonate buffer, pH 9.6. After overnight incubation at 4°C, the wells were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and blocked for 1 h with RPMI 1640 medium containing 10% FCS. Splenocytes from immunized mice were resuspended to 107 cells/ml and placed in twofold dilutions into the antibody-coated wells in the presence of a 1 μM concentration of the envelope-specific or control peptide. For the analysis of frequencies of IFN-γ-secreting cells specific for the env gene products, splenocytes were combined with Jurkat-A2/Kb cells infected with vPE16, vBD3, vV1, or vac at a ratio of 3:1 and placed in twofold dilutions into the antibody-coated wells. For each dilution duplicate samples were used. After 24 h of incubation at 37°C, the plates were washed six times with PBS containing 0.05% Tween 20 and incubated for 2 h with 50 μl of 1-μg/ml biotinylated MAb directed to mouse IFN-γ (MAB485; R&D Systems Inc.). The plates were washed and incubated for 1 h with 50 μl of 1:1,000-diluted streptavidin-conjugated alkaline phosphatase (SA-5100; Vector Laboratories, Inc., Burlingame, Calif.). After a final wash with PBS, spots were developed with an alkaline phosphatase BCIP-NBT (5-bromo-4-chloro-3-indolyl-1-phosphate-nitroblue tetrazolium) substrate (SK-5400; Vector Laboratories, Inc.) and counted under a stereomicroscope. The frequencies of IFN-γ-secreting cells were determined by regression analysis from a curve generated by plotting the number of spots versus the number of effector cells.
Generation of bulk cultures of envelope-specific CTLs for in vitro cytotoxicity assays.
Bulk cultures of envelope-specific CTLs were generated from splenic lymphocytes isolated at least 3 weeks following immunization of the HLA-A2/Kb transgenic mice. Splenocytes were cultured at 2 × 106 cells/ml in modified Eagle medium supplemented with 10% FCS in the presence of 1 μM of envelope-specific peptide and 10% T-cell stimulatory factor (T-STIM culture supplement; Collaborative Biomedical Products, Bedford, Mass.) as a source of exogenous IL-2. In some experiments, splenocytes from the immunized mice were stimulated with Ad-WT- or Ad-ΔV3-transduced syngeneic spleen cells (106 cells/ml) treated with mitomycin C (100 μg/ml; Sigma, St. Louis, Mo.). Three days after stimulation, cells were split and cultured in medium supplemented with recombinant mouse IL-2 (0.3 ng/ml; Pharmingen, San Diego, Calif.) prior to the cytotoxicity assay. The oligoclonal CTL lines specific for the envelope peptides D1 and D2 were generated by repeated restimulation with mitomycin C-treated naive HLA-A2/Kb splenocytes (2 × 106 cells/ml) in the presence of the respective peptides (1 μM) and exogenous IL-2.
Cytotoxicity assay.
Cytolytic activity of envelope-specific CTL lines established from the HLA-A2/Kb mice was analyzed after 6 days of cultures in a standard 4-h 51Cr release assay against Jurkat-A2/Kb cells (human T-cell leukemia line transfected with the chimeric construct A2/Kb) infected with vPE16, vBD3, or vV1. Control cells were infected with vac. In some experiments, the TAP-deficient human lymphoblastoid cell line T2 transfected with the A2/Kb construct (T2-A2/Kb) was used in the cytotoxic experiments after coating with envelope-specific and HLA-A2-restricted peptides. Both Jurkat-A2/Kb and T2-A2/Kb were provided by L. Sherman (36). The percent specific lysis was calculated as follows: ([cpm experimental release − cpm spontaneous release]/[cpm maximum release − cpm spontaneous release]) × 100. Maximum release was determined from supernatants of cells that were lysed by addition of 5% Triton X-100. Spontaneous release was determined from target cells incubated with medium only.
Protection against challenge with rVV expressing gp160 proteins of HIV-1 isolates.
On day 28 after the last immunization with the envelope-specific vaccine, mice were challenged i.p. with 2.5 × 107 PFU of vPE16, vBD3, or vV1. Five days later, mice were sacrificed and ovaries were removed, homogenized, sonicated, and assayed for vaccinia virus titer by plating serial 10-fold dilutions on human HuTK− 143B indicator cells, staining with crystal violet, and counting plaques at each dilution. To evaluate the contribution of CD8 and CD4 T cells to protection against rVV challenge, mice immunized with the envelope vaccine were treated i.p. with 100 μg of protein G-Sepharose-purified anti-CD4 MAb (clone GK1.5), anti-CD8 MAb (clone 53-6.72), or control rat IgG (ICN Biomedicals, Aurora, Ohio) starting 3 days and 1 day before and 1 day after the challenge with vaccinia recombinants as described (6, 44).
Data analysis.
Amino acid sequences of gp160 proteins of primary HIV-1 isolates were obtained through the accession numbers available from the HIV Sequence Database (http://hiv-web.lanl.gov). The percentage of amino acid sequence identity within immunogenic regions of gp160 proteins of HIV-1HXB2 and primary HIV-1 isolates was determined by the BLAST 2 program (http://www.ncbi.nlm.nih.gov/BLAST).
The significance of differences in numbers of IFN-γ-secreting CD8+ cells specific for the envelope epitopes or the env gene products induced by WT and ΔV3 gp160 vaccines was determined by the unpaired Student t test using JMP software (SAS Institute Inc., Cary, N.C.). Mixed model analysis of variance (54) was used to compare mean values of the rVV titers between control mice and those immunized with WT and ΔV3 vaccines after challenge with vPE16, vBD3, vV1 or vVK1.
RESULTS
Synthesis and processing of WT and ΔV3 envelope glycoproteins in rVV-infected cells.
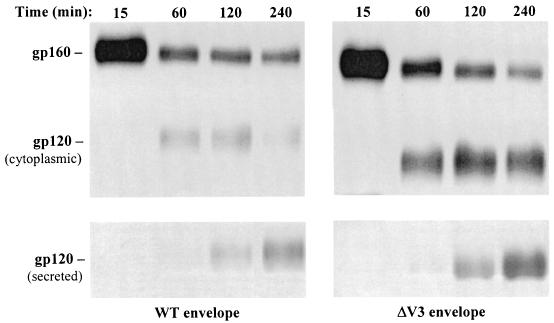
In the initial set of experiments, the biochemical properties of the envelope glycoproteins were characterized by a pulse-chase labeling analysis of the ΔV3 mutant and full-length (WT) gp160 (Fig. 1). HeLa cells were infected with rVV expressing the full-length or ΔV3 gp160 protein, labeled with [35S]methionine for 10 min, and chased with an excess of unlabeled methionine for the time indicated in Fig. 1. The envelope glycoproteins in cellular lysates and supernatants were immunoprecipitated with a mixture of sera from HIV-1-infected individuals and resolved by SDS-polyacrylamide gel electrophoresis. The WT and ΔV3 gp160 proteins could be distinguished based on a lower molecular weight of the ΔV3 mutant due to a replacement of the 31 aa of the V3 loop with a 3-aa sequence (Gly-Ala-Gly).
FIG. 1.
Pulse-chase, [35S]methionine labeling, and immunoprecipitation of HIV-1 envelope proteins expressed by rVV-infected cells. HeLa cells were infected for 2 h with rVV expressing the WT or ΔV3 envelope glycoprotein. The infected cells were then pulsed for 10 min with [35S]methionine and chased with an excess of unlabeled methionine for the time indicated. After the chase time, cells and culture media were harvested. The gp160 proteins were immunoprecipitated from cell lysates (top) and supernatants (bottom) with a mixture of sera from HIV-1-infected individuals followed by polyclonal rabbit anti-human Ig and protein A-Sepharose CL-4B.
At 15 min, the intensity of a band corresponding to ΔV3 gp160 protein was more than twofold higher than that expressed in cells infected with vWT. These results are consistent with those of previous studies showing that the form of the envelope glycoprotein with the variable loop deletion was expressed at higher levels than WT gp160 (10, 35). Processing of the proteins to yield the WT and ΔV3 gp120 was first apparent at 60 min and more so at 120 min. The gp160 protein bands were present at each time point throughout the experiment, consistent with previous results that only a fraction of the gp160 protein is processed to the constituent gp120 and gp41 subunits (48). Beginning at 60 min, the intensity of the ΔV3 mutant gp160 band was decreased by 71% relative to the value observed at 15 min, and at 240 min, the intensity decreased by over 90% as determined by densitometry. The processing rate of the WT gp160 protein was lower, having decreased at 60 min by 67% relative to the 15 min time point and differing by less than 8% at subsequent time points. The differences in synthesis and processing rates between the WT and ΔV3 mutant were also reflected in the levels of gp120 secreted in the culture media. The expression of the secreted form of ΔV3 gp120 was noticeable at 60 min and increased with longer incubation, whereas a faint band corresponding to WT gp120 in the medium was first apparent at 120 min (Fig. 1).
Breadth of CTL responses induced by WT and ΔV3 mutant of HIV-1IIIB gp160.
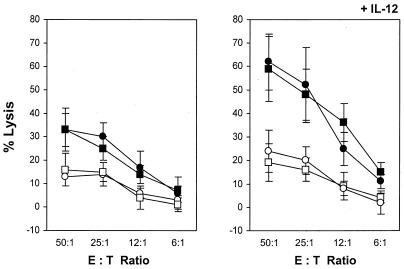
The effect of the V3 loop deletion on the level and repertoire of envelope-specific CTL responses was examined in the HLA-A2/Kb transgenic mice immunized by the prime-boost strategy with plasmid DNA and rVV expressing the full-length or the ΔV3 envelope protein. In some experiments, 10 μg of plasmid DNA encoding IL-12p40 and IL-12p35 was coadministered with the vaccine to augment the specific responses and improve evaluation of any subtle differences at the CTL levels. First, we analyzed envelope-specific CTL responses elicited by the WT and ΔV3 vaccines against Jurkat-A2/Kb cells infected with rVV expressing the homologous envelope glycoprotein of the HIV-1IIIB isolate (vPE16). Splenocytes from the immunized mice were stimulated for 6 days with syngeneic cells expressing the respective env gene products and analyzed for CTL activities against vPE16-infected Jurkat-A2/Kb cells. Cells infected with vac served as a negative control. As shown in Fig. 2 (left panel), mice immunized with the WT or ΔV3 vaccine elicited a level of CTL responses comparable to that of the homologous env gene products. Although the treatment with IL-12-encoded plasmid DNA augmented the level of cytotoxic activity to gp160, it had no effect on the overall profile of envelope-specific responses (Fig. 2, right panel).
FIG. 2.
Analysis of CTL responses directed to the homologous gp160 gene products in mice immunized by the DNA prime-rVV boost regimen with WT and ΔV3 mutant of HIV-1IIIB gp160. Splenocytes from the HLA-A2/Kb mice immunized with the WT (▪) and ΔV3 (•) envelope vaccines in the presence (right panel) or absence (left panel) of plasmid DNA encoding IL-12 gene products were stimulated with mitomycin C-treated syngeneic cells transduced with E1/E3 deletion Ad-WT or Ad-ΔV3. The level of envelope-specific CTL responses was analyzed after 6 days of cultures in a standard 51Cr release assay against Jurkat-A2/Kb cells infected with rVV expressing the HIV-1IIIB gp160 protein (vPE16). Control cells were infected with vac (open symbols). All determinations were made in triplicate samples, and the standard deviation was <10%. Results are presented as the means ± standard deviations (error bars) of four independent experiments. E:T, effector-to-target cell ratio.
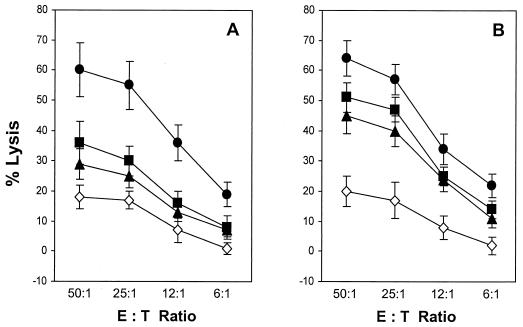
To investigate the ability of the ΔV3 mutant to induce cross-reactive CTL responses, splenocytes from the HLA-A2/Kb transgenic mice immunized with the WT or ΔV3 envelope vaccine and stimulated in vitro with syngeneic cells expressing the WT or ΔV3 mutant of gp160 were analyzed for lysis of Jurkat-A2/Kb targets infected with rVV expressing envelope glycoproteins of the primary HIV-1 isolates VI-06 (vV1) and 89.6 (vBD3). Target cells infected with vPE16 and vac served as positive and negative controls, respectively. As shown in Fig. 3A, the CTLs derived from animals immunized with the full-length envelope vaccine exhibited a rather restricted pattern of reactivity, in which Jurkat-A2/Kb cells expressing the heterologous env gene products were recognized at approximately half of the level detected for targets expressing the homologous gp160. The ΔV3 vaccine-induced CTLs showed increases in cross-reactivity, lysing vBD3- and vV1-infected target cells with ∼70% of the efficiency of that directed to cells expressing the homologous env gene products over a broad range of effector-to-target ratios (Fig. 3B).
FIG. 3.
Induction of cross-reactive CTL responses with WT and ΔV3 envelope vaccines. Splenocytes from the HLA-A2/Kb transgenic mice immunized with WT (A) and ΔV3 mutant (B) HIV-1IIIB gp160 were stimulated with mitomycin C-treated syngeneic splenocytes expressing the respective env gene products. The CTL activity against Jurkat-A2/Kb cells infected with rVV expressing the homologous (vPE16 [•]) or heterologous (vV1 [▪] and vBD3 [▴]) gp160 was analyzed in a standard 51Cr release assay. Control cells were infected with vac (◊). All determinations were made in triplicate samples, and the standard deviation was <10%. Results are presented as the means ± standard deviations (error bars) of four independent experiments. E:T, effector-to-target cell ratio.
Antigenic repertoire of CD8+ T-cell responses induced by WT and ΔV3 gp160 vaccines.
The comparable levels of CTL responses to homologous env gene products in mice immunized with WT and ΔV3 envelope vaccines raised the possibility that the absence of CTLs to V3 loop-specific epitopes in the ΔV3 mutant-vaccinated animals might be compensated by induction of higher CTL responses to epitopes located outside the V3 region. To analyze qualitative differences in CD8+ T-cell responses induced by WT and ΔV3 vaccines in the HLA-A2/Kb transgenic mice, we measured the number of IFN-γ-secreting CD8+ T cells in splenocytes stimulated with envelope-specific peptides. The envelope-specific peptides included peptides D1, aa 121 to 129 (KLTPLCVTL), and D2, aa 813 to 822 (SLLNATAIAV), which map to the N- and C-terminal regions of gp160, respectively. The V3 loop-specific peptides included peptides 29, aa 291 to 307 (SVEINCTRPNNNTRKRI); 35/36, aa 308 to 322 (RIQRGPGRAFVTIGK); and I10, aa 311 to 320 (RGPGRAFVTI). Figure 4 shows that the HLA-A2/Kb transgenic mice immunized with the full-length env gene products exhibited responses to all V3 loop-specific peptides, with the highest reactivity directed to peptides I10 and 35/36. The numbers of CD8+ T cells specific for peptides D2 and I10 were comparable, whereas D1-specific responses were 60% lower than those directed to peptide I10. In mice immunized with the ΔV3 construct, responses to the D1 peptide were threefold higher (P = 0.002) than that for the WT gp160-vaccinated mice, and the number of D2-specific CD8+ T cells increased by more than 40% (P = 0.012).
FIG. 4.
Cellular responses to envelope-specific peptides in the HLA-A2/Kb mice immunized with the WT and the ΔV3 mutant of HIV-1IIIB gp160. The number of IFN-γ-secreting, HIV-1 envelope peptide-specific CD8+ T cells in splenocyte cultures established from the immunized mice was determined by ELISPOT assay. The envelope peptides included peptides D1, aa 121 to 129 (KLTPLCVTL), and D2, aa 813 to 822 (SLLNATAIAV), which map to the N- and C-terminal regions of gp160, respectively. The V3 loop-specific peptides included peptides 29, aa 291 to 307 (SVEINCTRPNNNTRKRI); 35/36, aa 308 to 322 (RIQRGPGRAFVTIGK); and I10, aa 311 to 320 (RGPGRAFVTI). The p17-specific peptide with HLA-A2-binding motif (aa 77 to 85 [SLYNTVATL]) was used as a control. Results are presented as the means + standard deviations (error bars) of at least three independent experiments.
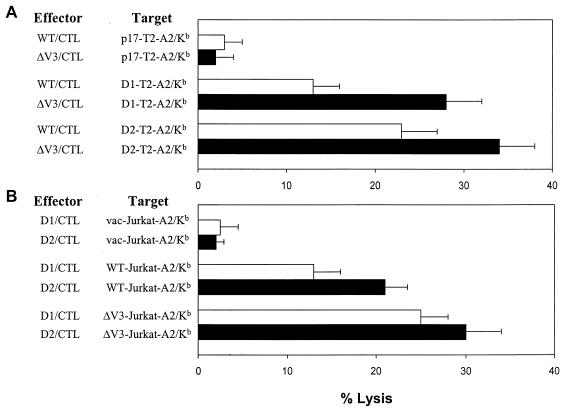
The enhancement of T-cell responses to epitopes D1 and D2 after immunization with the ΔV3 vaccine was further examined in the standard 51Cr release assay against peptide-coated T2-A2/Kb cells. Splenocytes from the HLA-A2/Kb transgenic mice, vaccinated either with the WT or ΔV3 gp160 of the HIV-1IIIB isolate, were stimulated for 6 days with syngeneic cells expressing the full-length or ΔV3 env gene products for the assessment of specific cytotoxic activities. At an effector-to-target ratio of 12:1, cultures derived from mice immunized with the ΔV3 mutant exhibited a roughly twofold increase in lysis of D1-coated target cells compared to cultures established from animals immunized with the WT env gene products (Fig. 5A). A similar but less pronounced effect was observed for the D2 peptide.
FIG. 5.
Analysis of CTL responses to the conserved D1 and D2 epitopes of the envelope glycoprotein in the HLA-A2/Kb mice immunized with WT and ΔV3 envelope vaccines. (A) Splenocytes from the HLA-A2/Kb mice immunized with the WT (open bars) and the ΔV3 mutant (solid bars) of HIV-1IIIB gp160 were stimulated with mitomycin C-treated syngeneic splenocytes expressing the respective env gene products and analyzed for CTL activity against T2-A2/Kb cells coated with the envelope-specific peptide D1 or D2. The effector-to-target cell ratio was 12:1. Control cells were coated with unrelated p17-specific and HLA-A2-restricted epitope. All determinations were made in triplicate samples, and the standard deviations was <10%. Results are presented as the means + standard deviations (error bars) of four independent experiments. (B) The cell surface occupancy of the epitope-HLA-A2 complexes was determined in 51Cr release assays using Jurkat-A2/Kb cells infected with rVV expressing WT or ΔV3 gp160 and oligoclonal CTL lines specific for peptides D1 (open bars) and D2 (solid bars). The effector-to-target ratio was 10:1. All CTL experiments were performed in triplicate samples, and the standard deviation was SD <10%. Cells infected with vac served as a negative control. Results are presented as the means + standard deviations (error bars) of three independent experiments.
Since the efficient processing and presentation of CTL epitopes requires a permissive protein context (43), we compared the abundance of the HLA-A2 complexes with epitopes D1 and D2 on cell surface of Jurkat-A2/Kb cells expressing either WT or ΔV3 env gene products. In this set of experiments, Jurkat-A2/Kb cells were infected for 4 h with vWT or vΔV3 recombinant virus to allow the expression of epitope-HLA-A2 complexes, radiolabeled with 51Cr, and combined with a D1- or D2-specific oligoclonal CTL line for the 51Cr release assay. Figure 5B shows that at an effector-to-target ratio of 10:1, the lysis of vWT-infected Jurkat-A2/Kb cells by D1-specific CTLs was approximately 60% of that mediated by CTLs specific for the D2 peptide. Targets expressing ΔV3 env gene products were lysed more efficiently by both epitope-specific CTL lines. The lysis of vΔV3-infected Jurkat-A2/Kb cells by D1-specific CTL line increased twice, and responses mediated by D2-specific CTLs were augmented by 30%.
Cellular responses induced by immunization with the WT and ΔV3 mutant of HIV-189.6 envelope glycoprotein.
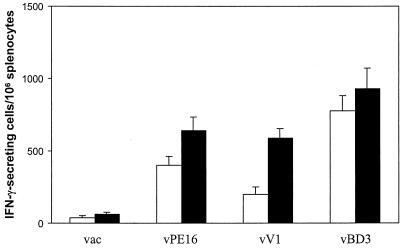
To investigate whether the profile of immune responses elicited by immunization with the ΔV3 mutant of HIV-1IIIB gp160 could be generalized to other HIV-1 primary isolates, we prepared the WT and ΔV3 mutant of HIV-189.6 envelope glycoprotein. As gp140 has been shown to have enhanced immunogenicity in comparison to gp160 gene products (35), the ΔV3 mutant was generated utilizing the sequence encoding WT gp140 of the HIV-189.6 virus. The HLA-A2/Kb transgenic mice were immunized with plasmid DNA expressing either WT or ΔV3 gp140, and the frequency of IFN-γ-secreting splenocytes was analyzed by ELISPOT assay against Jurkat-A2/Kb cells infected with vPE16, vV1, or vBD3. As shown in Fig. 6, the HIV-189.6 gp140-encoding plasmid DNA elicited a substantial level of envelope-specific cellular responses, thus eliminating the need for a booster with the viral vector. Similar to the profile of CD8+ T-cell responses in mice immunized with the HIV-1IIIB envelope vaccine, the highest numbers of IFN-γ-secreting cells were measured in cultures stimulated with the homologous env gene products. The responses directed to Jurkat-A2/Kb cells expressing the heterologous envelope of HIV-1IIIB or HIV-1VI-06 in WT gp140-immunized mice were two- to threefold lower than those expressing the homologous gp160 gene products. On the other hand, cross-reactive responses to vPE16- and vV1-infected Jurkat-A2/Kb cells were significantly higher in mice vaccinated with the ΔV3 mutant than with the WT envelope (P = 0.005 and P = 0.0001, respectively).
FIG. 6.
Cellular responses induced by immunization with WT and ΔV3 mutant of HIV-189.6 gp140. The HLA-A2/Kb transgenic mice immunized with plasmid DNA expressing the WT (open bars) and the ΔV3 mutant (solid bars) of the 89.6 gp140 protein were analyzed for numbers of IFN-γ-secreting splenocytes by ELISPOT assay with Jurkat-A2/Kb cells infected with vPE16, vV1, or vBD3. Control cells were infected with vac. Results are presented as the means + standard deviations (error bars) of at least three independent experiments.
Protection against challenge with rVV expressing homologous and heterologous envelope glycoproteins.
The significance of the WT and ΔV3 mutant vaccine-induced immune responses was further examined in protective studies against challenge with vPE16, vV1, or vBD3. First, we analyzed the antiviral protection in mice immunized with the envelope vaccines of the HIV-1IIIB isolate. For immunization with the WT and ΔV3 mutant of HIV-1IIIB gp160, mice were primed with a DNA vaccine and then were given intramuscular boosters containing 106 PFU of E1/E3 deletion Ad recombinant expressing either the WT or ΔV3 mutant, instead of rVV, to avoid cross-reactivity with vaccinia virus-specific antigens during challenge. Mice immunized with the WT and ΔV3 vaccines were challenged on day 28 by i.p. injection with 2.5 × 107 PFU of vPE16, vV1, or vBD3 as described (5). To determine whether the protection induced by immunization with the WT and ΔV3 vaccines was specific for the envelope glycoprotein, additional groups of immunized and control mice were challenged with rVV expressing the HIV-1IIIB gag-pol genes in addition to β-galactosidase (vVK1). Because the vaccinia virus replicates most efficiently in ovaries (1), 5 days after the challenge, the ovaries were removed and tested for the vaccinia virus titer on a monolayer of HuTK− 143B cell line.
As shown in Table 1, vaccinia virus titers in the ovaries of mice immunized with the HIV-1IIIB envelope vaccines and challenged with rVV expressing the homologous gp160 protein were reduced by a factor of 104 compared with unprimed control animals (P < 0.0001). Consistent with the profile of CTL responses, comparable levels of protection against the challenge with vPE16 were elicited with the WT- and ΔV3 mutant-specific vaccines. Some differences in the protection level were detected in the immunized animals that were challenged with rVVs expressing the heterologous envelope glycoproteins. In mice immunized with the WT envelope-specific vaccine, the resistance to transmission of vV1 virus expressing gp160 of the VI-06 primary isolate was approximately 100-fold higher than that of the control group. Immunization with the ΔV3 envelope was approximately 10-fold more effective in eliciting protection against challenge with vV1 than the full-length envelope vaccine (P = 0.007). A higher antiviral effect was also detected in ΔV3-immunized mice that were challenged with vBD3 expressing gp160 of the HIV-189.6 isolate than that elicited by vaccination with the full-length envelope (P = 0.02). There was no significant difference between vaccinia virus titers in the ovaries of control and immunized mice challenged with vVK1 virus, indicating that the reduction in viral titers in mice immunized with the envelope-specific vaccines could not be mediated by nonspecific inflammatory responses.
TABLE 1.
WT and ΔV3 envelope vaccine-induced protection against challenge with rVV expressing homologous and heterologous gp160 proteins
| Envelope vaccine | Immunization strategya | Virus titer (log10/ovaries)b
|
|||
|---|---|---|---|---|---|
| vVK1 | vPE16 | vV1 | vBD3 | ||
| Control | Unprimed | 8.90 ± 0.79 | 8.30 ± 0.84 | 8.92 ± 0.94 | 8.81 ± 0.55 |
| WT-EnvIIIB | DNA/ΔE1/E3 Ad | 9.21 ± 0.59 | 4.49 ± 0.42 | 6.89 ± 0.21 | 7.78 ± 0.18 |
| ΔV3-EnvIIIB | DNA/ΔE1/E3 Ad | 9.07 ± 0.64 | 4.21 ± 0.37 | 5.74 ± 0.48 | 7.20 ± 0.11 |
| WT-Env89.6 | DNA | 8.61 ± 0.44 | 6.98 ± 0.32 | 7.49 ± 0.35 | 6.01 ± 0.39 |
| ΔV3-Env89.6 | DNA | 8.73 ± 0.67 | 6.26 ± 0.23 | 6.48 ± 0.10 | 5.54 ± 0.71 |
The HLA-A2/Kb transgenic mice were immunized with the WT or the ΔV3 mutant of HIV-1IIIB gp160 and HIV-189.6 gp140. For immunization with the WT and ΔV3 mutant of HIV-1IIIB gp160, the DNA prime-boost strategy was applied with plasmid DNA used for priming and ΔE1/E3 Ad recombinant delivered as the booster. DNA vaccine was used for immunization with the WT and ΔV3 mutant of HIV-189.6 gp140.
On day 28 after immunization with the envelope-specific vaccine, mice were challenged i.p. with 2.5 × 107 PFU of vVK1, vPE16, vV1, or vBD3. Five days later, mice were sacrificed and ovaries were removed, homogenized, sonicated, and assayed for vaccinia virus titer by plating serial 10-fold dilutions on human HuTK− 143B indicator cells, staining with crystal violet, and counting plaques at each dilution. The values are presented as the mean log10 ± standard deviation of PFU per ovaries of four mice per group.
Immunization with the DNA vaccine expressing the full-length or the ΔV3 mutant of 89.6 gp140 also elicited protection against challenge with rVV expressing the homologous or the heterologous envelope glycoprotein (Table 1). As expected, the highest protection was detected in mice that were challenged with vBD3 expressing the homologous env gene products (P = 0.0004). Although a significant resistance was also detected against the heterologous challenge with vPE16 and vV1 (P = 0.0015 and P = 0.0031, respectively), the overall protection level was lower than that against the vBD3 recombinant virus. Similar to the protection profile in mice immunized with the ΔV3 envelope vaccine of the HIV-1IIIB isolate, animals vaccinated with the ΔV3 mutant of the HIV-189.6 isolate exhibited an increased resistance to the heterologous challenge with vPE16 and vV1 compared to those immunized with WT gp140 (P = 0.01 and P = 0.004, respectively).
Protection depends on the existence of CD8+ T cells.
Next, we analyzed the protective role of T-cell subsets against challenge with rVV expressing the homologous env gene products. To determine to what extent the elimination of CD4+ or CD8+ T cells would affect the viral replication in vivo, the transgenic mice immunized with the 89.6 gp140 vaccine were treated with CD4- or CD8-specific MAb at the time of the challenge. Anti-CD8 or anti-CD4 MAb was given 3 days and 1 day before and 1 day after the challenge with vaccinia virus recombinants. This treatment was capable of depleting CD4+ and CD8+ T cells in nonimmunized mice by >95% as determined by flow cytometric analysis (data not shown). As shown in Table 2, the 89.6 gp140 vaccine-primed and CD4+ T-cell subset-depleted mice inhibited replication of vBD3 recombinant virus in ovaries at a level comparable to that measured in the envelope vaccine-immunized mice that received rat IgG at the time of challenge. In contrast, depletion of CD8+ T cells at the time of challenge almost completely eliminated the antiviral protection in the immunized mice. In the latter group of animals, virus titers in the ovaries were comparable with those measured in the immunized mice that were challenged with vVK1 recombinant. Results of these experiments indicated that protection against rVV challenge in the HLA-A2/Kb transgenic mice was largely dependent on the presence of CD8+ T cells.
TABLE 2.
Effect of anti-CD4 and anti-CD8 antibody treatment on replication of vBD3 vaccinia virus recombinant in ovaries of mice immunized with HIV-189.6 gp140 DNA vaccine
| Challengea | Treatmentb | Virus titerc (log10/ovaries) | Protectiond (Δ log10) |
|---|---|---|---|
| vVK1 | 8.20 ± 0.65 | 0 (control) | |
| vBD3 | IgG | 6.17 ± 0.20 | 2.03 |
| vBD3 | Anti-CD4 | 6.48 ± 0.47 | 1.72 |
| vBD3 | Anti-CD8 | 7.65 ± 0.53 | 0.52 |
The HLA-A2/Kb transgenic mice were immunized with plasmid DNA expressing the WT or the ΔV3 mutant of HIV-189.6 gp140. On day 28 after immunization, mice were challenged i.p. with 2.5 × 107 PFU of vVK1 or vBD3. The viral titer in ovaries was analyzed 5 days after the challenge.
To evaluate the contribution of CD8 and CD4 T cells to protection against rVV challenge, the immunized mice were treated i.p. with 100 μg of anti-CD4 MAb (clone GK1.5), anti-CD8 MAb (clone 53-6.72), or control rat IgG on days 3 and 1 before and day 1 after the challenge with vaccinia virus recombinants.
The rVV titers were determined in ovaries of three mice per group (mean log10 ± standard deviation).
Protection is reduction of vBD3 titers (Δlog10 PFU) in ovaries compared with the value for the control group challenged with vVK1 virus.
DISCUSSION
While much has been learned about humoral responses induced by the envelope glycoprotein, relatively little is known about the elements of gp160 that modulate the immunogenicity of major histocompatibility complex class I-restricted CTL epitopes. The vaccine-induced CTLs are not restricted by limitations such as the inability of antibodies induced to previously cryptic epitopes on modified gp160 to bind to the same structures on native virions. Furthermore, the magnitude and repertoire of CTL responses can be altered at the level of antigen processing and presentation. Our results demonstrated that deletion of CTL epitopes in the highly variable V3 region induced qualitative changes at the level of envelope peptide-specific CD8+ T-cell responses in the HLA-A2/Kb transgenic mice. Although these changes did not have a significant effect on CTL responses to the homologous gp160, perhaps due to a deletion of some HLA-A2-restricted CTL epitopes located in the V3 loop, they were associated with an increased recognition of target cells expressing heterologous env gene products. Considering the variation in the amino acid sequence of the V3 region between the heterologous gp160 and the envelope used for priming, it is likely that the higher CTL activities observed with heterologous env gene products in ΔV3 mutant-immunized mice might be attributed to increases in responses to epitopes located outside the V3 loop of gp120.
As the principal factor that regulates CTL responses is the quantity of the epitope-major histocompatibility complexes displayed on the cell surface (reviewed in reference 59), the increased level of D1- and D2 epitope-HLA-A2 complexes on the surface of Jurkat-A2/Kb expressing ΔV3 gp160 in comparison to the WT envelope might have contributed to the enhanced immunogenicity of these peptides in ΔV3 mutant-vaccinated mice. It is possible that a reduced presentation of the conserved epitopes of gp160 in cells expressing the WT envelope glycoprotein is due to a competition with V3 loop-specific epitopes during antigen processing and presentation. Alternatively, the increased expression of ΔV3 gp160 compared with the WT counterpart in rVV-infected cells could augment the concentration of conserved epitopes available for CTL recognition. Although further studies are required to determine which of the mechanisms contributes to the increased immunogenicity of the conserved epitopes in the ΔV3 mutant, results presented here suggest that one possible approach to broaden the repertoire of envelope-specific cellular activity is to redirect the responses to highly conserved epitopes by modifications of the variable sequences within gp120.
Our analysis of cellular responses induced by immunization with the envelope vaccine of either the laboratory-adapted variant or the primary isolate of HIV-1 is in agreement with the previous data which demonstrated that envelope proteins possess divergent, strain-specific CTL epitopes with limited ability for cross-reactivity and protection (38). Thus, the efforts to improve the cross-reactivity of envelope-specific vaccine might require either a polyvalent vaccine composed of envelope glycoproteins from multiple primary isolates or a vaccine in which the structure of the envelope is modified to elicit cellular responses to conserved epitopes that are otherwise poorly immunogenic. Based on the HIV Molecular Database (30), the majority of CTL epitopes with multiple HLA binding motifs are clustered in five regions of gp160 that differ in genetically diverse viral species. Analyses of intra- and interclade envelope sequence in the immunogenic regions of primary isolates revealed a high degree of conservation among CTL epitopes that map to the C1 region of gp120 and the gp41 ectodomain (Table 3). This suggests that the ability to direct immune responses to these highly conserved regions may increase the level of cross-clade protection. Although the effect of the V3 loop deletion on CTL responses to epitopes in the gp41 ectodomain was not analyzed in our studies, we demonstrated that there is an increase in the level of specific responses to the C1 region of the envelope glycoprotein in the ΔV3 mutant-immunized mice. Additional experiments will determine whether deletions or modifications of other variable regions of gp120 induce changes in the repertoire of CTL responses and affect protection against challenge with rVV expressing gp160 proteins of primary HIV-1 isolates.
TABLE 3.
Amino acid sequence homology within immunogenic regions of gp160 proteins of HIV-1HXB2 and primary HIV-1 isolates
| HIV-1 consensus | No. of HIV-1 isolates | % Sequence homology (mean ± SD)a in region
|
||||
|---|---|---|---|---|---|---|
| aa 30-130 | aa 296-331 | aa 370-440 | aa 567-598 | aa 770-856 | ||
| A | 10 | 86 ± 1 | 63 ± 6 | 71 ± 4 | 90 ± 3 | 62 ± 2 |
| B | 10 | 93 ± 2 | 69 ± 3 | 79 ± 4 | 93 ± 2 | 87 ± 3 |
| C | 10 | 89 ± 2 | 64 ± 4 | 64 ± 5 | 89 ± 3 | 65 ± 3 |
| D | 10 | 87 ± 2 | 49 ± 6 | 70 ± 4 | 96 ± 3 | 78 ± 2 |
| AE | 10 | 84 ± 3 | 52 ± 4 | 64 ± 3 | 87 ± 1 | 69 ± 3 |
| F1 | 5 | 88 ± 1 | 63 ± 5 | 58 ± 4 | 94 ± 3 | 70 ± 3 |
| G | 9 | 83 ± 2 | 64 ± 2 | 60 ± 4 | 93 ± 3 | 66 ± 2 |
| O | 9 | 64 ± 2 | 32 ± 5b | 43 ± 3 | 59 ± 3 | 49 ± 3 |
The immunogenic regions of the HIV-1HXB2 envelope glycoprotein have been determined based on accumulation of CTL epitopes with multiple HLA class I- binding motifs (30). They are located within the C1 region of gp120 between residues 30 and 130 (18 CTL epitopes); the V3 loop between residues 296 and 331 (12 CTL epitopes); the C3, V4, and C4 regions between residues 370 and 440 (13 CTL epitopes); the gp41 ectodomain between residues 567 and 598 (10 CTL epitopes); and the C-terminal region on gp41 between residues 770 and 856 (20 CTL epitopes). The number of CTL epitopes between the immunogenic regions of the envelope glycoprotein is less than 10. The percentage of amino acid sequence identity within the immunogenic regions of gp160 proteins between HIV-1HXB2 and primary HIV-1 isolates was determined by using the BLAST 2 program based on the accession numbers available from the HIV Sequence Database.
In the case of HIV-1 isolates from clade O, a region from residues 275 to 340 of gp160 of the HIV-1HXB2 isolate was aligned because no significant similarity was found for this region between residues 296 and 331.
Consistent with the notion that both cellular and humoral responses will be required for an effective AIDS vaccine, understanding of the immunogenicity of envelope glycoproteins from primary HIV-1 isolates will be necessary for the development of broadly protective envelope vaccines. Despite different constraints for the induction of cross-reactive humoral and cellular responses, approaches are focused on redirecting the envelope-specific responses toward highly conserved epitopes that can be recognized on more than one strain of HIV-1. Towards that goal, modifications including partial or complete deletions of the variable sequences of gp120 that elicited enhanced cross-reactivity of envelope-specific humoral responses (49) may augment the breadth of cellular responses. Additionally, we have observed that immunization with gp140, which is more effective in inducing neutralizing antibodies than the complete env gene products (35), elicited substantial levels of specific cellular responses. Further studies are necessary to determine whether structural modifications of the envelope glycoprotein that are optimal for cross-protective cellular activities concur with those required for eliciting broadly neutralizing antibody responses.
The concept of removal of variable and potentially immunosuppressive HIV-1 genes has been explored in vaccine strategies against AIDS with attenuated viruses (8). Although the live-attenuated vaccine approach was found to be effective, concern for safety is the key factor that requires further evaluation of such vaccine for use in humans (3, 9, 56). Other attractive strategies for HIV-1 vaccine design are based on the polytope genetic vaccines consisting of several CTL epitopes (55) or vaccines based on a mixture of multiepitopic lipopetides corresponding to regulatory or structural HIV-1 antigens (21). Although these vaccines are also aimed at inducing immunity to selected regions that are highly conserved, it remains unclear whether responses to few epitopes are sufficient for biologically significant immunity in humans. Additionally, the ability to induce CTLs that recognize selected epitopes of HIV-1 is likely to vary among individuals due to differences in the HLA antigen expression (20). In fact, a detailed analysis of the CTL response to optimally defined CTL epitopes restricted by HLA A and B alleles in HIV-infected individuals revealed that HLA type alone does not predict CTL responses and that numerous potential epitopes may not be targeted by CTLs in a given individual (13). These results further stress the need for boosting both the breadth and the magnitude of HIV-1-specific immunity in AIDS vaccines. Thus, the use of genetic vaccines deprived of sequences of the envelope glycoprotein that enhance the ability of HIV-1 to evade the host immune responses in combination with other regulatory or structural HIV-1 proteins may have significant potential in increasing cross-protective immunity.
Acknowledgments
We are grateful to Bernard Moss, Patricia Earl, Katherine Luzuriaga, Ronald Collman, and Linda Sherman for reagents. We thank the Gene Therapy Center at the University of North Carolina at Chapel Hill for preparing the E1/E3 deletion adenovirus recombinants expressing envelope glycoproteins.
This work was supported by research grants from the Japan Health Sciences Foundation and the National Institute of Allergy and Infectious Diseases to D. Kozbor (AI/HD39148 and AIDE48370) and to G. Trinchieri (AI34412). Toshio Naito is a Research Scholar of the Japan Health Sciences Foundation.
REFERENCES
- 1.Alexander-Miller, M. A., G. R. Leggatt, and J. A. Berzofsky. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 93:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander-Miller, M. A., K. C. Parker, T. Tsukui, C. D. Pendleton, J. E. Coligan, and J. A. Berzofsky. 1996. Molecular analysis of presentation by HLA-A2.1 of a promiscuously binding V3 loop peptide from the HIV-envelope protein to human cytotoxic T lymphocytes. Int. Immunol. 8:641-649. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., Y. S. Jeong, D. Penninck, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, S. W., D. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Haynes, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95:1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder, D., and T. M. Kundig. 1991. Antiviral protection by CD8+ versus CD4+ T cells. CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent IL. J. Immunol. 146:4301-4307. [PubMed] [Google Scholar]
- 7.Binley, J. M., R. W. Sanders, B. Class, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogers, W. M., H. Niphuis, P. ten Haaft, J. D. Laman, W. Koornstra, and J. L. Heeney. 1995. Protection from HIV-1 envelope-bearing chimeric simian immunodeficiency virus (SHIV) in rhesus macaques infected with attenuated SIV: consequences of challenge. AIDS 9:F13-F18. [PubMed] [Google Scholar]
- 9.Bolognesi, D. P. 1994. Not yet, it is too early to consider use of a live-attenuated virus vaccine against HIV-1. J. NIH Res. 6:55-57. [Google Scholar]
- 10.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho, M. W., Y. B. Kim, M. K. Lee, K. C. Gupta, W. Ross, R. Plishka, A. Buckler-White, T. Igarashi, T. Theodore, R. Byrum, C. Kemp, D. C. Montefiori, and M. A. Martin. 2001. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques. J. Virol. 75:2224-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dadaglio, G., A. Leroux, P. Langlade-Demoyen, E. M. Bahraoui, F. Traincard, R. Fisher, and F. Plata. 1991. Epitope recognition of conserved HIV envelope sequences by human cytotoxic T lymphocytes. J. Immunol. 147:2302-2309. [PubMed] [Google Scholar]
- 13.Day, C. L., A. K. Shea, M. A. Altfeld, D. P. Olson, S. P. Buchbinder, F. M. Hecht, E. S. Rosenberg, B. D. Walker, and S. A. Kalams. 2001. Relative dominance of epitope-specific cytotoxic T-lymphocyte responses in human immunodeficiency virus infected person with shared HLA alleles. J. Virol. 75:6279-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 15.Dunn, C. S., B. Hurtrel, C. Beyer, L. Gloeckler, T. N. Ledger, C. Moog, M. P. Kieny, M. Mehtali, D. Schmitt, J. P. Gut, A. Kirn, and A. M. Aubertin. 1997. Protection of SVImac-infected macaques against superinfection by a simian immunodeficiency virus expressing envelope glycoprotein of HIV type 1. AIDS Res. Hum. Retrovir. 13:913-922. [DOI] [PubMed] [Google Scholar]
- 16.Dupuis, M., S. K. Kundu, and T. C. Merigan. 1995. Characterization of HLA-A 0201-restricted cytotoxic T cell epitopes in conserved regions of the HIV type 1 gp160 protein. J. Immunol. 155:2232-2239. [PubMed] [Google Scholar]
- 17.Earl, P. L., C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J. Virol. 68:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earl, P. L., A. W. Hügin, and B. Moss. 1990. Removal of cryptic transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of recombinant vaccinia virus. J. Virol. 64:2448-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari, G., W. Humphrey, M. J. McElrath, J.-L. Excler, A.-M. Duliege, M. L. Clemens, L. C. Corey, D. P. Bolognesi, and K. J. Weinhold. 1997. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc. Natl. Acad. Sci. USA 94:1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gahéry-Ségard, H., G. Pialoux, B. Charmeteau, S. Sermet, H. Porcelet, M. Raux, A. Tartar, J.-P. Levy, H. Gras-Masse, and J.-G. Guillet. 2000. Multiepitopic B- and T-cell responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine. J. Virol. 74:1694-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrity, R. R., G. Rimmelzwaan, A. Minassian, W. P. Tsai, G. Lin, J. J. de Jong, J. Goudsmit, and P. L. Nara. 1997. Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J. Immunol. 159:279-289. [PubMed] [Google Scholar]
- 23.Gherardi, M. M., and M. Esteban. 1999. Mucosal and systemic immune responses induced after oral delivery of vaccinia virus recombinants. Vaccine 17:1074-1083. [DOI] [PubMed] [Google Scholar]
- 24.Goulder, P. J., S. L. Rowland-Jones, A. J. McMichael, and B. D. Walker. 1999. Anti-HIV cellular immunity: recent advances toward vaccine design. AIDS 13:S121-S136. [PubMed] [Google Scholar]
- 25.Hu, S. L., J. Klaniecki, T. Dykers, P. Sridhar, and B. M. Travis. 1991. Neutralizing antibodies against HIV-1 BRU and SF2 isolates generated in mice immunized with recombinant vaccinia virus expressing HIV-1 (BRU) envelope glycoprotein and boosted with homologous gp160. AIDS Res. Hum. Retrovir. 7:615-620. [DOI] [PubMed] [Google Scholar]
- 26.Karakostas, V., K. Nagashima, M. A. Gonda, and B. Moss. 1989. Human immunodeficiency virus-like particles produced by vaccinia virus expression vector. Proc. Natl. Acad. Sci. USA 86:8964-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman, R. S., M. V. Davies, L. C. Wasley, and D. Michnick. 1991. Improved vectors for stable expression of foreign genes in mammalian cells by use of the untranslated leader sequence from EMC virus. Nucleic Acids Res. 19:1485-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kmieciak, D., I. Bednarek, M. Takiguchi, T. J. Wasik, J. Bratosiewicz, A. Wierzbicki, H. Teppler, J. Pientka, S. H. Hsu, Y. Kaneko, and D. Kozbor. 1998. The effect of epitope variation on the profile of cytotoxic T lymphocyte responses to the HIV envelope glycoprotein. Int. Immunol. 10:1789-1799. [DOI] [PubMed] [Google Scholar]
- 29.Kmieciak, D., T. J. Wasik, H. Teppler, J. Pientka, S. H. Hsu, H. Takahashi, K. Okumura, Y. Kaneko, and D. Kozbor. 1998. The effect of deletion of the V3 loop of gp120 on cytotoxic T cell responses and HIV gp120-mediated pathogenesis. J. Immunol. 160:5676-5683. [PubMed] [Google Scholar]
- 30.Korber, B. T. M., J. P. Moore, C. Brander, B. D. Walker, B. F. Haynes, and R. Koup (ed.). 1999. HIV molecular immunology database. Theoretical Biology and Biophysics, Los Alamos National Library, Los Alamos, N.Mex.
- 31.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwong, P. D., R. Wyatt, Q. J. Sattentau, J. Sodroski, and W. A. Hendrickson. 2000. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J. Virol. 74:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaFace, D. M., M. Vestberg, Y. Yang, R. Srivastava, J. DiSanto, N. Flomenberg, S. Brown, L. A. Sherman, and P. A. Peterson. 1995. Human CD8 transgene regulation of HLA recognition by murine T cells. J. Exp. Med. 182:1315-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu, S., R. Wyatt, J. F. Richmond, F. Mustafa, S. Wang, J. Weng, D. C. Montefiori, J. Sodroski, and H. L. Robbinson. 1998. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/2 and V3 regions. AIDS Res. Hum. Retrovir. 14:151-155. [DOI] [PubMed] [Google Scholar]
- 36.Lustgarten, J., M. Theobald, C. Labadie, D. LaFace, P. Peterson, M. L. Disis, M. A. Cheever, and L. A. Sherman. 1997. Identification of Her-2/Neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD8. Hum. Immunol. 52:109-118. [DOI] [PubMed] [Google Scholar]
- 37.Mackett, M., G. L. Smith, and B. Moss. 1982. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc. Natl. Acad. Sci. USA 79:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montefiori, D. C., K. A. Reimann, M. S. Wyand, K. Manson, M. G. Lewis, R. G. Collman, J. G. Sodroski, D. P. Bolognesi, and N. L. Letvin. 1998. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J. Virol. 72:3427-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore, J. P., and J. Binley. 1998. HIV. Envelope's letters boxed into shape. Nature 393:630-631. [DOI] [PubMed] [Google Scholar]
- 40.Moore, J. P., Y. Cao, D. D. Ho, and R. A. Koup. 1994. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J. Virol. 68:5142-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore, J. P., Q. J. Sattentau, R. Wyatt, and J. Sodroski. 1994. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J. Virol. 68:469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mylin, L. M., R. H. Bonneau, J. D. Lippolis, and S. S. Tevethia. 1995. Hierarchy among multiple H-2b-restricted cytotoxic T-lymphocyte epitopes within simian virus 40 T antigen. J. Virol. 69:6665-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuda, K., A. Ihata, S. Watabe, E. Okada, T. Yamakawa, K. Hamajima, J. Yang, N. Ishii, M. Nakazawa, K. Okuda, K. Nakajima, and K. Q. Xin. 2001. Protective immunity against influenza A virus by immunization with DNA plasmid containing influenza M gene. Vaccine 19:3681-3691. [DOI] [PubMed] [Google Scholar]
- 45.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13:S137-S162. [PubMed] [Google Scholar]
- 46.Pikora, C. A., J. L. Sullivan, D. Panicali, and K. Luzuriaga. 1997. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J. Exp. Med. 185:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 48.Ruff, A. L., F. G. Guarnieri, K. Staveley-O'Carrol, R. F. Siliciano, and J. T. August. 1997. The enhanced immune response to the HIV gp160/LAMP chimeric gene product targeted to the lysosome membrane protein trafficking pathway. J. Biol. Chem. 272:8671-8678. [DOI] [PubMed] [Google Scholar]
- 49.Sanders, R. W., L. Schiffner, A. Master, F. Kajumo, Y. Guo, T. Dragic, J. P. Moore, and J. M. Binley. 2000. Viable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J. Virol. 74:5091-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi, H., Y. Nakagawa, G. R. Leggatt, Y. Ishida, T. Saito, K. Yokomuro, and J. A. Berzofsky. 1996. Inactivation of human immunodeficiency virus (HIV)-1 envelope-specific CD8+ cytotoxic T lymphocytes by free antigenic peptide: a self-veto mechanism? J. Exp. Med. 183:879-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verrier, F., S. Burda, R. Belshe, A.-M. Duliege, J.-L. Excler, M. Klein, and S. Zolla-Pazner. 2000. A human immunodeficiency virus prime-boost immunization regimen in humans induces antibodies that show interclade cross-reactivity and neutralize several X4-, R5-, and dualtropic clade B and C primary isolates. J. Virol. 74:10025-10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitiello, A., D. Marchesini, J. Furze, L. A. Sherman, and R. W. Chesnut. 1991. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in trangenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J. Exp. Med. 173:1007-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wersto, R. P., E. R. Rosenthal, P. K. Steh, N. T. Eissa, and R. E. Donahue. 1998. Recombinant, replication-defective adenovirus gene transfer vectors induce cell cycle dysregulation and inappropriate expression of cyclin proteins. J. Virol. 72:9491-9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winer, B. J. 1971. Design and analysis of factorial experiments, p. 224-251. In W. Maytham, A. Shapiro, and J. Stern (ed.), Statistical principals in experimental design. McGraw Hill, New York, N.Y.
- 55.Woodberry, T., J. Gardner, L. Mateo, D. Eisen, J. Medveczky, I. A. Ramshaw, S. A. Thomson, R. A. Ffrench, S. L. Elliott, H. Firat, F. A. Lemonnier, and A. Suhrbier. 1999. Immunogenicity of a human immunodeficiency virus (HIV) polytope vaccine containing multiple HLA A2 HIV CD8+ cytotoxic T-cell epitopes. J. Virol. 3:5320-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyand, M. S., K. H. Manson, A. A. Lackner, and R. C. Desrosiers. 1997. Resistance of neonatal monkeys to live attenuated vaccine strains of simian immunodeficiency virus. Nat. Med. 3:32-36. [DOI] [PubMed] [Google Scholar]
- 57.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 58.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 67:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yewdell, J. W., and J. R. Bennink. 1992. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv. Immunol. 52:1-123. [DOI] [PubMed] [Google Scholar]