Abstract
Pit2 is a type III sodium-dependent phosphate transporter and the cell surface receptor for amphotropic murine leukemia virus. Indirect arguments have previously suggested that retrovirus receptor assembly play a role in triggering membrane fusion. Using CHO cells expressing physiological amounts of functional versions of human Pit2 fused to various tagging epitopes, we provide evidence that Pit2 forms assemblies at the cell surface. Living cells were exposed to cross-linking reagents and protein extracts were treated with trifluoroacetic acid (TFA), a chemical that destroys all protein interactions but covalent links. Assemblies were also detected in the absence of cross-linking and TFA treatment, indicating that they are partially resistant to detergent denaturation. The formation of homo-oligomers was documented by the coimmunoprecipitation of differently tagged molecules. The amounts of Pit2 assemblies detected in the presence or in the absence of cross-linking reagents varied with extracellular inorganic phosphate concentration ([Pi]). Variation of signal intensity was in the range of twofold, occurred in the absence of de novo protein synthesis and took place at the cell surface. These results indicate that Pit2 assemblies exhibit variable conformations at the surface of living cells. Susceptibility to virus infection and phosphate uptake also vary with extracellular [Pi]. A model is proposed in which cell surface Pit2 assemblies switch from a compacted to an expanded configuration in response to changes of extracellular [Pi], and possible relationships with the variation of biological activities are discussed.
Pit1 and Pit2 have been identified as the cell surface receptors for the gibbon ape leukemia virus and the amphotropic murine leukemia virus (A-MLV), respectively. They were identified as the mammalian representatives of type III sodium-phosphate symporters (NaPi-III). Pit1 and Pit2 contain a domain (PD1131) duplicated at the amino- and carboxy-terminal moieties and which shares homology with sequences found in prokaryotes, fungi, yeasts, and plants (47, 48). This homology domain defines the Pit family, which currently includes 37 members. In addition to the various versions of Pit1 and Pit2 described for mammals, a sodium-dependent, or proton-dependent, phosphate or sulfate transporter activity has been demonstrated for several members of the Pit family, including Escherichia coli (15), Acetinobacter johnsonii (52), Bacillus subtilis (37), Rhizobium meliloti (2), Arabidopsis thaliana (14), Neurospora crassa (54), and Saccharomyces cerevisiae (39). We previously showed that human Pit2 is a glycoprotein that forms 12 transmembrane domains and has extracellular N- and C-terminal extremities. The duplicated PD1131 domains are likely in opposite topology with respect to the plasma membrane plan (48).
NaPi-III transporters do not share homology or similarity with phosphate transporters belonging to the other known families, NaPi-I and NaPi-II. In contrast with NaPi-II transporters, which are expressed in specialized structures like the brush border membranes of kidney and intestinal cells and which participate in phosphate homeostasy, NaPi-III likely represents a general phosphate exchange system between the cells and the extracellular medium. Pit1 and Pit2 are ubiquitously expressed in mammals, although mRNA levels differ depending on tissues. Both the phosphate transport and the virus receptor activities are modulated in response to variations of extracellular phosphate concentration ([Pi]). Persistent starvation increases Pit2 and Pit1 mRNA amounts (8, 9, 27, 45). In addition, we have shown previously that changes of Pit2 activity occur within minutes in response to [Pi] variation (46). Retrovirus entry and phosphate uptake are, respectively, 2.5- and 4.5-fold more efficient in phosphate-deprived conditions than at 10 mM. Adaptation to [Pi] occurs without modification of the number of Pit2 molecules expressed at the cell surface. It is therefore presumable that structural modifications affecting the activity of cell surface Pit2 molecules occur in response to the variation of extracellular [Pi].
We presently ignore which modifications accompany the switch between the active and inactive states of Pit2. Phosphorylation is likely to play a role since Pit2 phosphate uptake is modulated by protein kinase C (25) and phosphate transport is affected by protein kinase A in mouse fibroblasts and HeLa cells (34, 41, 43, 44). Modifications of the connection of Pit2 with the actin network could also affect activity (46).
Multimerization has been previously reported for members of the large family of porter molecules to which Pit2 belongs. Initial studies performed by radiation inactivation suggested that NaPi-II transporters could be oligomeric (4, 57) and that oligomerization might vary in response to transmembrane Na+ or H+ gradients (24). Although monomers appeared to be functional (29), a correlation was suspected between the presence of oligomers and phosphate transport activity (24, 32, 57). There is also indirect evidence suggesting a cooperation between several MLV receptors for processing fusion (3, 33, 49). CD4-coreceptor interactions have been largely documented for human immunodeficicency virus (HIV) entry (1, 23, 56), and the cooperation of four to six coreceptor molecules seems required for fusion to occur (30).
We examined whether human Pit2 forms assemblies when expressed in CHO cells. Cell treatment with cross-linking reagents revealed the existence of cell surface Pit2 complexes. Coimmunoprecipitation experiments provided evidence for Pit2 self-assembly. The detection of Pit2 assemblies varied with extracellular [Pi]. Data showed that Pit2 undergoes conformational changes within assemblies present at the surface of living cells. These changes may be related with the biological activity of the phosphate transporter-retrovirus receptor.
MATERIALS AND METHODS
Antibodies and chemicals.
Monoclonal antibodies (MAb) P5D4 (anti-VSV-G tag) and 9E10 (anti-Myc) were purchased from Sigma (St. Louis, Mo.). The rabbit polyclonal serum directed against the intracellular loop of human Pit2 (anti-Pit2) was a generous gift of S. Khumann and D. Kabat (Oregon Health & Science University, Portland, Oreg.). Secondary antibodies coupled to horseradish peroxidase were from Amersham Pharmacia Biotech. The chemical cross-linkers dimethyl adipimidate (DMA), dimethyl suberimidate (DMS), and bis[sulfosuccinimidyl] suberate (BS3) were purchased from Pierce (Rockford, Ill.). One milliliter of anhydrous trifluoroacetic acid (TFA) vials were obtained from Pierce. A combination of protease inhibitors (Complete) was obtained from Boehringer (Mannheim, Germany). Protein N-glycosydase F (PNGase F) was purchased from New England Biolabs and used as described previously (48).
Cell lines, plasmids, and transfections.
CHO and CHO-Pit2-V cells have been previously described (46). Cells were cultured in minimal essential medium alpha (Gibco) supplemented with 10% fetal bovine serum (FBS) and G418 (1 mg/ml) (CHO-Pit2). Hemagglutinin-Pit2-M (HA-Pit2-M) bears a C-terminal Myc tag and an N-terminal HA tag and was described previously (48). Cell transfection was performed with the Lipofectamine PLUS reagent (Gibco). Subconfluent CHO cells in 10-cm-diameter culture plates were incubated with 6 μg of plasmid DNA for 2 h, washed, and analyzed 24 h later. For incubation in defined phosphate and/or sulfate concentrations, cell culture plates were washed once with HeBS buffer [150 mM NaCl, 20 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic) acid (HEPES), 2.7 mM KCl, 1.3 mM CaCl2], twice with NH buffer (150 mM NaCl, 20 mM HEPES), and then incubated for 30 min in medium with defined Na2HPO4, NaH2PO4, and Na2SO4 concentrations in phosphate-free RPMI 1640 (ICN, Costa Mesa, Calif.) supplemented with 10% dialyzed FBS (Gibco) and 25 mM HEPES. Nondialyzed FBS contains less than 3 mM phosphate and less than 0.5 mM sulfate. After dialysis of FBS against 3 volumes of NaCl, [Pi] in phosphate-free RPMI supplemented with 10% dialyzed FBS was <0.1 mM and sulfate concentration was approximately that of RPMI (0.4 mM).
Virus infection experiments.
Amphotropic pseudotype stock of a retrovirus vector containing the nls-lacZ gene expressed under the control of the long terminal repeat were prepared from the TelCeB6-AF7 cells (infectious titer: 5 × 106 β-galactosidase positive foci/ml on NIH 3T3 cells) (12). Cells (105 in six-well plates) maintained at physiological [Pi] were switched to medium containing various [Pi]. After 30 min at 37°C, serial dilutions (from 10−1 to 10−6 infectious U/ml) of the nls-lacZ retrovirus vector were added for 30 min in the presence of polybrene (8 μg/ml). Cells were then incubated for 4 h with fresh medium containing an equivalent [Pi]. Cells were washed and further cultivated for 48 h in normal culture medium prior to 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining and scoring of β-galactosidase-positive foci.
SDS-PAGE.
Cells were washed twice with NH buffer, adjusted to 0, 1, or 10 mM Pi when required, and scrapped in the same buffer. Cells were lysed in NH buffer containing 1% Triton X-100 and protease inhibitors. Nuclei were pelleted after a 30-min incubation at 4°C. Soluble material was isolated as the supernatant of cell extracts centrifuged at 4°C (13,000 × g, 30 min) or at 100,000 × g for 1 h at 4°C. The gel loading buffer contained 5% β-mercaptoethanol. Samples were not heated before loading. Samples were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8% or 4 to 12% gradient polyacrylamide gels) (NuPAGE; Invitrogen).
TFA treatment.
SDS was added to cell lysates (5 to 30 μg of protein) to a final concentration of 5%. Samples were dried in a Speed-Vac concentrator and resuspended in 100 to 300 μl of anhydrous TFA under a chemical hood. TFA was then evaporated under airflow. Dried film on tube walls was resuspended in 20 to 30 μl of Laemmli buffer without SDS. Samples were analyzed immediately by SDS-PAGE.
Western blottings.
Samples separated on SDS-PAGE were transferred to polyvinylidene difluoride membranes. After blocking with phosphate-buffered saline (PBS)-0.1% Tween-5% nonfat milk, membranes were incubated for 1 h at room temperature with the primary antibody (dilutions: anti-Pit2 antibody, 1:1,000; 9E10, 1:1000), washed, and revealed with a horseradish peroxidase-coupled secondary antibody (1:3,500) and enhanced chemiluminescence ECL+ (Amersham).
Immunoprecipitation.
Cells were lysed in 1% Triton X-100-NH buffer at 4°C, then incubated overnight at 4°C with the anti-VSV-G MAb P5D4 (2 μg). Immune complexes were precipitated with protein G-agarose for 2 h at 4°C, washed in NH buffer-1% Triton X-100, and eluted in NH buffer-5% SDS-50 mM dithiothreitol (DTT) without heating. Samples were treated with TFA before analysis on an SDS-4 to 12% gradient polyacrylamide gel.
Chemical cross-linking.
Cells were washed twice in NH buffer adjusted to 0, 1, or 10 mM Pi, scrapped in the same buffer, and divided into aliquots (106 cells/ml) which were incubated for 30 min at 4°C with the cross-linking reagent. Addition of Tris (pH 8) to a final concentration of 50 mM quenched the reaction. After 15 min on ice, treated cells were washed and lysed in 1% Triton X-100-NH buffer for 30 min on ice. Soluble extracts were treated with TFA, run on a 4 to 12% gradient SDS polyacrylamide gel, and analyzed by Western blotting.
RESULTS
The synthesis of a specific inhibitor that interferes with the interaction between hamster Pit2 and the amphotropic envelope impairs infection of CHO cells with A-MLV (7, 26, 40). CHO cells become susceptible to A-MLV infection when human Pit2 is expressed. We constructed an expression vector encoding a tagged version of human Pit2, in which an epitope of the VSV-G protein is fused at the C-terminal extremity (Pit2-V). A CHO cell clone expressing this construct (CHO-Pit2-V) has been isolated (46). Increased phosphate uptake and susceptibility to A-MLV infection indicated that human Pit2 is functional in these cells. The number of A-MLV envelope binding sites at the cell surface of mouse NIH 3T3 cells, human TE671 cells, and CHO-Pit2-V cells is in the same range of magnitude (105 to 106 binding sites/cell) (3, 46). Thus, the density of Pit2-V at the surface of CHO-Pit2-V cells is within the physiological range.
Detection of Pit2 assemblies in reducing SDS-PAGE.
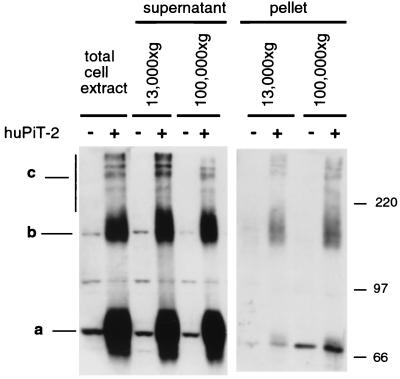
Pit2 expression was analyzed by Western blotting of CHO and CHO-Pit2-V cells by using a rabbit polyclonal antibody directed against the large intracellular loop of human Pit2. Cell proteins were extracted in 1% Triton X-100-NH buffer at 4°C before analysis by SDS-PAGE in the presence of 5% β-mercaptoethanol (Fig. 1). A signal at 73 kDa detected in parental CHO cell extracts likely corresponds to the recognition of hamster Pit2. Additional bands were also revealed in CHO cells, the nature of which has not been determined. Several intense signals were detected in CHO-Pit2-V cell extracts in addition to the expected 73-kDa Pit2 signal (Fig. 1, band a). They include a band at 150 kDa (Fig. 1, band b) and three or four bands of higher molecular mass (Fig. 1, band c). Similar patterns were observed for supernatants of 13,000 × g or 100,000 × g centrifugations (Fig. 1, supernatant). These signals were much less intense in centrifugation pellets, though material loaded in these lanes corresponds to the totality of cell extracts in comparison with 5% for the supernatants (Fig. 1, pellet). We conclude that human Pit2 was efficiently solubilized in 1% Triton X-100-NH buffer and that high-molecular-mass Pit2 signals were not generated by insoluble aggregates. Detection in the presence of β-mercaptoethanol indicated that they did not involve disulfide bounds.
FIG. 1.
Detection of human Pit2 by SDS-PAGE and Western blotting. CHO (−) and CHO-Pit2-V (+) cells were grown in the presence of 1 mM Pi. Cell extracts were prepared in 1% Triton X-100-NH buffer and analyzed by Western blotting using a rabbit anti-human Pit2 serum. For the supernatants, 5% of total cell extract volume or of the volume of supernatants from a centrifugation at 13,000 × g for 30 min or at 100,000 × g for 1 h was analyzed. For the pellets, all material pelleted from cell extracts was analyzed. Samples were not heated before loading on the gel. Pit2 species are labeled a, b, and c. Molecular mass markers are in kilodaltons.
High-molecular-mass Pit2 signals were equally well revealed when Triton X-100 (a nonionic analog of lysophospholipid) was replaced by other detergents, including n-octylglucosid (an analog of glycolipid), deoxycholic acid (an anionic analog of cholesterol), and CHAPS (a zwitterionic analog of cholesterol) (not shown). Thus, bands b and c signals appeared at least partially resistant to detergent denaturation.
We concluded that high-molecular-mass Pit2 signals could be regarded either as artifacts resulting from the formation of soluble Pit2 aggregates in cell extracts or as an indication that Pit2 forms assemblies in living cells with potential biological relevance. We addressed this question by performing chemical cross-linking experiments in living cells.
Chemical cross-linking.
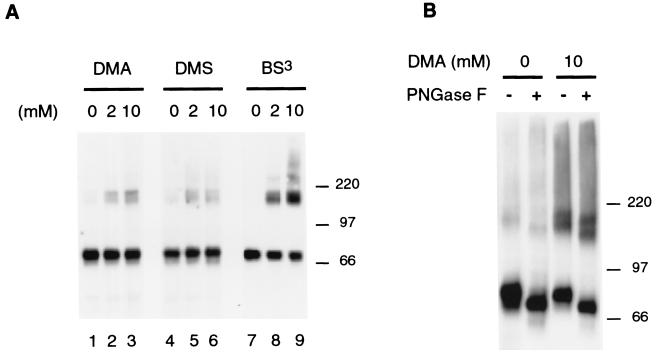
Chemical cross-linking reagents form covalent links between neighboring peptides. They can be applied to living cells. Intact CHO-Pit2-V cells were exposed to cross-linking reagents, and cell extracts were treated with TFA, an organic solvent that destroys all noncovalent protein interactions. Pit2 signals were then analyzed by Western blotting. In the absence of prior treatment with a cross-linking reagent, high-molecular-mass Pit2 signals disappeared when cell extracts were treated with TFA (Fig. 2a, lanes 1, 4, and 7), indicating that they do not involve covalent peptidic links. Living cells were exposed to membrane-permeable (DMA or DMS) or to membrane-impermeable (BS3) cross-linking reagents. These chemicals form covalent links between peptides located within a distance of 8.6 to 11.4 Å. A signal similar to band b was detected in TFA-treated extracts from cells incubated with the cross-linking reagents (Fig. 2a). The intensity of this signal increased with DMA and BS3 concentration. Signals of higher molecular mass than band b, possibly corresponding to band c, were detected with 10 mM BS3. At least with BS3, the appearance of high-molecular-mass signals was accompanied by a reduction of the intensity of the monomeric 73-kDa Pit2 signal. This experiment shows that Pit2 molecules can be cross-linked in living cells, indicating that they are located in a very close environment. This result suggests that the b and c signals revealed in intact CHO-Pit2-V cells may correspond, at least in part, to these molecular complexes. Data obtained with BS3, which was the most efficient cross-linking reagent in these experiments, indicate that Pit2 assemblies may be as abundant as Pit2 monomers and that they are located at the cell surface.
FIG. 2.
Cross-linking of human Pit2. CHO-Pit2-V cells grown in the presence of 1 mM Pi were incubated without cross-linking reagent (0) or with a cross-linking reagent in NH buffer for 30 min at 4°C before lysis with 1% Triton X-100-NH buffer. Five micrograms of protein of the supernatant of a centrifugation at 100,000 × g for 1 h were treated with TFA before resuspension in Laemmli sample buffer and analysis by SDS-PAGE. Pit2 signals were revealed by Western blotting using a rabbit anti-Pit2 serum. (A) Cells were exposed to a 2 or 10 mM concentration of a membrane-permeable (DMA and DMS) or membrane-impermeable (BS3) reagent. (B) Cells were exposed to 10 mM DMA and protein extracts were treated (+) or not treated (−) with PNGase F (1,000 U), an enzyme that hydrolyzes N-linked oligosaccharide chains. Molecular mass markers are in kilodaltons.
To further investigate the nature of cross-linked materials, extracts of CHO-Pit2-V cells treated with 10 mM DMA were treated with PNGase F, an enzyme that hydrolyzes N-linked oligosaccharide chains. We have previously shown that human Pit2 is N-glycosylated on asparagine 81 (48). PNGase treatment shifted the migration of Pit2 monomers to a slightly lower molecular mass and did similarly on cross-linked material, consistently with the presence of human Pit2 molecules in these complexes (Fig. 2b).
Coimmunoprecipitation of differently tagged Pit2 molecules.
The apparent molecular mass of band b (150 kDa), as well as that of the majority of the cross-linked material, was consistent with either the association of two Pit2 molecules or the association of Pit2 with a protein of equal molecular mass. We therefore examined whether Pit2 actually forms homodimers.
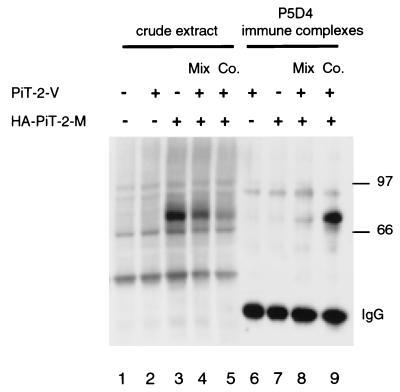
An expression vector was constructed that encodes human Pit2 with an influenza HA epitope tag fused at the N-terminal extremity and a Myc epitope tag fused at the C-terminal extremity (HA-Pit2-M) (48). HA-Pit2-M confers susceptibility to A-MLV infection when expressed in CHO cells, indicating that the tagging epitopes did not alter biological activity (data not shown). Naive CHO cells were transiently transfected with either Pit2-V, HA-Pit2-M, or both vectors. Crude extracts of transfected cells were analyzed by Western blotting after TFA treatment. A polyclonal anti-Pit2 serum directed against the intracellular loop detected equal amounts of Pit2 in all extracts (not shown). The 9E10 anti-Myc MAb revealed a 73-kDa signal in cells transfected with HA-Pit2-M alone (Fig. 3, lane 3) and a less intense signal when cells were transfected with both vectors (Fig. 3, lane 5) or when extracts of cells transfected with HA-Pit2-M alone were mixed with equal amounts of extracts from cells transfected with Pit2-V alone (Fig. 3, lane 4). A fraction of these extracts was incubated with P5D4, a MAb directed against the VSV-G tag borne by Pit2-V. Immune complexes were precipitated and analyzed by Western blotting after TFA treatment for the presence of the Myc tag. A 73-kDa Pit2 specific signal was detected only when both vectors had been transfected in the same cells (Fig. 3, lane 9). The signal indicated that molecular complexes bearing the Myc epitope had been immunoprecipitated together with Pit2 molecules bearing the VSV-G epitope. Association between Pit2-V and HA-Pit2-M molecules did not occur when expression vectors were introduced into different cells, and these cells mixed together before lysis (Fig. 3, lane 8). These results demonstrate that Pit2-V and HA-Pit2-M have the capacity to form homo-oligomers in transiently transfected CHO cells. Reciprocal coimmunoprecipitation experiments could not be performed because P5D4 does not recognize Pit2-V on Western blots.
FIG. 3.
Coimmunoprecipitation of two differently tagged versions of Pit2. CHO cells were transfected (+) or not transfected (−) either with 6 μg of plasmid DNA encoding Pit2-V (a version of human Pit2 with a C-terminal VSV-G tag) or HA-Pit2-M (a version of human Pit2 with an N-terminal influenza virus HA tag and a C-terminal Myc tag) or with 3 μg of each plasmid DNA (Co.). Cell extracts were prepared 24 h later in 1% Triton X-100-NH buffer at 4°C from individual cultures or from a mixture of cells that received one of each plasmid DNA (Mix). Thirty micrograms of protein of each extract were treated by TFA and analyzed by Western blotting for the detection of the Myc tag using the MAb 9E10 (crude extracts). Myc tag signals are visible in lanes 3, 4, and 5. Less-intense signals in lanes 4 and 5 were due to the dilution of the HA-Pit2-M with equal amounts of Pit2-V. Aliquots of 100 μl of each extract were incubated overnight with the anti-VSG MAb P5D4 and immunoprecipitated with protein G-coated agarose beads (P5D4 immune complexes). Immune complexes were then eluted from beads and treated with TFA before analysis by Western blotting for the detection of the Myc tag. An intense Myc signal is visible in lane 9 only. Signals labeled IgG correspond to the detection of eluted P5D4 heavy chains. Molecular mass markers are in kilodaltons.
Pit2 oligomers are modulated by extracellular [Pi].
We previously reported that the activity of Pit2 as a sodium-phosphate transporter and as the A-MLV receptor is rapidly modulated in response to changes in extracellular [Pi] (46). Modulation occurs within minutes and without change in the number of cell surface Pit2 molecules. Though posttranslational modifications are presumably associated with the modulation of Pit2 activity, they have not been identified so far. We examined whether a relationship could be found between the detection of cell surface Pit2 assemblies and Pit2 activity as a retrovirus receptor.
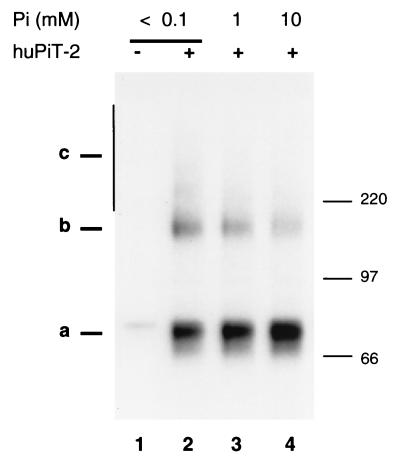
CHO-Pit2-V cells grown in the presence of physiological [Pi] (1 mM) were switched for 30 min in culture medium containing low-concentration (<0.1 mM) or high-concentration (10 mM) phosphate medium. Susceptibility to A-MLV infection was measured by exposing cells to an amphotropic MLV vector carrying the E. coli lacZ gene and scoring β-galactosidase-positive cell foci 48 h later. Consistently with our previous observations (46), infection was twice as efficient at 0.1 mM Pi (52 × 105 foci/ml) than at 10 mM Pi (21 × 105 foci/ml). Pit2 expression was examined by Western blottings in a similar experiment. Cells were treated with cycloheximide (100 μg/ml) for 1 h before switching [Pi] and during incubation in modified [Pi], with the aim of blocking protein neosynthesis. Small amounts of proteins were loaded on gels in order to facilitate the detection of variations in the intensity of Pit2 monomer signals and high-molecular-mass forms. In these conditions, Pit2 oligomers were mostly detected as 150-kDa species, presumably representing Pit2 dimers. Figure 4 shows that the intensity of Pit2 dimer signals decreased and that of Pit2 monomer signals increased when [Pi] was raised.
FIG. 4.
Detection of human Pit2 by SDS-PAGE after cell incubation with various [Pi]. CHO (−) and CHO-Pit2-V (+) cells were grown in the presence of 1 mM Pi. Cycloheximide (100 μg/ml) was added to culture medium for 1 h, then cells were washed with NH buffer and incubated in the presence of <0.1, 1, or 10 mM Pi and cycloheximide in culture medium for 30 min. Cell extracts were prepared in 1% Triton X-100-NH buffer and analyzed by Western blotting using a rabbit anti-human Pit2 serum. Pit2 species are indicated as a, b, and c signals. Molecular mass markers are in kilodaltons. The membrane was scanned for light emission, and the ratios of dimer-to-monomer signal intensities were measured: lane 2, 30%; lane 3, 20%; and lane 4, 10%.
In order to confirm this observation and to verify that changes of Pit2 oligomerization were associated with changes of [Pi] and not of another ion, a similar experiments was performed in which both the extracellular concentrations of Pi and that of sodium sulfate were modified. Results shown in Fig. 5 indicated that low [Pi] was associated with increased amounts of high-molecular-mass Pit2 signals, whereas changes in sulfate concentration had no effect.
FIG. 5.
Detection of human Pit2 by SDS-PAGE after cell incubation at various Pi and Na2SO4 concentrations . CHO (−) and CHO-Pit2-V (+) cells were grown in the presence of 1 mM Pi, washed with NH buffer, and incubated with various concentrations of phosphate and sulfate in culture medium for 30 min. Cell extracts were prepared in 1% Triton X-100-NH buffer and analyzed by Western blotting using a rabbit anti-human Pit2 serum. Pit2 species are indicated as a, b, and c signals. The film was overexposed for a better analysis of the b and c signals. Molecular mass markers are in kilodaltons.
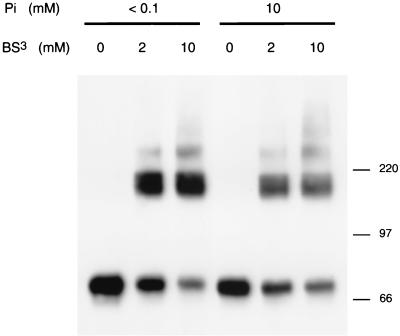
To examine whether changes in extracellular [Pi] affect cell surface Pit2 assemblies in living cells, CHO-Pit2-V cells grown in the presence of physiological [Pi] were switched to <0.1 or 10 mM Pi for 30 min and treated with the cross-linking reagent BS3 at the defined [Pi]. Cell extracts were treated with TFA before analysis by Western blotting. Results showed decreased monomer signals and increased oligomer signals with increasing BS3 concentrations at both Pi concentrations. However, for equivalent concentration of BS3, the amounts of cross-linked species were higher at low [Pi] than at high [Pi] (Fig. 6). This experiment indicates that more Pit2 molecules forming assemblies at the surface of living cells were accessible to BS3 when cells were phosphate deprived.
FIG. 6.
Cross-linking of human Pit2 at various [Pi]. CHO-Pit2-V cells were grown in the presence of 1 mM Pi, washed with NH buffer, and incubated in the presence of <0.1 or 10 mM Pi in culture medium for 30 min. Cells were then exposed to 2 or 10 mM concentrations of the cross-linking reagent BS3 in NH buffer containing 0 or 10 mM Pi for 30 min at 4°C or were not treated. Cells were lysed with 1% Triton X-100-NH buffer. Five micrograms of protein of the supernatant of a centrifugation at 100,000 × g for 1 h was treated with TFA before resuspension in Laemmli sample buffer and analysis by SDS-PAGE. Pit2 signals were revealed by Western blotting using a rabbit anti-Pit2 serum. Molecular mass markers are in kilodaltons.
DISCUSSION
Pit2 is a highly hydrophobic 12-transmembrane receptor (48). The propensity of Pit2 to form molecular aggregates after detergent solubilization and heating of cell extracts has been previously reported (8). Hydrophobic interactions resistant to SDS could therefore account for the observation of high-molecular-mass signals reacting with anti-Pit2 antibodies in extracts of CHO-Pit2-V cells, even in the absence of heating and although Pit2 is not overexpressed in these cells. Disappearance of these signals after TFA treatment indicated that they do not involve covalent links, thus opening the possibility to investigate the action of chemical cross-linking reagents. Results obtained with BS3, a membrane-impermeable chemical forming covalent links between amine residues located 11.4 Å apart at the cell surface, were especially demonstrative, as they indicated that Pit2 molecules participate to molecular complexes at the plasma membrane of living cells. In physiological conditions, a majority of cell surface Pit2 molecules reacted with BS3, forming covalently linked assemblies. Pit2 signals with an equivalent high molecular mass were also detected in extracts of cells not subjected to cross-linking reaction and not treated with TFA. They likely represent Pit2 assemblies partly resistant to detergent denaturation. The conservation of molecular interactions during SDS-PAGE has previously been reported for other transmembrane proteins, such as NHE1 and NHE3 (17), mASCT2 (38), SNAREs (31), integrins (59), and phospholamban (11). However, we cannot exclude that nonspecific aggregates of Pit2 also participate in these signals.
The most abundant cross-linked species detected by anti-Pit2 antibodies, as well as the most abundant high-molecular-mass signals observed in Western blottings performed in the absence of TFA and cross-linking reaction (the b signal), migrated as 150-kDa complexes. As this apparent molecular mass corresponds to the expected size of Pit2 homodimers, we searched for evidence indicating that Pit2 could self-associate. The association of Pit2 molecules bearing different tags was demonstrated by coimmunoprecipitation, indicating that Pit2 has the capacity to form homodimers. However, as the detection of coimmunoprecipitated species required transient overexpression of each tagged version of Pit-2, we cannot rule out that self-association was facilitated or even forced in experimental conditions. Control experiments indicated that if this happened, Pit2 assemblies were formed in living cells and not in cell extracts (Fig. 3, lanes 8 and 9). Nevertheless, the possibility remains that Pit2 associates with proteins with molecular masses of 70 kDa that are not Pit2. Efforts are presently under way to identify such proteins.
Human Pit2 does not contain sequence that could form coiled-coil or leucine zipper structures, as frequently found in cytosolic or nuclear proteins forming homo- or heterodimers. Crystallography studies have shown that the sequence GXXXG (in which G indicates a glycine and X indicates any amino acid), which is present in the transmembrane domains of glycophorine A, is an important motif for the dimerization of this molecule (36). This sequence has also been recognized as a transmembrane dimerization motif in other proteins (51). Three GXXXG motifs can be found in the transmembrane domains of Pit2 (respectively in TM-IV, TM-V, and TM-IX, according to the topology that we proposed previously [48]), suggesting that interactions between Pit2 molecules might involve transmembrane regions. Alternatively, with respect to the fact that Pit2 is linked to the actin network (46), cytoskeletal proteins could mediate interactions between two or several Pit2 molecules and actin filaments. Interactions between multitransmembrane proteins may also involve lipids (6). Structural studies of bacteriorhodopsin revealed trimers with lipids binding to and linking monomers, suggesting that lipids stabilize bacteriorhodopsin trimers in membranes (16, 19, 35).
Oligomerization has been described for few transporters, including NaPi-II phosphate transporters (4, 24, 32, 57), the erythrocytic glucose transporter GLUT1 (10, 22, 60), the NA+/H+ exchangers NHE1 and NhaA (13, 17, 20, 55), the serotonin transporter (28), the brain glutamate transporters GLAST, GLT, and EAAC (21), and the lactose permease of Streptococcus (18, 53). Whether oligomerization is important for transporter function remains uncertain in most cases. By analogy with the mechanisms governing the fusion of influenza virus with cell membranes, it is thought that receptor assembly is important for triggering retrovirus entry. Fusion mediated by the influenza protein HA results from the formation of an assembly of at least eight molecules, of which only two or three must undergo conformational changes (5). A similar mechanism is suspected for HIV. Cell infection with HIV requires the cooperation of four to six CCR5 molecules (30). In cells that contain only a trace of coreceptors and a vast excess of CD4, the formation of ternary complexes implies recruiting distant coreceptors (42). A direct link between syncitia formation and receptor assembly has also been proposed for MLVs (49), as well as indirect evidence for receptor cooperation in the processing of particle entry (3, 33).
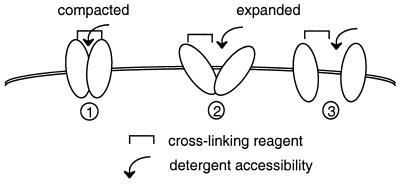
Cross-linking studies with BS3 revealed that signals corresponding to Pit2 oligomers were more intense in cells that had been incubated in medium containing less than 0.1 mM Pi prior to and during exposure to the cross-linking reagent than in cells incubated in the presence of 10 mM Pi. Variation of signal intensity was in the range of twofold, occurred within few minutes, and took place at the cell surface. This observation suggests that more cell surface Pit2 assemblies were accessible to BS3 cross-linking when extracellular [Pi] was low. We interpret these data as an indication that at least a fraction of cell surface Pit2 oligomers underwent conformational changes in response to the modification of extracellular [Pi]. Similarly, analysis of cell extracts in the absence of cross-linking reaction and TFA treatment revealed that Pit2 signals corresponding to oligomers were more intense after cell incubation at <0.1 mM than at 1 mM Pi and more at 1 mM than at 10 mM Pi. We assume that these changes could result from conformational modifications affecting susceptibility to detergent denaturation, as has been shown for integrins (59). These changes occurred in the absence of protein neosynthesis and were likely reversible. Thus, Pit2 assemblies, especially those located at the cell surface, may switch between two conformational configurations. In the configuration predominating at low [Pi], the proportion of individual Pit2 molecules that can be cross-linked is high and the accessibility to detergent is restricted, whereas the opposite is true for the configuration predominating at high [Pi]. A model can be proposed in which Pit2 assemblies switch between compacted and relaxed configurations depending on extracellular [Pi] (Fig. 7).
FIG. 7.
Schematic representation of the configuration of cell surface Pit2 assemblies at various [Pi]. (1) At low [Pi], most Pit2 assemblies are in a compact configuration. The proximity of Pit2 monomers allows cross-linking of monomers and restricts accessibility to detergent. This configuration is associated with active phosphate uptake and is compatible with A-MLV particle entry. (2 and 3) At higher [Pi], more Pit2 assemblies adopt a relaxed configuration. Distance between monomers impairs efficient cross-linking and facilitates accessibility to detergent. These configurations are associated with inactive phosphate uptake and are not suitable for A-MLV entry. The expanded configuration may be viewed either as shown in schema 2, where assemblies are maintained, or as shown in schema 3, where they are fully dissociated.
The detection of larger amounts of Pit2 assemblies was associated with increased susceptibility to A-MLV infection. Similarly, we showed previously that the uptake of extracellular radiolabeled phosphate is also more active in phosphate-deprived conditions (46). The relationships between the cell surface configurations of Pit2 and the biological activity of the transporter are unclear at this point. There are only few reports showing that the biological activity of a transporter can be affected by the modulation of cell surface assemblies. Assembly and disassembly with stomatin determine the activity of the glucose transporter GLUT-1 (58). Calcium transport through the sarcoplasmic Ca2+-ATPase is regulated through a balance between the amounts of monomers and oligomers, which is controlled by the phosphorylation of phospholamban (11, 50). As an alternative point of view, one may consider that the increase detection of Pit2 oligomers is the consequence, and not the cause, of the activation of phosphate uptake in conditions of phosphate deprivation. Active phosphate uptake would require that Pit2 assemblies are in a compact configuration that is more efficiently detected by cross-linking reagents than the inactive relaxed state. It is known that transporters cycle between two conformational states with their substrate binding site alternatively exposed outside and inside the cell. The compact and relaxed configurations of Pit2 may correspond to the exposure of the phosphate binding site outside and inside the cell, respectively. Phosphate deprivation would favor exposure of the substrate binding site, and thus of the compact configuration, at the cell surface. This configuration may be more favorable to inducing viral envelope rearrangements required for membrane fusion triggering.
Acknowledgments
We are grateful to Shawn Kuhmann for the generous gift of the rabbit anti-human Pit2 serum and to D. Kabat for helpful discussions.
This work was supported by grants from the Agence National de Recherche contre le SIDA (ANRS). C.S. is a fellow of the Ministère de l'Enseignement Supérieur et de la Recherche, and E.G. is a fellow of the Fondation pour la Recherche Médicale.
REFERENCES
- 1.Alfano, M., H. Schmidtmayerova, C.-A. Amella, T. Pushkarsky, and M. Bukrinsky. 1999. The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains. J. Exp. Med. 190:597-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardin, S. D., R. T. Voegele, and T. M. Finan. 1998. Phosphate assimilation in Rhizobium (Sinorhizobium) meliloti: identification of a pit-like gene. J. Bacteriol. 180:4219-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini, J. L., P. Rodrigues, R. Müller, O. Danos, and J. M. Heard. 1996. Receptor-binding properties of a purified fragment of the 4070A amphotropic murine leukemia virus envelope glycoprotein. J. Virol. 70:4387-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beliveau, R., M. Demeule, H. Ibnoul-Khatib, M. Bergeron, G. Beauregard, and M. Potier. 1988. Radiation-inactivation studies on brush-border-membrane vesicles. General considerations, and application to the glucose and phosphate carriers. Biochem. J. 252:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentz, J. 2000. Membrane fusion mediated by coiled coils: a hypothesis. Biophys. J. 78:886-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breyton, C., C. Tribet, J. Olive, J. P. Dubacq, and J. L. Popot. 1997. Dimer to monomer conversion of the cytochrome b6 f complex. Causes and consequences. J. Biol. Chem. 272:21892-21900. [DOI] [PubMed] [Google Scholar]
- 7.Chaudry, G. J., K. B. Farrel, Y.-T. Ting, C. Schmitz, Y. S. Lie, C. J. Petropoulos, and M. V. Eiden. 1999. Gibbon ape leukemia virus receptor functions of type III phosphate transporters from CHO-K1 are disrupted by two distinct mechanisms. J. Virol. 73:2916-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien, M. L., J. L. Foster, J. L. Douglas, and J. V. Garcia. 1997. The amphotropic murine leukemia virus receptor gene encodes a 71-kilodalton protein that is induced by phosphate depletion. J. Virol. 71:4564-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien, M. L., E. O'Neill, and J. V. Garcia. 1998. Phosphate depletion enhances the stability of the amphotropic murine leukemia virus receptor mRNA. Virology 240:109-117. [DOI] [PubMed] [Google Scholar]
- 10.Coderre, P. E., E. K. Cloherty, R. J. Zottola, and A. Carruthers. 1995. Rapid substrate translocation by the multisubunit, erythroid glucose transporter requires subunit associations but not cooperative ligand binding. Biochemistry 34:9762-9773. [DOI] [PubMed] [Google Scholar]
- 11.Cornea, R. L., L. R. Jones, J. M. Autry, and D. D. Thomas. 1997. Mutation and phosphorylation change the oligomeric structure of phospholamban in lipid bilayers. Biochemistry 36:2960-2967. [DOI] [PubMed] [Google Scholar]
- 12.Cosset, F. L., Y. Takeuchi, J. L. Battini, R. A. Weiss, and M. K. Collins. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Counillon, L., and J. Pouyssegur. 2000. The expanding family of eucaryotic Na+/H+ exchangers. J. Biol. Chem. 275:1-4. [DOI] [PubMed] [Google Scholar]
- 14.Daram, P., S. Brunner, C. Rausch, C. Steiner, N. Amrhein, and M. Bucher. 1999. Pht2;1 encodes a low-affinity phosphate transporter from Arabidopsis. Plant Cell 11:2153-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elvin, C. M., N. E. Dixon, and H. Rosenberg. 1986. Molecular cloning of the phosphate (inorganic) transport (pit) gene of Escherichia coli K12. Identification of the pit+ gene product and physical mapping of the pit-gor region of the chromosome. Mol. Gen. Genet. 204:477-484. [DOI] [PubMed] [Google Scholar]
- 16.Essen, L., R. Siegert, W. D. Lehmann, and D. Oesterhelt. 1998. Lipid patches in membrane protein oligomers: crystal structure of the bacteriorhodopsin-lipid complex. Proc. Natl. Acad. Sci. USA 95:11673-11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fafournoux, P., J. Noel, and J. Pouyssegur. 1994. Evidence that Na+/H+ exchanger isoforms NHE1 and NHE3 exist as stable dimers in membranes with a high degree of specificity for homodimers. J. Biol. Chem. 269:2589-2596. [PubMed] [Google Scholar]
- 18.Friesen, R. H., J. Knol, and B. Poolman. 2000. Quaternary structure of the lactose transport protein of Streptococcus thermophilus in the detergent-solubilized and membrane-reconstituted state. J. Biol. Chem. 275:33527-33535. [DOI] [PubMed] [Google Scholar]
- 19.Fyfe, P. K., K. E. McAuley, A. W. Roszak, N. W. Isaacs, R. J. Cogdell, and M. R. Jones. 2001. Probing the interface between membrane proteins and membrane lipids by X-ray crystallography. Trends Biochem. Sci. 26:106-112. [DOI] [PubMed] [Google Scholar]
- 20.Gerchman, Y., A. Rimon, M. Venturi, and E. Padan. 2001. Oligomerization of NhaA, the Na+/H+ antiporter of Escherichia coli in the membrane, and its functional and structural consequences. Biochemistry 40:3403-3412. [DOI] [PubMed] [Google Scholar]
- 21.Haugeto, O., K. Ullensvang, L. M. Levy, F. A. Chaudhry, T. Honore, M. Nielsen, K. L. Lehre, and N. C. Danbolt. 1996. Brain glutamate transporter proteins form homomultimers. J. Biol. Chem. 271:27715-27722. [DOI] [PubMed] [Google Scholar]
- 22.Hebert, D. N., and A. Carruthers. 1992. Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependant interconversions of tetrameric and dimeric GLUT1. J. Biol. Chem. 267:23829-23838. [PubMed] [Google Scholar]
- 23.Iyengar, S., J. E. K. Hildreth, and D. Schwartz. 1998. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J. Virol. 72:5251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jette, M., V. Vachon, M. Potier, and R. Béliveau. 1996. The renal sodium/phosphate symporters: evidence for different functional oligomeric states. Biochemistry 35:15209-15214. [DOI] [PubMed] [Google Scholar]
- 25.Jobbagy, Z., Z. Olah, G. Petrovics, M. V. Eiden, B. D. Leverett, N. M. Dean, and W. B. Anderson. 1999. Up-regulation of the Pit-2 phosphate transporter/retrovirus receptor by protein kinase Cɛ. J. Biol. Chem. 274:7067-7071. [DOI] [PubMed] [Google Scholar]
- 26.Kadan, M. J., S. Sturm, W. F. Anderson, and M. A. Eglitis. 1992. Detection of receptor-specific murine leukemia virus binding to cells by immunofluorescence analysis. J. Virol. 66:2281-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavanaugh, M. P., D. G. Miller, W. Zhang, W. Law, S. L. Kozak, D. Kabat, and A. D. Miller. 1994. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. USA 91:7071-7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilic, F., and G. Rudnik. 2000. Oligomerization of serotonin transporter and its functional consequences. Proc. Natl. Acad. Sci. USA 97:3106-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohler, K., I. C. Forster, G. Lambert, J. Biber, and H. Murer. 2000. The functional unit of the renal type IIa Na+/Pi cotransporter is a monomer. J. Biol. Chem. 275:26113-26120. [DOI] [PubMed] [Google Scholar]
- 30.Kuhmann, S. E., E. J. Platt, S. L. Kozak, and D. Kabat. 2000. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74:7005-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laage, R., J. Rohde, B. Brosig, and D. Langosch. 2000. A conserved membrane-spanning amino acid motif drives homomeric and supports heteromeric assembly of presynaptic SNARE proteins. J. Biol. Chem. 275:17481-17487. [DOI] [PubMed] [Google Scholar]
- 32.Lambert, G., M. Traebert, J. Biber, and H. Murer. 2000. Cleavage of disulfide bonds leads to inactivation and degradation of the type IIa, but not type IIb, sodium phosphate cotransporter expressed in Xenopus laevis oocytes. J. Membr. Biol. 176:143-149. [DOI] [PubMed] [Google Scholar]
- 33.Lavillette, D., B. Boson, S. J. Russell, and F. L. Cosset. 2001. Activation of membrane fusion by murine leukemia viruses is controlled in cis or in trans by interactions between the receptor-binding domain and a conserved disulfide loop of the carboxy terminus of the surface glycoprotein. J. Virol. 75:3685-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehel, C., Z. Olah, H. Mischak, J. F. Mushinski, and W. B. Andersen. 1994. Overexpressed protein kinase C-delta and -epsilon subtypes in NIH-3T3 cells exhibit differential subcellular localization and differential regulation of sodium-dependant phosphate uptake. J. Biol. Chem. 269:4761-4766. [PubMed] [Google Scholar]
- 35.Luecke, H., B. Schobert, H. T. Richter, J. P. Cartailler, and J. K. Lanyi. 1999. Structure of bacteriorhodopsin at 1.55 Å resolution. J. Mol. Biol. 291:899-911. [DOI] [PubMed] [Google Scholar]
- 36.MacKenzie, K. R., J. H. Prestegard, and D. M. Engelman. 1997. A transmembrane helix dimer: structure and implications. Science 276:131-133. [DOI] [PubMed] [Google Scholar]
- 37.Mansilla, M. C., and D. de Mandoza. 2000. The Bacillus subtilis cysP gene encodes a novel sulphate permease related to the inorganic phosphate transporter (Pit) family. Microbiology 146:815-821. [DOI] [PubMed] [Google Scholar]
- 38.Marin, M., C. S. Tailor, A. Nouri, and D. Kabat. 2000. Sodium-dependent neutral amino acid transporter type 1 is an auxiliary receptor for baboon endogenous retrovirus. J. Virol. 74:8085-8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez, P., and B. L. Persson. 1998. Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol. Gen. Genet. 258:628-638. [DOI] [PubMed] [Google Scholar]
- 40.Miller, D. G., and A. D. Miller. 1992. Tunicamycin treatment of CHO cells abrogates multiple blocks to retrovirus infection, one of which is due to a secreted inhibitor. J. Virol. 66:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olah, Z., C. Lehel, and W. B. Andersen. 1993. Differential effects of activation of protein kinase C and cyclic-AMP-dependant protein kinase on sodium-dependant phosphate uptake in NIH 3T3 cells. Biochem. Biophys. Acta 1176:333-338. [DOI] [PubMed] [Google Scholar]
- 42.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raymond, J. R., A. Fargin, J. P. Middleton, J. M. Graff, D. M. Haupt, M. G. Caron, R. J. Lefkowitz, and V. W. Dennis. 1989. The human 5-HT1A receptor expressed in HeLa cells stimulates sodium-dependant phosphate uptake via protein kinase C. J. Biol. Chem. 264:21943-21950. [PubMed] [Google Scholar]
- 44.Raymond, J. R., J. P. Middleton, and V. W. Dennis. 1990. HeLa cells express cAMP-inhibitable sodium-dependant phosphate uptake. Am. J. Physiol. 258:F433-F437. [DOI] [PubMed] [Google Scholar]
- 45.Richardson, C., and A. Bank. 1996. Developmental-stage-specific expression and regulation of an amphotropic retroviral receptor in hematopoietic cells. Mol. Cell. Biol. 16:4240-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues, P., and J. M. Heard. 1999. Modulation of phosphate uptake and amphotropic murine leukemia virus entry by posttranslational modifications of PIT-2. J. Virol. 73:3789-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saier, M. H., B. H. Eng, S. Fard, J. Garg, D. A. Haggerty, W. J. Hutchinson, D. L. Jack, E. C. Lai, H. J. Liu, D. P. Nusinew, A. M. Omar, S. S. Pao, I. T. Paulsen, J. A. Quan, M. Sliwinski, T. Tseng, S. Wachi, and G. B. Young. 1999. Phylogenetic characterization of novel transport protein families revealed by genome analysis. Biochem. Biophys. Acta 1422:1-56. [DOI] [PubMed] [Google Scholar]
- 48.Salaün, C., P. Rodrigues, and J. M. Heard. 2001. Transmembrane topology of PiT-2, a phosphate transporter-retrovirus receptor. J. Virol. 75:5584-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siess, D. C., and S. L. K. Kozak, D. 1996. Exceptional fusogenicity of Chinese hamster ovary cells with murine retroviruses suggests roles for cellular factor(s) and receptor clusters in the membrane fusion process. J. Virol. 70:3432-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simmerman, H. K., and L. R. Jones. 1998. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol. Rev. 78:921-947. [DOI] [PubMed] [Google Scholar]
- 51.Ubarretxena-Belandia, I., and D. M. Engelman. 2001. Helical membrane proteins: diversity of functions in the context of simple architecture. Curr. Opin. Struct. Biol. 11:370-376. [DOI] [PubMed] [Google Scholar]
- 52.van Veen, H. W. 1997. Phosphate transport in prokaryotes: molecules, mediators and mechanisms. Antonie Leeuwenhoek 72:299-315. [DOI] [PubMed] [Google Scholar]
- 53.Veenhoff, L. M., E. H. Heuberger, and B. Poolman. 2001. The lactose transport protein is a cooperative dimer with two sugar translocation pathways. EMBO J. 20:3056-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Versaw, W. K., and R. L. Metzenberg. 1995. Repressible cation-phosphate symporters in Neurospora crassa. Proc. Natl. Acad. Sci. USA 92:3884-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams, K. A. 2000. Three-dimensional structure of the ion-coupled transport protein NhaA. Nature 403:112-115. [DOI] [PubMed] [Google Scholar]
- 56.Xiao, X., L. Wu, T. S. Stantchev, Y.-R. Feng, S. Ugolini, H. Chen, Z. Shen, J. L. Riley, C. C. Broder, Q. Sattentau, and D. S. Dimitrov. 1999. Constitutive cell surface association between CD4 and CCR5. Proc. Natl. Acad. Sci. USA 96:7496-7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao, Y., C. J. Boyer, E. Vincent, A. Dugre, V. Vachon, M. Potier, and R. Béliveau. 1997. Involvement of disulphide bonds in the renal sodium/phosphate co-transporter NaPi-2. Biochem. J. 323:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, J. Z., W. Abbud, R. Prohaska, and F. Ismail-Beigi. 2001. Overexpression of stomatin depresses GLUT-1 glucose transporter activity. Am. J. Physiol. Cell Physiol. 280:C1277-C1283. [DOI] [PubMed] [Google Scholar]
- 59.Zolotarjova, N. I., G. F. Hollis, and R. Wynn. 2001. Unusually stable and long-lived ligand-induced conformations of integrins. J. Biol. Chem. 276:17063-17068. [DOI] [PubMed] [Google Scholar]
- 60.Zottola, R. J., E. K. Cloherty, P. E. Coderre, A. Hansen, D. N. Hebert, and A. Carruthers. 1995. Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry 34:9734-9747. [DOI] [PubMed] [Google Scholar]