Abstract
CD4+ Th1 responses to virus infections are often necessary for the development and maintenance of virus-specific CD8+ T-cell responses. However, in the present study with Friend murine retrovirus (FV), the reverse was also found to be true. In the absence of a responder H-2b allele at major histocompatibility complex (MHC) class II loci, a single H-2Db MHC class I allele was sufficient for the development of a CD4+ Th1 response to FV. This effect of H-2Db on CD4+ T-cell responses was dependent on CD8+ T cells, as demonstrated by depletion studies. A direct effect of CD8+ T-cell help in the development of CD4+ Th1 responses to FV was also shown in vaccine studies. Vaccination of nonresponder H-2a/a mice induced FV-specific responses of H-2Dd-restricted CD8+ cytotoxic T lymphocytes (CTL). Adoptive transfer of vaccine-primed CD8+ T cells to naive H-2a/a mice prior to infection resulted in the generation of FV-specific CD4+ Th1 responses. This novel helper effect of CD8+ T cells could be an important mechanism in the development of CD4+ Th1 responses following vaccinations that induce CD8+ CTL responses. The ability of MHC class I genes to facilitate CD4+ Th1 development could also be considerable evolutionary advantage by allowing a wider variety of MHC genotypes to generate protective immune responses against intracellular pathogens.
Recovery from virus infections often requires the development of both cell-mediated and humoral immune responses which are regulated by CD4+ helper T cells. CD4+ helper T cells have been divided into two major functional types, Th1 and Th2. Th1 cells have an integral role in protective immunity against virus infections, providing help for the development and maintenance of both cytotoxic T lymphocyte (CTL) and neutralizing antibody responses and also directly suppressing virus replication by the secretion of gamma interferon (IFN-γ) (5, 23, 43). Th2 responses also provide help for the generation of neutralizing antibody responses but can suppress the development of antiviral Th1 responses through the production of immunosuppressive cytokines like interleukin-10 (IL-10) and/or transforming growth factor β (TGF-β) (8, 36). Thus, protection against retrovirus infection which requires both cell-mediated and humoral effector mechanisms (19) may be favored by the development of a Th1 rather than a Th2 CD4+ T-cell response.
Although many factors can direct CD4+ T cells toward a Th1 or Th2 response (9), the regulation of these responses during infection in vivo is not well understood. One factor that has been shown to affect the development of CD4+ Th1 or Th2 responses in vivo is the major histocompatibility complex (MHC) genotype of the host (34). In fact, MHC regulation of these responses can influence whether or not a host recovers from infection with a specific pathogen (2, 22). This has been best studied in mice, where the MHC genotypes of inbred strains can determine resistance or susceptibility to infection (28, 43). For example, in the mouse model of Friend retrovirus (FV) infection, the MHC genotype regulates recovery from FV-induced splenomegaly (18, 24). The infection of H-2a/a mice with FV induces rapid polyclonal erythroblast proliferation, which results in splenomegaly, erythroleukemia, and death (29). In contrast, H-2b/b mice initially develop splenomegaly but then spontaneously recover and live for a normal life span (6). Heterozygous H-2a/b mice can recover from FV-induced splenomegaly when they are infected with small amounts of virus (15 to 150 spleen focus-forming units [SFFU]) but do not recover after infection with large amounts of virus (1,500 SFFU) (18, 29). Recovery in H-2b/b and H-2a/b mice is dependent upon the generation of strong immune responses, including FV-specific CD8+ CTLs, neutralizing antibody, and CD4+ T cells (19).
The present study examined the effects of different subregions of MHC on FV-specific CD4+ T-cell subsets. As expected, an FV-specific Th1 response was associated with MHC class II genes of the H-2b haplotype, since MHC class II molecules present peptides which are antigenic to CD4+ T cells (33, 35). However, in the absence of H-2b alleles at the MHC class II loci, a strong FV-specific Th1 response was also associated with an H-2b allele at the MHC class I locus, H-2D. This surprising effect was mediated by CD8+ T cells, which appeared to exert a helper effect on the CD4+ T-cell response to this virus.
MATERIALS AND METHODS
Mice.
(C57BL/10 × A.BY)F1 (H-2b/b), (B10.A × A.BY)F1 (H-2a/b), (B10.A × AWySnJ)F1 (H-2a/a), (B10.A[2R] × AWySnJ)F1 (H-2h2/a), and (B10.A[18R] × AWySnJ)(H-2i18/a) mice were produced in the animal care facilities at Rocky Mountain Laboratories and were 8 to 16 weeks of age at experimental onset. Mice of parental strains C57BL/10, B10.A, AWySnJ, and A.BY were purchased from Jackson Laboratories (Bar Harbor, Maine). All animal experiments were carried out in accordance with the regulations of the Animal Care and Use Committee of the Rocky Mountain Laboratories and the guidelines of the National Institutes of Health.
Virus.
In most experiments, the B-tropic, polycythemia-inducing strain of FV (FV-B) was used for mouse inoculations (7). However, in some experiments, prior to infection with FV-B, (B10.A × AWySnJ)F1 mice were vaccinated with the N-tropic form of FV (FV-N), which acts like an attenuated immunogenic live-virus vaccine in this mouse strain (15). The virus used for animal inoculations consisted of a 10% (wt/vol) homogenate of spleens from FV-infected BALB/c mice prepared in 0.2 M phosphate-buffered saline (PBS) containing 2 mM EDTA (7, 11, 15). Infectious titers, expressed in SFFU per milliliter, were determined in a spleen focus assay as previously described (7). Mice were infected with 15 or 150 SFFU of FV by intravenous (i.v.) injection of virus stock diluted in phosphate-buffered balanced salt solution containing 2% fetal calf serum (FCS). Purified FV used for in vitro stimulation was prepared from concentrated culture supernatants from AA41 FV-erythroleukemia cells grown in the Cellmax artificial capillary cell culture system in Cellulosic MPS cartridges with a molecular mass cutoff of 30 kDa (Spectrum, Laguna Hills, Calif.) and purified as described below (4). AA41 cells were grown at high density (1 × 108 to 3 × 108 cells/cartridge) and were fed every other day with 1 liter of RPMI medium containing 2 × 10−5 M β-mercaptoethanol and 5% FCS. Supernatant (15 ml) was harvested from the cartridge daily, spun at 500 × g for 15 min to remove cells and debris, and frozen at −20°C. To collect virus particles, polyethylene glycol was added to pooled culture supernatants at a final concentration of 6%, and the resulting mixture was incubated overnight and centrifuged at 10,000 × g for 30 min. Pelleted virus was further purified over a 15 to 60% step sucrose gradient and a 15 to 60% continuous sucrose gradient (29), and the concentration was determined by an FV-specific enzyme-linked immunosorbent assay (ELISA) (38). Purified FV was inactivated by UV irradiation by incubating purified virus at a distance of 6 to 7 cm underneath a UV lamp for 20 min. Inactivation of FV was determined by spleen focus assay prior to the use of the virus in culture.
Cell separation.
T-cell subsets were purified by positive selection with the MidiMACS separation system (Miltenyi Biotech, Bergisch Gladbach, Germany). Spleen cells, depleted of red blood cells by lysis with ammonium chloride-Tris, were incubated with anti-CD4 magnetic beads for 15 min at 4°C. CD4+ cells were then selected by using LS+Midi columns according to the manufacturer's instructions. For the isolation of CD8+ T cells, anti-CD8 beads were used according to the protocol described above. Positively selected CD4+ and CD8+ T-cell populations were more than 94% positive for their specific cell types as determined by flow cytometry. Spleen cells (105) were stained directly with phycoerythrin-labeled anti-CD3 (145-2C11), fluorescein isothiocyanate- labeled anti-CD4 (GK1.5), fluorescein isothiocyanate-labeled anti-CD8 (Ly-2), and/or phycoerythrin-labeled anti-NK1.1 (PK136). All antibodies were purchased from BD Pharmingen (San Diego, Calif.). Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.).
Measurement of cytokine production by FV-specific CD4+ T cells.
Spleens were removed from H-2b/b, H-2a/b, H-2h2/a, H-2i18/a, and H-2a/a mice at 8 to 9 days postinfection with 15 or 150 SFFU of FV, prior to the development of splenomegaly. CD4+ T cells were purified from individual spleens with the Midi-MACS separation system. Purified CD4+ T cells were washed in RPMI medium with 10% FCS and counted. CD4+ T cells were cultured in 24-well plates at 3 × 106 spleen cells/ml/well with 3 × 106 irradiated syngeneic spleen cells from naive mice per well as antigen-presenting cells (APCs) and with or without 5 μg of purified FV per ml. Culture supernatants were harvested at 72 h and analyzed for cytokine production by antigen capture ELISA. For the detection of IFN-γ, anti-mouse IFN-γ monoclonal antibody (MAb) R4-6A2 (BD Pharmingen), diluted to 2 μg/ml in sodium carbonate buffer, was used as the coating antibody and biotinylated XMG1.2 (BD Pharmingen), diluted to 0.5 μg/ml in 2% bovine serum albumin-PBS, was used as the detection antibody. For IL-10 detection, anti-mouse IL-10 MAb JES5-2AS (BD Pharmingen) at 2 μg/ml was used as the coating antibody and biotinylated SXC-1 (BD Pharmingin) at 0.5 μg/ml was used as the detection antibody.
Treatment of mice with antibodies.
The anti-IFN-γ (XMG1.2)-producing hybridoma (American Type Culture Collection [ATCC]), the anti-IL-10 receptor (anti-IL-10R; 1B1.3a)-producing hybridoma (generous gift from Robert Coffman, DNAX, Palo Alto, Calif.), and the anti-CD8 (163.4)-producing hybridoma (ATCC) were grown in Cellmax artificial capillary cell culture systems (Spectrum) (4). Cells were grown at a high density (1 × 108 to 3 × 108 cells/cartridge) in Cellulosic MPS cartridges with a molecular mass cutoff of 30 kDa and were fed every other day with 1 liter of RPMI medium containing 1% FCS. Supernatant (15 ml) was harvested from the cartridge every other day, spun at 500 × g for 15 min to remove cells and debris, and frozen at −20°C. Supernatants were pooled, analyzed for antibody concentration and activity, and then aliquoted and stored at −20°C. For treatment of mice with anti-IFN-γ or anti-IL-10 R, mice were given 0.5 ml of the appropriate Cellmax supernatants or 0.5 ml of rat immunoglobulin (Ig) at 0.5 mg/ml every three days from the day of infection until day 21 postinfection.
In vivo depletion of CD8+ T cells.
CD8+ T cells were depleted from [B10.A(2R) × AWySnJ]F1 mice by treatment with anti-CD8 Cellmax supernatant (antibody 163.4) at 5, 3, and 1 day before infection with FV-B and at 2 and 5 days after infection. Depletion of CD8+ T cells from anti-CD8-treated [B10.A(2R) × AWySnJ]F1 mice was confirmed by flow cytometry analysis.
Transfer of CD8+ T cells.
(B10.A × AWySnJ)F1 mice were vaccinated with 150 SFFU of FV-N (15). At 8 days postvaccination, CD8+ T cells were purified from the spleens of either naive or vaccinated mice by positive selection with Midi-MACS beads. More than 95% of the purified CD8+ T cells were positive for both CD3 and CD8, and fewer than 2% were positive for CD4 or NK1.1. CD8+ T cells (1 × 107 to 1.5 × 107) from naive or vaccinated mice were transferred i.v. to naive (B10.A × AWySnJ)F1 mice. As an additional control, the flowthrough from the Medi-MACs columns (CD8-negative cells) was also transferred to naive (B10.A× AWySnJ)F1 mice. One day after transfer, all mice were infected with 15 SFFU of virulent FV-B. At 8 to 9 days postinfection, CD4+ T cells were purified and analyzed for FV-specific cytokine production.
Treatment of mice with IFN-γ.
H-2a/a mice were given 105 U of IFN-γ (Leinco Technologies, St. Louis, Mo.) in a 100-μl volume intraperitoneally. Mice were injected with IFN-γ on the day before infection with 15 SFFU of FV-B, on the day of infection, and on days 2, 4, and 6 postinfection.
RESULTS
Cytokine production by CD4+ T cells after infection with FV is influenced by MHC.
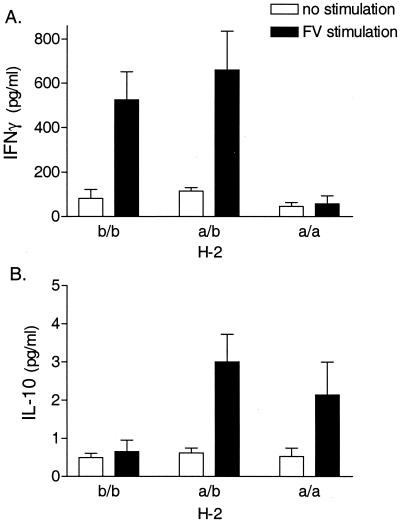
To determine the influence of the MHC genotype on FV-specific CD4+ T-cell responses, purified splenic CD4+ T cells from FV-infected congenic H-2b/b, H-2a/b, and H-2a/a mice were cultured with syngeneic APCs in the presence or absence of FV for 72 h. Culture supernatants were then analyzed for cytokine production by ELISA. As IFN-γ is an important factor in recovery from virus infections (36), we initially looked for FV-specific IFN-γ production by these cells. FV stimulation of CD4+ T cells from infected H-2b/b and H-2a/b mice induced a significant increase in IFN-γ production (Fig. 1A). In contrast, FV stimulation of CD4+ T cells from infected H-2a/a mice did not induce IFN-γ production (Fig. 1A). Thus, FV-specific production of IFN-γ by CD4+ T cells after infection with a low dose of FV required the presence of at least one H-2b gene.
FIG. 1.
FV-specific production of IFN-γ (A) and IL-10 (B) by purified CD4+ T cells. CD4+ T cells were purified from spleens of H-2b/b, H-2a/b, or H-2a/a mice at 8 days postinfection with FV-B. The CD4+ T cells were cultured with irradiated syngeneic spleen cells as APCs in the presence or absence of purified UV-inactivated FV. After 72 h, culture supernatants were analyzed for cytokine production by IFN-γ- or IL-10-specific ELISA. Data shown are the average values ± standard errors of the means (SEM) for four mice per group.
The predominant Th2 cytokine, IL-4, was not produced at levels detectable by ELISA by CD4+ T cells from any of the mice tested (data not shown). IL-2 and IL-5 were also not consistently detectable in the culture supernatants of these CD4+ T cells at 24, 48, or 72 h postculture (data not shown). However, FV stimulation of CD4+ T cells from H-2a/b and H-2a/a mice did induce IL-10 production (Fig. 1B), indicating the presence of either an FV-specific CD4+ Th2 or a regulatory Tr1 response (31). This increase in IL-10 production was not detected in CD4+ T cells from H-2b/b mice (Fig. 1B), indicating that an H-2a allele was necessary for FV-specific production of IL-10 by CD4+ T cells.
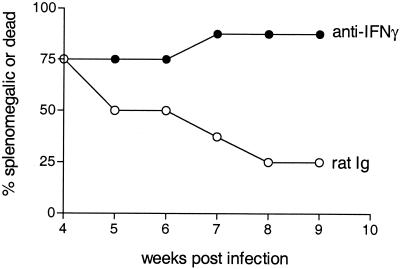
To determine the role of IFN-γ in recovery from FV-induced splenomegaly, we treated H-2a/b mice with the IFN-γ-neutralizing MAb XMG1.2 or a control antibody for 3 weeks after FV infection. At 9 weeks postinfection, >80% of H-2a/b mice treated with anti-IFN-γ still had FV-induced splenomegaly, compared to 25% of control mice treated with rat Ig (Fig. 2). Similar results were also observed after anti-IFN-γ treatment of H-2b/b mice, demonstrating that IFN-γ is an essential component for recovery from FV-induced splenomegaly (37). To determine if IL-10 was responsible for susceptibility to FV-induced erythroleukemia, H-2a/a mice were treated with an anti-IL-10R MAb, 1B1.3a, during the first 3 weeks after FV infection. Treatment with anti-IL-10R did not inhibit or enhance the development of splenomegaly in H-2a/a mice (data not shown). The same treatment protocol with anti-IL-10R has been shown to reduce nonspecific immunosuppression in FV-infected H-2a/a mice (13). Thus, the lack of an effect of anti-IL-10R on recovery from splenomegaly indicates that the production of IL-10 alone is not responsible for the lack of recovery in H-2a/a mice.
FIG. 2.
Anti-IFN-γ treatment of H-2a/b mice during infection with FV. H-2a/b mice were given either 0.5 ml of a 0.5-mg/ml concentration of rat Ig or anti-IFN-γ (XMG1.2) every 3 days starting on the day of infection until 4 weeks postinfection with FV-B. Splenomegaly was then followed by weekly palpations of all mice. Results are given as percentages of mice that were splenomegalic or dead (8 mice per group).
FV-specific CD4+ T-cell production of IFN-γ is influenced by MHC class I and class II genes.
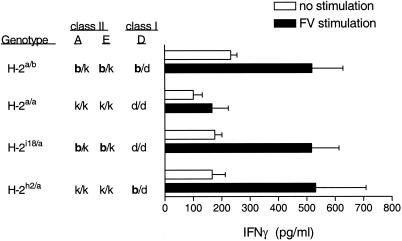
To determine whether the generation of IFN-γ-producing CD4+ T cells is controlled by the MHC class I or class II genes, mice with recombinations between the H-2E and H-2D loci were tested for FV-specific CD4+ T-cell responses. CD4+ T cells from FV-infected H-2i18/a mice (Kb/k Ab/k Eb/k Dd/d) produced significant levels of IFN-γ after in vitro restimulation (Fig. 3). As the MHC class I gene K does not appear to be involved in the immune response to FV (6, 30), these results demonstrated H-2b class II regulation of the CD4+ T-cell response.
FIG. 3.
Influence of MHC class I and class II genes on IFN-γ production. Purified CD4+ T cells from spleens of H-2a/b, H-2a/a, H-2h2/a, and H-2i18/a mice infected with FV-B were cultured with APCs and purified FV as described for Fig. 1. Data shown are the average values + SEM for four to eight mice per group.
H-2h2/a mice (Kk/k Ak/k Ek/k Db/d) differ from the non-IFN-γ-producing H-2a/a mice (Kk/k Ak/k Ek/k Dd/d) solely at the MHC class I H-2D loci (6). Surprisingly, CD4+ T cells from H-2h2/a mice did produce IFN-γ in response to FV stimulation (Fig. 3). This indicated that a single b allele at the H-2D class I gene was sufficient to generate an IFN-γ-producing CD4+ T-cell response, even in the absence of b alleles at the MHC class II genes. Other genes such as the gene coding for tumor necrosis factor alpha are segregated with H-2Db in recombinant H-2h2 mice (B10.A(2R)). However, the difference in the CD4+ T-cell responses between H-2h2/a mice and H-2a/a mice is most likely due to allelic differences in the H-2D gene, because in previous studies using bm14 mutant mice, FV-specific T-cell proliferation and recovery from FV-induced splenomegaly mapped to the H-2Db gene (18, 30, 32).
CD8+ T cells can influence the generation of FV-specific IFN-γ-producing CD4+ T cells.
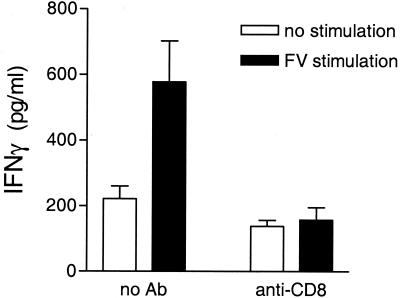
As CD8+ T cells, rather than CD4+ T cells, recognize antigen in the context of MHC class I molecules, the influence of H-2Db on the generation of IFN-γ-producing CD4+ T cells suggested an indirect effect by CD8+ T cells (6, 14). To test for effects of CD8+ T cells on FV-specific CD4+ T-cell responses in H-2h2/a mice, we depleted CD8+ T cells in these mice prior to infection with FV. CD4+ T cells purified from CD8-depleted mice did not produce IFN-γ upon restimulation in vitro, while CD4+ T cells from nondepleted mice did (Fig. 4). This effect did not appear to be due to the depletion of CD8+ CD11c+ dendritic cells, as the same treatment protocol that was used to deplete CD8+ cells in this study did not appear to reduce the number of CD8+ CD11c+ cells in the spleen. For example, in non-CD8-depleted mice, 0.26% of the total spleen cells were CD8+ CD11c+, while in anti-CD8-treated mice, 0.34 to 0.56% of the total spleen cells were CD8+ CD11c+, as measured by flow cytometry. Thus, the development of FV-specific IFN-γ-producing CD4+ T cells in H-2h2/a mice required the presence of CD8+ T cells.
FIG. 4.
Effect of anti-CD8 treatment of H-2h2/a mice on IFN-γ production by CD4+ T cells. H-2h2/a mice were given 0.5 ml of anti-CD8 Cellmax supernatant on days −5, −3, −1, +2, and +5 relative to the day of infection with 15 SFFU of FV-B. At 8 days postinfection, CD4+ T cells were purified from the spleens of infected mice and analyzed for IFN-γ production as described for Fig. 1. Data shown are the average values + SEM for eight mice per group and are the combined results of two separate experiments.
CD8+ T cells from vaccinated H-2a mice can influence IFN-γ production by CD4+ T cells.
The depletion studies described above suggest that CD8+ T cells may influence the development of CD4+ Th1 responses to FV. Therefore, we wanted to determine whether the transfer of CD8+ T cells could also induce CD4+ Th1 responses to FV. We could not use H-2h2/a mice for these studies, as they are already capable of making CD4+ Th1 responses to FV infection (Fig. 3). Instead, we used H-2a/a mice, as they do not normally generate CD4+ Th1 or CD8+ CTL responses to FV (Fig. 1) (6) but can be induced to generate protective immune responses (6) after vaccination with a live attenuated form of FV (FV-N) (10). Although H-2Dd-restricted CD8+ T cells probably interact with epitopes different than those of the H-2Db-restricted CD8+ T cells used in the studies described above, CD8+ T cells from vaccinated H-2a/a mice do lyse FV-transformed, H-2Dd target cells, indicating an antigen-specific Dd-restricted CTL response to FV (Table 1) (6). Therefore, we used CD8+ T cells from vaccinated H-2a/a mice to determine whether the transfer of primed CD8+ T cells could influence the generation of CD4+ Th1 responses to FV.
TABLE 1.
FV-specific CTL responses in H-2a/a mice after vaccination with FV-Na
| Infectious agent | No. of CTL-positiveb mice/total no. tested for:
|
|
|---|---|---|
| AA41 (KkDd) cells | D1B (KdDd) cells | |
| FV-B | 0/8 | 0/8 |
| FV-N | 10/22 | 9/22 |
H-2a/a mice [(B10.A × A/WySn)F1] were infected with 100 SFFU of FV-B or FV-N. At 14 to 21 days postinfection, spleen cells were used in a direct CTL assay with 51Cr-labelled target cells at a ratio of 200:1 (spleen cells/target cells). Assay mixtures were incubated for 19 h at 34°C (D1B target cells) or for 5 h at 37°C (AA41 target cells) as previously described (6). No lysis was observed when Y57-2C (H-2b) FV-transformed cells were used as targets for these H-2a/a mice in a 5-h, 37°C assay.
CTL responses were determined for AA41 and D1B target cells. See reference 6 for details.
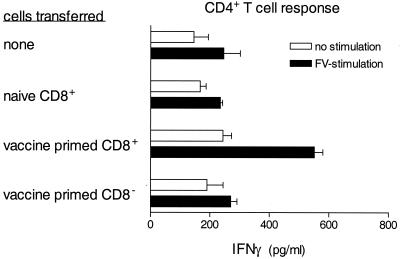
CD8+ cells were purified from the spleens of H-2a/a mice at 8 days after vaccination with the attenuated virus, FV-N. More than 95% of these cells were CD8+, and less than 2% were CD4+ or NK1.1+. More than 99.5% of the CD8+ cells were also positive for CD3+, indicating that almost all of these cells were CD8+ T cells. Purified CD8+ T cells from vaccinated and naive H-2a/a mice were adoptively transferred to naive H-2a/a mice prior to infection with virulent FV-B. At 9 days postinfection, CD4+ T cells from recipient mice were analyzed for FV-specific IFN-γ production. CD4+ T cells from H-2a/a mice given vaccine-primed CD8+ T cells produced IFN-γ in response to FV stimulation (Fig. 5). In contrast, those given CD8+ T cells from unvaccinated H-2a/a mice prior to infection did not (Fig. 5). To rule out the influence of contaminating CD8− cells or small amounts of vaccine virus, the same number of cells from the CD8− fraction of vaccinated H-2a/a mice was also transferred to naive H-2a/a mice prior to infection with FV. The transfer of immune CD8− cells did not induce the production of IFN-γ by FV-specific CD4+ T cells (Fig. 5). Thus, vaccine-primed CD8+ T cells were able to induce the generation of an IFN-γ-producing CD4+ T-cell response following FV infection.
FIG. 5.
Transfer of immune CD8+ T cells affects IFN-γ production by CD4+ T cells. CD8+ T cells were purified by positive selection from the spleens of H-2a/a mice at 8 days postvaccination with FV-N. More than 95% of these cells were positive for both CD8 and CD3, as analyzed by flow cytometry. Approximately 1.5 × 107 CD8+ T cells from FV-N-vaccinated or unvaccinated H-2a/a mice were transferred to each naive H-2a/a mouse. As an additional control, a similar number of CD8− cells were also transferred to naive H-2a/a mice. At day 1 posttransfer, all groups were infected with 15 SFFU of FV-B. At 8 days postinfection, CD4+ T cells were purified and analyzed for IFN-γ production as described for Fig. 1. Data shown are the average values + SEM for four to eight mice per group and are the combined results from two separate experiments.
Treatment with anti-IFN-γ inhibits CD8+ T-cell regulation of FV-specific CD4+ T-cell response.
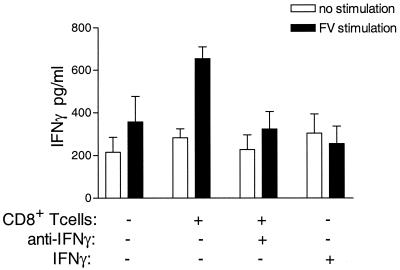
Activated CD8+ T cells may affect the CD4+ T-cell response through the production of cytokines. CD8+ cells isolated from vaccinated H-2a/a mice had higher IFN-γ mRNA levels after anti-CD3 stimulation in vitro than CD8+ cells from naive H-2a/a mice (data not shown). Possibly, CD8+ T cells function in a manner similar to that of natural killer cells, which indirectly affect CD4+ Th1 responses through the production of IFN-γ (17, 40). To determine whether CD8+ T cells regulate CD4+ T-cell responses by the production of IFN-γ, mice that were given vaccine-primed CD8+ T cells were treated with anti-IFN-γ (XMG1.2). In vivo neutralization of IFN-γ negated the effect of adoptively transferred CD8+ T cells on endogenous CD4+ T cells (Fig. 6). Thus, IFN-γ was necessary for the helper effect of CD8+ T cells on the IFN-γ-producing CD4+ T-cell response. To see if IFN-γ could replace the effect of vaccine-primed CD8+ T cells, H-2a/a mice were treated with five doses of IFN-γ over an 8-day period beginning the day before infection. Although this dose of IFN-γ was sufficient to decrease viremia levels in treated mice (data not shown), it did not induce an IFN-γ-producing CD4+ T-cell response. Thus, IFN-γ was important in the CD8+ regulation of FV-specific CD4+ T-cell responses, but exogenous IFN-γ was not sufficient to induce an FV-specific IFN-γ-producing CD4+ T-cell response in these experiments.
FIG. 6.
Anti-IFN-γ blocks CD8+ T-cell help. Vaccine-primed CD8+ T cells were purified by positive selection and transferred to naive H-2a/a mice as described for Fig. 1. One day posttransfer, mice were infected with 15 SFFU of FV-B. Mice were then given 0.5 ml of either anti-IFN-γ Cellmax supernatant or rat Ig every other day starting on the day of infection. Alternatively, one group of H-2a/a mice were given 105 U of IFN-γ intraperitoneally on the day before infection, the day of infection, and on days 2, 4, and 6 postinfection. At 8 days postinfection, CD4+ T cells were purified and analyzed for IFN-γ production as described for Fig. 1. Data shown are the average values + SEM for eight mice per group and are the combined results from two separate experiments.
DISCUSSION
The present study demonstrates a unique mechanism by which MHC class I genes can influence the development of protective CD4+ Th1 responses. CD4+ T-cell responses are normally regulated by the ability of MHC class II molecules to present the appropriate antigen to CD4+ T cells (34, 35), and accordingly in this study, an H-2b allele at the class II loci correlated with the induction of a CD4+ Th1 response to low-dose FV infection (Fig. 3). However, in the absence of an H-2b allele at the MHC class II loci, a single H-2b allele at the MHC class I H-2D gene was sufficient for the generation of an FV-specific CD4+ Th1 response (Fig. 3). This effect of the MHC class I genotype on CD4+ T-cell responses appeared to occur through the regulation of CD8+ T-cell responses by MHC class I genes, as depletion of CD8+ T cells prevented the development of CD4+ Th1 responses (Fig. 4). A similar effect of CD8+ T-cell depletion suppressing the development of a CD4+ Th1 response has also been observed in DNA vaccination studies against Leishmania major (17). Thus, CD8+ T cells may have a greater role in the host immune response against pathogens than was previously thought, providing both antigen-specific lysis of infected cells and help for the development of CD4+ Th1 responses.
IFN-γ appears to be a critical component of CD8+ T-cell help for the development of CD4+ Th1 responses, as treatment with anti-IFN-γ abolished the CD4+ Th1 response induced by the transfer of vaccine-primed CD8+ T cells (Fig. 6). IFN-γ produced by CD8+ T cells may influence the CD4+ T-cell response by upregulating the antigen-presenting capabilities of APCs (16), by inducing APCs to produce IL-12, a cytokine known to induce Th1 responses (27, 45), or by suppressing the development of a Th2 response (44). The role of IFN-γ does not appear to be suppression of virus production (16, 23), as treatment with soluble IFN-γ suppressed viremia levels but did not induce CD4+ Th1 responses (Fig. 6). Although technical aspects may be responsible for the lack of an effect by soluble IFN-γ on CD4+ T-cell responses, this result suggests that IFN-γ production may be only part of the mechanism by which CD8+ T cells provide help for CD4+ Th1 responses. Other mediators, such as chemokines, may also be involved in driving CD4+ T cells toward a Th1 response (25).
In the present study using a low dose of FV, the development of CD4+ Th1 responses to FV required either the presence of an H-2b allele at the MHC class II loci (Fig. 3) or the presence of activated CD8+ T cells (Fig. 4 and 5). However, in a previous study using a high dose of FV, a single mutation (bm14) in the H-2Db gene was sufficient to reduce CD4+ Th1 responses, even in the presence of another H-2Db allele and two b alleles at the MHC class II loci (37). Possibly, during high-dose infection, both strong stimulation of FV-specific CD4+ T cells through the MHC class II H-2b alleles and CD8+ T-cell help may be required to induce CD4+ Th1 responses. The effect of the H-2Dbm14 mutation on CD4+ Th1 responses after high-dose infection may be due to a lower number of FV-specific CD8+ T cells in H-2Db/bm14 mice than in H-2Db/b mice (20, 37), resulting in decreased CD8+ T-cell help.
The effect of the virus dose on the immune response may also explain why H-2a/a mice can generate a protective immune response after inoculation with FV-N but not FV-B (4, 12, 15) (Fig. 5). Due to the FV-1b/b genotype of the mice used in our experiments, FV-N replicates at a much lower rate than FV-B and does not induce massive splenomegaly in these mice (15). This lower rate of virus spread may allow a weak immune response time to develop before being overpowered by high levels of virus and virus-infected cells. Additionally, the high levels of virus produced after infection with FV-B, but not FV-N, may lead to activation-induced cell death or clonal exhaustion of FV-reactive T cells, as observed with high antigenic doses of pathogens in other systems (1, 3, 26).
A direct effect of CD8+ T-cell help for the development of CD4+ Th1 responses to FV was shown by the adoptive transfer of CD8+ cells to naive H-2a/a mice. Although the majority of CD8+ cells used in the transfer studies were CD3+, a minor population (<0.5%) were CD8+ CD3−. These CD8+ CD3− cells may have been dendritic cells, as subsequent experiments have shown similar percentages of cells being positive for both CD8+ and CD11c+ in purified CD8+ cell populations. Adoptive transfer of primed CD8α+ dendritic cells has been shown to induce IFN-γ production by CD4+ T cells (41). However, it is unlikely that the dendritic cells in the CD8+ population are responsible for the influence of vaccine-primed CD8+ cells on the FV-specific CD4+ Th1 response, as the transfer of CD8− cells did not induce CD4+ Th1 responses (Fig. 5), even though this population of cells also contained dendritic cells (data not shown). Although the types of dendritic cell (CD8α+ versus CD8α−) in these two populations are probably different, both populations are equally efficient at inducing antigen-specific CD4+ Th1 responses after i.v. injection in vivo (41). Since no Th1 response was induced by dendritic cells in the CD8− population (Fig. 5), it is unlikely that dendritic cells in the CD8+ population are responsible for the observed induction of CD4+ Th1 responses (Fig. 5).
In the present study, allelic differences in the MHC class I gene H-2D influenced the CD4+ T-cell response to viral infection via the activation of CD8+ T cells. The ability of an MHC class I allele to influence virus-specific CD4+ T-cell responses may be an evolutionary advantage in controlling virus infections. Both class I and class II MHC genes have high degrees of polymorphism, allowing their respective molecules to bind and present a wide range of antigenic peptides (46). However, due to this high degree of polymorphism, the host may not always have the correct alleles in both MHC class I and class II genes to generate both virus-specific CD8+ CTL and CD4+ Th1 responses. As several viruses, including FV, require both CD4+ and CD8+ T-cell responses to control virus infection (39, 42), the absence of appropriate alleles in either the MHC class I or class II genes may be detrimental to the host. The ability of MHC class I alleles to influence the CD4+ T-cell response may compensate for MHC class II alleles that generate only weak CD4+ Th1 responses to viral infection. This would allow for a more effective immune response to a greater number of pathogens.
REFERENCES
- 1.Bancroft, A. J., K. J. Else, and R. K. Grencis. 1994. Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. Eur. J. Immunol. 24:3113-3118. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell, J. M. 1996. Genetic susceptibility to leishmanial infections: studies in mice and man. Parasitology 112(Suppl.):S67-S74. [PubMed] [Google Scholar]
- 3.Bretscher, P. A., G. Wei, J. N. Menon, and H. Bielefeldt-Ohmann. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257:539-542. [DOI] [PubMed] [Google Scholar]
- 4.Britt, W. J., and B. Chesebro. 1983. H-2D control of recovery from Friend virus leukemia: H-2D region influences the kinetics of the T lymphocyte response to Friend virus. J Exp Med. 157:1736-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cher, D. J., and T. R. Mosmann. 1987. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J. Immunol. 138:3688-3694. [PubMed] [Google Scholar]
- 6.Chesebro, B., M. Miyazawa, and W. J. Britt. 1990. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu. Rev. Immunol. 8:477-499. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro, B., K. Wehrly, and J. Stimpfling. 1974. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly: mapping of a gene within the major histocompatibility complex. J Exp Med. 140:1457-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffman, R. L., B. W. Seymour, D. A. Lebman, D. D. Hiraki, J. A. Christiansen, B. Shrader, H. M. Cherwinski, H. F. Savelkoul, F. D. Finkelman, and M. W. Bond. 1988. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 102:5-28. [DOI] [PubMed] [Google Scholar]
- 9.Constant, S. L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297-322. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1999. Protection against establishment of retroviral persistence by vaccination with a live attenuated virus. J. Virol. 73:3753-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittmer, U., and K. J. Hasenkrug. 2000. Different immunological requirements for protection against acute versus persistent Friend retrovirus infections. Virology 272:177-182. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer, U., B. Race, and K. J. Hasenkrug. 1999. Kinetics of the development of protective immunity in mice vaccinated with a live attenuated retrovirus. J. Virol. 73:8435-8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmer, U., B. Race, K. E. Peterson, I. M. Stromnes, R. J. Messer, and K. J. Hasenkrug. 2002. Essential roles for CD8+ T cells and gamma interferon in protection of mice against retrovirus-induced immunosuppression. J. Virol. 76:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty, P. C., and J. P. Christensen. 2000. Accessing complexity: the dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 18:561-592. [DOI] [PubMed] [Google Scholar]
- 15.Earl, P. L., B. Moss, R. P. Morrison, K. Wehrly, J. Nishio, and B. Chesebro. 1986. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science 234:728-731. [DOI] [PubMed] [Google Scholar]
- 16.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 17.Gurunathan, S., L. Stobie, C. Prussin, D. L. Sacks, N. Glaichenhaus, D. J. Fowell, R. M. Locksley, J. T. Chang, C. Y. Wu, and R. A. Seder. 2000. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J. Immunol. 165:915-924. [DOI] [PubMed] [Google Scholar]
- 18.Hasenkrug, K. J., and B. Chesebro. 1997. Immunity to retroviral infection: the Friend virus model. Proc. Natl. Acad. Sci. USA 94:7811-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasenkrug, K. J., and U. Dittmer. 2000. The role of CD4 and CD8 T cells in recovery and protection from retroviral infection: lessons from the Friend virus model. Virology 272:244-249. [DOI] [PubMed] [Google Scholar]
- 20.Hasenkrug, K. J., G. J. Spangrude, J. Nishio, D. M. Brooks, and B. Chesebro. 1994. Recovery from Friend disease in mice with reduced major histocompatibility complex class I expression. J. Virol. 68:2059-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmi, S., J. Geliebter, R. A. Zeff, R. W. Melvold, and S. G. Nathenson. 1988. Three spontaneous H-2Db mutants are generated by genetic micro-recombination (gene conversion) events. Impact on the H-2-restricted immune responsiveness. J. Exp. Med. 168:2319-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill, A. V. 1996. Genetic susceptibility to malaria and other infectious diseases: from the MHC to the whole genome. Parasitology 112(Suppl.):S75-S84. [DOI] [PubMed] [Google Scholar]
- 23.Iwashiro, M., K. Peterson, R. J. Messer, I. M. Stromnes, and K. J. Hasenkrug. 2001. CD4+ T cells and gamma interferon in the long-term control of persistent friend retrovirus infection. J. Virol. 75:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabat, D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1-42. [DOI] [PubMed] [Google Scholar]
- 25.Kim, J. J., L. K. Nottingham, J. I. Sin, A. Tsai, L. Morrison, J. Oh, K. Dang, Y. Hu, K. Kazahaya, M. Bennett, T. Dentchev, D. M. Wilson, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J. Clin. Investig. 102:1112-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauritzsen, G. F., P. O. Hofgaard, K. Schenck, and B. Bogen. 1998. Clonal deletion of thymocytes as a tumor escape mechanism. Int. J. Cancer 78:216-222. [DOI] [PubMed] [Google Scholar]
- 27.Macatonia, S. E., C. S. Hsieh, K. M. Murphy, and A. O'Garra. 1993. Dendritic cells and macrophages are required for Th1 development of CD4+ T cells from alpha beta TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-gamma production is IFN-gamma-dependent. Int. Immunol. 5:1119-1128. [DOI] [PubMed] [Google Scholar]
- 28.Mayrand, S. M., P. A. Healy, B. E. Torbett, and W. R. Green. 2000. Anti-Gag cytolytic T lymphocytes specific for an alternative translational reading frame-derived epitope and resistance versus susceptibility to retrovirus-induced murine AIDS in F(1) mice. Virology 272:438-449. [DOI] [PubMed] [Google Scholar]
- 29.Miyazawa, M., J. Nishio, and B. Chesebro. 1988. Genetic control of T cell responsiveness to the Friend murine leukemia virus envelope antigen. Identification of class II loci of the H-2 as immune response genes. J. Exp. Med. 168:1587-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazawa, M., J. Nishio, K. Wehrly, G. Jay, R. W. Melvold, and B. Chesebro. 1992. Detailed mapping of the Rfv-1 gene that influences spontaneous recovery from Friend retrovirus-induced leukaemia. Eur. J. Immunogenet. 19:159-164. [DOI] [PubMed] [Google Scholar]
- 31.Moore, K. W., Malefyt R. de Waal, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 32.Morrison, R. P., P. L. Earl, J. Nishio, D. L. Lodmell, B. Moss, and B. Chesebro. 1987. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature 329:729-732. [DOI] [PubMed] [Google Scholar]
- 33.Murray, J. S., J. Madri, T. Pasqualini, and K. Bottomly. 1993. Functional CD4 T cell subset interplay in an intact immune system. J. Immunol. 150:4270-4276. [PubMed] [Google Scholar]
- 34.Murray, J. S., J. Madri, J. Tite, S. R. Carding, and K. Bottomly. 1989. MHC control of CD4+ T cell subset activation. J. Exp. Med. 170:2135-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray, J. S., C. Pfeiffer, J. Madri, and K. Bottomly. 1992. Major histocompatibility complex (MHC) control of CD4 T cell subset activation. II. A single peptide induces either humoral or cell-mediated responses in mice of distinct MHC genotype. Eur. J. Immunol. 22:559-565. [DOI] [PubMed] [Google Scholar]
- 36.O'Garra, A., and K. Murphy. 1994. Role of cytokines in determining T-lymphocyte function. Curr. Opin. Immunol. 6:458-466. [DOI] [PubMed] [Google Scholar]
- 37.Peterson, K. E., M. Iwashiro, K. J. Hasenkrug, and B. Chesebro. 2000. Major histocompatibility complex class I gene controls the generation of gamma interferon-producing CD4+ and CD8+ T cells important for recovery from Friend retrovirus-induced leukemia. J. Virol. 74:5363-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulsen, D. J., S. J. Robertson, C. A. Favara, J. L. Portis, and B. W. Chesebro. 1998. Mapping of a neurovirulence determinant within the envelope protein of a polytropic murine retrovirus: induction of central nervous system disease by low levels of virus. Virology 248:199-207. [DOI] [PubMed] [Google Scholar]
- 39.Robertson, M. N., G. J. Spangrude, K. Hasenkrug, L. Perry, J. Nishio, K. Wehrly, and B. Chesebro. 1992. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J. Virol. 66:3271-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scharton, T. M., and P. Scott. 1993. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlecht, G., C. Leclerc, and G. Dadaglio. 2001. Induction of CTL and nonpolarized Th cell responses by CD8alpha(+) and CD8alpha(−) dendritic cells. J. Immunol. 167:4215-4221. [DOI] [PubMed] [Google Scholar]
- 42.Selin, L. K., S. M. Varga, I. C. Wong, and R. M. Welsh. 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J. Exp. Med. 188:1705-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sher, A., and R. L. Coffman. 1992. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 10:385-409. [DOI] [PubMed] [Google Scholar]
- 44.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stobie, L., S. Gurunathan, C. Prussin, D. L. Sacks, N. Glaichenhaus, C. Y. Wu, and R. A. Seder. 2000. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA 97:8427-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeager, M., and A. L. Hughes. 1999. Evolution of the mammalian MHC: natural selection, recombination, and convergent evolution. Immunol. Rev. 167:45-58. [DOI] [PubMed] [Google Scholar]