Abstract
Recombinant adeno-associated viruses (rAAV) are highly efficient vectors for gene delivery into the central nervous system (CNS). However, host inflammatory and immune responses may play a critical role in limiting the use of rAAV vectors for gene therapy and functional genomic studies in vivo. Here, we evaluated the effect of repeated injections of five rAAV vectors expressing different genetic sequences (coding or noncoding) in a range of combinations into the rat brain. Specifically, we wished to determine whether a specific immune or inflammatory response appeared in response to the vector and/or the transgene protein after repeated injections under conditions of mannitol coinjection. We show that readministration of the same rAAV to the CNS is possible if the interval between the first and second injection is more than 4 weeks. Furthermore, our data demonstrate that rAAV vectors carrying different genetic sequences can be administered at intervals of 2 weeks. Our data therefore suggest that the AAV capsid structure is altered by the vector genetic sequence, such that secondary structures of the single-stranded genome have an impact on the antigenicity of the virus. This study provides guidelines for more rational design of gene transfer studies in the rodent brain and, in addition, suggests the use of repeated administration of rAAV as a viable form of therapy for the treatment of chronic diseases.
Vectors based on recombinant adeno-associated virus (rAAV) are currently being evaluated as tools for human gene therapy. Before human studies with rAAV can be initiated, studies to define the relationship between injection parameters, transgene expression, and toxicity must be determined. Among the many features that make rAAV an ideal vector for gene therapy is its ability to infect both dividing and nondividing cells and its long-term expression in tissues such as brain, skeletal muscle, lung, and liver (19, 24, 31, 35, 39, 46). These studies show that use of rAAV in animal models has not been associated with significant toxicity. However, like other viral vectors, transgene expression following readministration of rAAV may be compromised because of the potential host's immune response to structural components of the vector and/or the transgenic protein itself. Any host response to the virus in regard to antigen-specific immunity as well as any preexisting immunity to the virus due to naturally acquired infections will be of substantial importance (5).
The immune response to rAAV has not been studied extensively, although it is becoming clear that immune-tolerated vectors are preferred for most gene therapy applications. It was reported that the cellular (4) as well as the humoral immune response (1, 25, 50) to rAAV appears to depend on the route of administration. Additional studies using single rAAV injections reported in general a limited immune response or one without diminution of gene expression in brain (36) as well as in muscle (11, 13, 23, 33, 51) and liver (34). However, conflicting results were reported after readministration of rAAV into rabbit or mouse lung, muscle, or liver, which showed ineffective gene transfer (28, 29, 49, 51), yet readministration to the rabbit airway has met with success (2). Some of these contradictions may be due to different vector purities and administration methods and generally reflect the necessity to collect more data relating to host immune responses after vector administration(s). A cell-mediated immune response to the transgene expressed from AAV is seldom seen, and only few exceptions have been reported so far (4, 37). However, most reports agree that a humoral immune response against the virion and not the transgene product is responsible for blocking readministration (11, 30).
So far, few studies have analyzed the immune response to rAAV delivered to the nervous system. In one study, antibodies to AAV capsid proteins were low at 2 and 4 months after intracerebral injection and did not prevent transgene delivery upon reinjection of AAV (36). Several studies report that intracerebral administration of rAAV vectors does not induce infiltration of microglia or astrogliosis (7, 39). These results indicate that rAAV is well-tolerated in the rat brain, similar to results for lentivirus (3) or adenovirus, viruses for which preexisting antiadenoviral immunity does not harm transgene expression (47). One study performed in rhesus macaques reported a lack of a toxic reaction after treatment of wild-type AAV intranasally, intravenously, and intramuscularly (30). With regard to rhesus macaque responses being immunologically similar to those of humans, these results are encouraging.
In the human, antigen-specific immunity has been studied in healthy subjects and cystic fibrosis patients, revealing that almost all had antibodies to AAV serotype 2 (AAV2) derived from naturally acquired infections, but only 5% of patients had peripheral lymphocytes that proliferated in response to AAV antigens (10). Furthermore, an unstable AAV antibody response was reported in humans, allowing lifelong reinfection or reactivation of persisting virus, possibly due to partial immunotolerance after an infection (20). To understand preexisting immunity to AAV in humans, which may limit AAV-mediated gene delivery, the first domains of the AAV2 capsid containing immunogenic epitopes have been mapped (41, 48).
Transgene transfer into the central nervous system (CNS) to date has relied mainly on the use of rAAV2, with only a few studies reporting how other rAAV serotypes perform as CNS gene delivery vehicles. AAV2 and alternative serotypes (9, 42, 44, 49) differ in their capsid structures, suggesting that a possible immune reaction might be different for each AAV type (17). Indeed initial data on rAAV-mediated gene transfer into mouse muscle using distinct AAV serotypes reported significant differences in transgene expression (8).
Using rAAV vector technology with the established microprocessor-driven syringe for vector delivery (6) and ultraslow microperfusion (38), we wanted to address concerns that host immune responses to rAAV have the potential to interfere with efficient gene transfer. We wanted to specifically characterize (i) whether there is an ideal time window for repeated administration of rAAV vectors and (ii) any parameters that modulate a host immune response against rAAV delivery into the mammalian brain in vivo.
MATERIALS AND METHODS
Generation of rAAV.
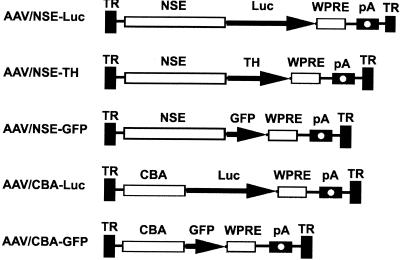
The plasmids rAAV/NSE-Luc-WPRE (AAV/NSE-Luc), rAAV/NSE-GFP-WPRE (AAV/NSE-GFP), rAAV/CBA-Luc-WPRE (AAV/CBA-Luc), rAAV/CBA-GFP-WPRE (AAV/CBA-GFP), and rAAV/NSE-TH-WPRE (AAV/NSE-TH) were created using standard molecular cloning procedures (Fig. 1). Luciferase (Luc), tyrosine hydroxylase (TH), and enhanced green fluorescent protein (GFP) (Clontech) cDNAs were subcloned into an expression cassette consisting of a 1.1-kb composite promoter (CBA) composed of the cytomegalovirus (CMV) immediate-early enhancer linked to chicken β-actin promoter sequences (43) or a rat neuron-specific enolase (NSE) 1.8-kb promoter fragment, woodchuck posttranscriptional regulatory element (WPRE) (18), and a simian virus 40 poly(A) signal. Expression cassettes were subcloned into the pSUB201 rAAV backbone flanked by AAV2 inverted terminal repeats (ITRs). The plasmids were subsequently packaged using the helper-free packaging system previously described (26) and heparin column purified according to the method of Clark et al. (12), with minor modifications, to yield high-titer rAAV2. The physical titers of virus stocks were measured using an AAV titration enzyme-linked immunosorbent assay (ELISA) kit (Progen, Heidelberg, Germany). The genomic titers of virus stocks were determined using the Perkin-Elmer-Applied Biosystems (Foster City, Calif.) prism 7700 sequence detector system as described previously (12).
FIG. 1.
AAV vectors. AAV ITRs (TR), coding regions (Luc, TH, and GFP), promoters (NSE and CBA), regulatory elements (WPRE), and polyadenylation sequences (pA) are indicated. All vectors shown contain AAV2 ITR.
The plasmid rAAV5/NSE-EGFP-WPRE (AAV5/EGFP) consisted of the identical expression cassette but was flanked with AAV5 ITRs instead of the AAV2 ITR sequences. AAV5 was packaged as previously described (9), and the titer was determined using dot blot analysis.
Vector purity was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A 10-μl aliquot of each virus stock was separated on a 12% gel under reducing, denaturing conditions and stained with Coomassie brilliant blue dye. In addition, viral capsid proteins were confirmed by standard immunoblot procedures using the mouse anti-VP1, -2, -3 antibody (1:500 dilution; Research Diagnostic Inc.) and endotoxin levels were determined in selected stocks by the BioReliance Laboratory, Rockville, Md., using the high-sensitivity quantitative chromogenic Limulus amebocyte lysate assay.
Animals and vector administration.
Male Wistar rats (280 to 300 g) were anesthetized with ketamine-xylazine (60 and 6 mg per kg of body weight, respectively). An additional one-third of the initial dose of anesthetic was administered during the surgical procedure. The experiments were carried out at the University of Auckland in accordance with Guidelines for Animal Care, and protocols were approved by the University of Auckland Animal Ethics Committee.
Aliquots (2 μl; 7 × 109 particles/μl) of virus were injected stereotactically and sequentially into the left and right hemispheres of the midstriatum with 1 μl of 20% mannitol (as described in reference 38) in phosphate-buffered saline (PBS) (3 μl total volume). Intervals between first and second injections administered contralaterally were 2 or 4 weeks or 3 months depending on the experiment. The injection coordinates (anterior-posterior axis + 0.4 mm, medial-lateral axis ± 3.0 mm, doroventral axis − 6.6 mm) were measured from the bregma, using Paxinos and Watson's Rat Brain Atlas. An Ultra Microsyringe pump controlled by a microprocessor (World Precision Instruments, Inc.) (6) was used to deliver the vector plus mannitol at a rate of 66 nl/min with a 10-μl Hamilton syringe. Animals were left to recover for 4 weeks after the last operation before analysis. Blood was taken at 7, 14, and 24 days after the first injection and before sacrifice and used for ELISA. In addition to blood samples, cerebrospinal fluid (CSF) samples were taken from some of the animals to determine the presence of antibodies (n = 4 to 6). To collect CSF, rats were anesthetized as described above. A 27-gauge needle connected to a 1-ml syringe was introduced into the cerebellomedullary cistern, and 50 to 70 μl of CSF was collected at 3, 7, and 28 days after the first injection and was used for ELISAs.
ELISA analysis of serum.
To detect serum antibodies generated against the transgenes Luc or to AAV capsid proteins, an ELISA was performed using 96-well immunoassay plates (Nunc). The plates were coated with rAAV/CBA-Luc (109 rAAV particles/well; 50 μl); or luciferase protein (Promega; 0.5 μg/well; 50 μl); and incubated at 37°C overnight, which was followed by a 2-h incubation with PBS containing 0.1% Tween 20 (PBST)-1% fetal calf serum.
The pooled sera from the rats were diluted in series (1:20, 1:40, 1:80, 1:160, 1:320, 1:640, 1:1,280; 100 μl total volume per well) and incubated at 4°C overnight. Sera from naive and orally rAAV-vaccinated rats were used as negative and positive controls. Plates were washed three times for 5 min with 200 μl of PBST. Following a 1-h incubation at room temperature with peroxidase-conjugated anti-rat secondary antibody (dilution 1:15,000; Biolab) and a wash step as described above, 100 μl of TMB substrate (Pierce) was applied, 20 min later the solution was quenched with 100 μl of 2 M H2SO4, and absorption at 450 nm was determined in a Victor 1420 multilabel counter (Wallac).
Luciferase assay.
After 4 weeks, animals were sacrificed under deep anesthesia, and their brains were removed, immediately frozen on dry ice, and stored at −80°C prior to sectioning. The brains were cut as a whole hemisphere or cryostat cut and were stored at −80°C. Luciferase activity was measured in lumens per second using a luciferase assay kit (Promega) on a Wallac Jet luminescence counter as previously described (38, 52). According to the standard measurements, 7 lm/s equaled 1 pg of luciferase protein.
Antibody and immunoneutralization assays.
The sera from three animals that received injections to the brain, obtained at 2 weeks after the first injection, were pooled and prepared by incubating blood at 4°C overnight or at room temperature for 1 h followed by removal of the clot by centrifugation. To adjust the amount of serum necessary to neutralize the virus, 2, 20, 50, or 100 μl of serum was added to 1 μl of virus and directly added to HEK293 cells (plated the previous day at 105 cells per well in 24-well plates) for transduction. Addition of 2, 20, or 50 μl of serum to 1 μl of virus did not change the transgene expression in this assay indicating that the amount of antibodies present was not sufficient for neutralizing antibody formation. Therefore, 100 μl of serum was used in the following experiments, and the specific viruses used are described below. Two to three days following infection, cells were either fixed and stained for expression analysis (immunocytochemistry). This assay was performed in duplicate.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (38). In brief, rats were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. After being immersed in fixative overnight, brains were cryoprotected in 30% sucrose in PBS. Cryostat-cut sections 35 μm in thickness were rinsed in PBS containing 0.2% Triton X-100 (PBS-Triton) before being incubated in 1% H2O2 in 50% methanol for 1 h. After being rinsed again in PBS-Triton, sections were incubated overnight at room temperature with monoclonal antibodies against GFAP (dilution, 1:1,000; Sigma), T-cell helper/macrophage CD-4 (ox-35; dilution, 1:100; Pharmingen), isolectin B4 peroxidase-conjugated (dilution 1:50; Sigma), CD-8 suppressor or cytotoxic cells (dilution, 1:50; Pharmingen), or polyclonal antibodies against EGFP (dilution, 1:2,000; Clontech) or luciferase (dilution, 1:10,000; CorTex). Sections were processed using commercial secondary antibodies, and immunoreactivity was analyzed as previously described (53). Immunofluorescent and DAB labeling was detected using an Olympus AX70 light microscope attached to a REAL-14 digital camera with a Micro color filter (Photometrics) supported by IPLab image manager software (Scananlytics, Fairfax, Va.) or a stereomicroscope attached to a DC camera supported by IM1000 Image Manager software (Leica Microsystem). The images were processed using Photoshop 6.0 (Adobe Systems, Mountain View, Calif.).
Statistical analysis.
Data are given as means ± standard errors of the means (SEM) and were analyzed using Student's t test and analysis of variance using the Statview statistical software.
RESULTS
Relationship of the time between the first and second rAAV administration and transduction efficiency.
As the main goal of this study was to analyze immune responses to rAAV2, it was necessary to work with pure virus solutions to reduce the influence of contaminating proteins on a host response. Therefore, our virus plasmids were packaged using the helper-free packaging system described previously (26). Heparin column affinity-purified virus stocks were generated according to the method of Clark et al. (12), with minor modifications, to achieve a high-titer rAAV2 yield as previously demonstrated (12). These heparin column-purified vector stocks were shown to have >95% purity based on Coomassie protein gels, and endotoxin levels were assayed in selected viral stocks showing that there was no detectable amount of endotoxin present.
In order to confirm that equal amounts of viral vectors were used in each experiment, two independent methods were performed to assay the physical as well as the genomic titer of the rAAV vector stocks. The genomic titer of the viral vector stocks used was 2 × 1011 particles/ml.
We conducted a first set of experiments injecting groups of rats with the luciferase-expressing vector AAV/NSE-Luc followed by a second injection of the same vector after different time intervals into the striatum (Fig. 2). The control group received only a single injection of AAV/NSE-Luc at the second time point. Two microliters of AAV/NSE-Luc (7 × 109 particles/μl) was administered via stereotactic injection into a single midstriatum site (n = 4 to 6 rats per group).
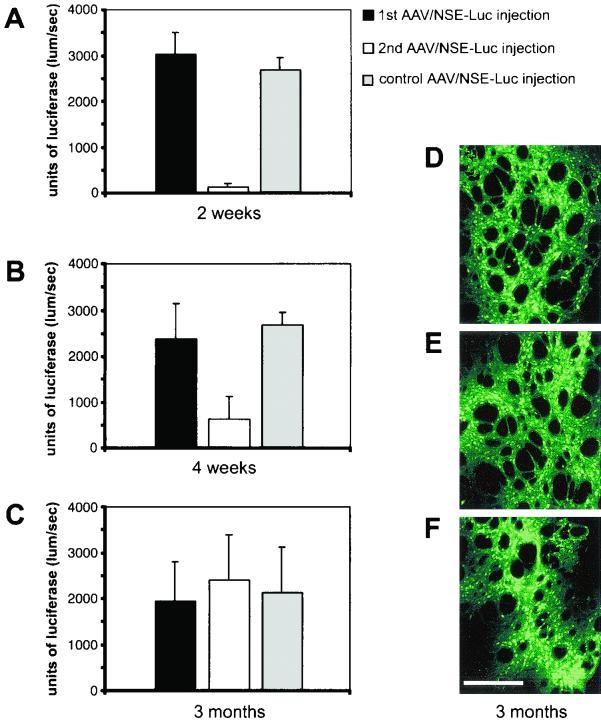
FIG. 2.
Repeated rAAV injections into rat brain at different time points and effects of a host immune response on transgene expression. The luciferase-expressing AAV/NSE-Luc vector was injected into rat striatum, and a second rAAV injection followed after 2 weeks (A), 4 weeks (B), or 3 months (C). The control groups of rats received a single injection at the second time point only. All rAAVs were coinjected with mannitol, and luciferase enzyme activity was determined 4 weeks after the second injection. Note the 10- and 5-fold decreases in luciferase activity in the groups that received the second rAAV injection after 2 and 4 weeks, respectively (A and B), suggesting the presence of a host humoral immune response that could have an impact on the transgene expression levels. In the groups that received the second rAAV administration 3 months (C) after the first injection, transgene activity was as high as in the control groups, indicating a lack or minor effect of a host immune response. The level of luciferase expression was dependent on the time period between the first and second injection (n = 4 to 6 per group; total n = 41; F(7, 33) = 7.703; P < 0.0001). Error bars, SEM. (D to F) Fluorescent microscopic images showing autofluorescence of EGFP (green) of representative striatal sections after repeated administration of AAV/NSE-GFP virus 3 months apart. (D and E) Transgene expression at the first injection site (3-month transgene expression period) (D) and at the second injection site (1-month transgene expression period) (E). (F) EGFP expression in a section of a control brain that received a single injection at the second time point only (1-month transgene expression period). Note the homogenous and constantly high expression level in all sections. Scale bar, 150 μm.
After 2 or 4 weeks or 3 months, rats received an additional rAAV injection into the contralateral site. Four weeks after the second injection, expression of luciferase was detected in both hemispheres, measuring enzyme activity with a luciferase assay as described previously (38). Interestingly, we found that for a given transgene, expression of the foreign protein was diminished by approximately 5- to 10-fold in those groups of animals in which the second injection was done within a 2- to 4-week interval (Fig. 2A and B). On the contrary, there was no reduction in the expression when the second injection was administered 3 months (Fig. 2C) after the first injection. Thus, these data indicate that intervals between rAAV injections of 3 months lead to stable and high-level transduction efficiency from the repeated injection (n = 4 to 6 per group; total n = 41; F(7, 33) = 7.703; P < 0.0001). This was confirmed immunohistochemically by analyzing transgene expression of striatal sections after repeated administration after 3 months (Fig. 2D to F) of a GFP-expressing virus. These data showed the same homogeneous distribution of GFP-expressing cells in the striatum at both the first (Fig. 2D, 3-month transgene expression period) and the second (Fig. 2E, 1 month) injection sites, being indistinguishable from striatal cells of the control group that received a single injection at the second time point only (Fig. 2F, 1 month).
The most dramatic and highly significant effect was visible after a period of 2 weeks between injections (Fig. 2A; n = 4 to 6 per group; total n = 14; F(2, 11) = 106.354; P < 0.0001), suggesting a host immune response following the first rAAV injection that could have an impact on the transgene expression levels and may limit rAAV-mediated gene delivery.
To investigate this in more detail, in parallel we repeatedly coinjected rAAVs differing in their promoters or transgenes into rat striatum with a period of 2 weeks between injections to evaluate any influence of the viral expression cassette on a humoral immune response (Fig. 3).
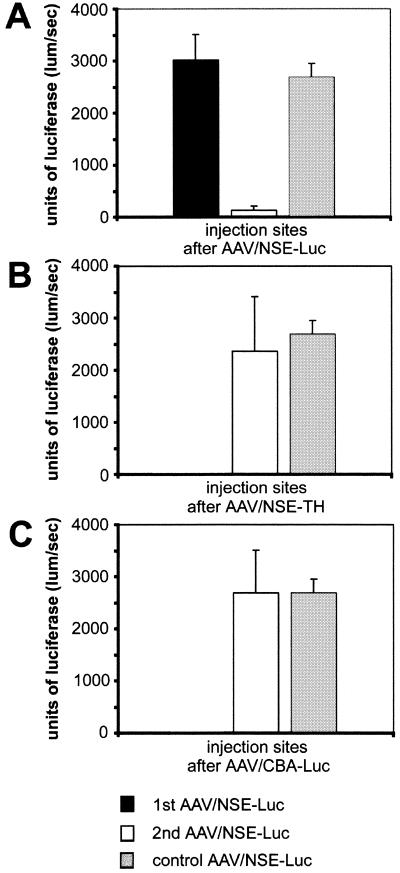
FIG. 3.
Repeated rAAV injections into rat striatum after 2 weeks and effects of a host immune response on transgene expression. Rats received a first injection of AAV/NSE-Luc vector (A), AAV/NSE-TH (B) or AAV/CBA-Luc (C) followed by a second injection of AAV/NSE-Luc after 2 weeks. The control groups received only a single injection of AAV/NSE-Luc at the second time point. All rAAVs were coinjected with mannitol, and luciferase enzyme activity was determined 4 weeks after the second injection. Note the 10-fold reduction in luciferase enzyme activity if the same virus was used for the second injection (A) and that no decrease in luciferase enzyme activity was observed if AAV/NSE-TH (B) or AAV/CBA-Luc (C) was injected prior to the AAV/NSE-Luc vector. The level of AAV/NSE-Luc expression was dependent on the second virus injected (n = 4 to 6 per group; total n = 20; F(3, 16) = 15.319; P < 0.0001). Error bars, SEM.
The luciferase-expressing vector AAV/NSE-Luc was used to determine transgene expression quantitatively and was injected in the second site in all animals, including the controls. The group of rats that received the same AAV/NSE-Luc vector 2 weeks apart showed a 10-fold decrease in luciferase enzyme activity as suggested above due to the host immune response to the viral capsid (Fig. 3A). However, no reduction in luciferase enzyme activity was visible if a rAAV with a different transgene (TH instead of Luc [Fig. 3B]) or surprisingly with a different promoter (CBA instead of NSE [Fig. 3C]) was injected 2 weeks prior to the AAV/NSE-Luc vector, indicating no negative effect on transgene expression of the potential immune response to the first rAAV injected. Overall, these data showed that a host immune response limiting rAAV-mediated transgene expression was only created if identical vectors, containing the identical genome, were readministered within a 2-week interval (n = 4 to 6 per group; total n = 20; F(3, 16) = 15.319, P < 0.0001). The transgene expression levels at the first injection sites were not effected, indicating that there was no cytotoxic effect or cell-mediated immune response against any viral or virus-encoded proteins.
Interestingly, our results are strongly suggestive that the outer viral capsid structure is influenced by the vector genome, including the cDNA, promoter, and other regulatory sequences. The genetic sequence differences may be sufficient to lead to a humoral immune response to the first rAAV not recognizing a second different rAAV injected, with antibodies generated against the first vector unable to recognize and neutralize the second vector even if it is only altered by the promoter sequence.
Injecting rAAV5 and 2 weeks later rAAV2 did not change GFP transgene expression levels.
Having hypothesized that structural differences in rAAV may account for protection against antibody-mediated reduction of expression, we now wanted to evaluate effects that rAAVs of different serotypes may evoke on a host immune response. Therefore, rAAV of serotypes 5 and 2 with identical expression cassettes containing EGFP were coinjected 2 weeks apart, and autofluorescence of EGFP was determined 4 weeks after the second virus injection. These results showed that EGFP autofluorescence was homogeneously distributed and present in all animals (Fig. 4, n = 6 per group), thus confirming our hypothesis that structural differences in rAAV vectors used (including different serotypes) allow successful gene transfer following a second injection after 2 weeks.
FIG. 4.
Injecting rAAV5 and 2 weeks later rAAV2 did not change GFP transgene expression levels. Rats received a first injection of AAV5/EGFP followed by a second injection of AAV/NSE-GFP after 2 weeks. The control group received only a single injection of AAV/NSE-GFP at the second time point. The fluorescent microscopy images show autofluorescence of EGFP of representative striatal sections 4 weeks after the second virus administration. Note the homogenous and constantly high expression level in all sections, indicating that virus spread and transgene expression was not effected after injecting rAAV5 followed by rAAV2 after 2 weeks. Scale bar, 300 μm.
Effects of rAAVs differing in their transgenes on neutralizing antibody formation and transgene expression in vitro.
Our data suggested that neutralizing antibodies were generated in response to a previous exposure to the virus, with titers at 2 weeks sufficient to almost completely neutralize expression upon reinjection. Neutralizing antibodies generally bind to the virus surface to prevent virus attachment to cellular receptors and uptake into the cell. Thus, the question arose whether a humoral antibody response might be responsible for the blocked transgene activity when the time between the two viral injections was 4 weeks or less. To assess the effect of neutralizing antibodies on readministration of rAAV vectors, we analyzed the pooled sera from rats that received injections into the brain, which were taken 2 weeks after the first injection of AAV/CBA-Luc vectors, and compared these to the pooled sera from naive rats.
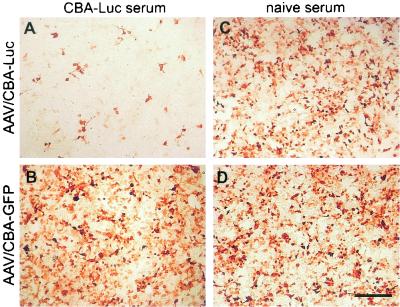
A 100-μl aliquot of serum was added to 1 μl of virus, the mixture was incubated on HEK293 cells, and transgene expression was determined immunocytochemically after 2 to 3 days using antibodies against the Luc or GFP transgene (Fig. 5). Our results show that the AAV/CBA-Luc serum, from the rats that received injections in the brain, incubated with AAV/CBA-Luc virus reduced Luciferase expression (Fig. 5A). Incubation of the same serum with AAV/CBA-GFP virus, however, did not reduce GFP expression (Fig. 5B), indicating the presence of AAV/CBA-Luc-specific antibodies in the serum of rats that received injections of AAV/CBA-Luc in the brain. Incubation of serum from naive rats with each virus was used as a control and did not reduce the corresponding transgene expression (Fig. 5C and D), indicating the absence of cross-reactivity. An ELISA was performed to detect serum antibodies reactive with Luc or the viral capsid. The animals that received injections in the brain had relatively low anti-AAV capsid antibody titers in the serum (Table 1) or CSF as well as low titers of antibody to the specific transgene at all time points. Despite the serum antibody titers of the rats that received injections in the brain being just at the threshold for detection, the amount of antibodies present was still sufficient to influence rAAV-mediated transgene expression.
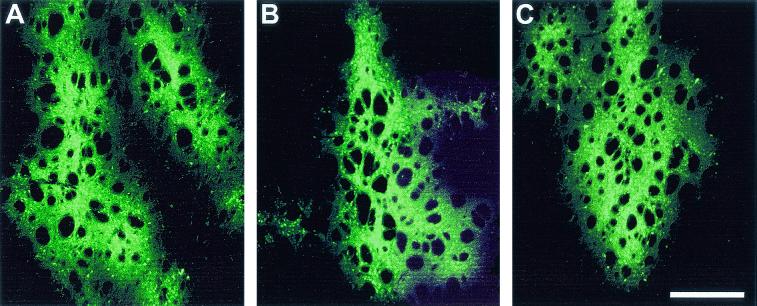
FIG. 5.
Immunoneutralization assay showing effects of rAAVs differing in their transgenes on neutralizing antibody formation and transgene expression. The sera from three animals that received AAV/CBA-Luc injections in the brain (A and B) or from three naive animals (C and D) obtained at 2 weeks after injection were pooled, and 100 μl was added to 1 μl of virus (AAV/CBA-Luc [A and C] or AAV/CBA-GFP [B and D]) and directly incubated on HEK293 cells for transduction. Immunocytochemistry was performed after 2 to 3 days to determine transgene expression using antibodies against the Luc (A and C) or GFP transgene (B and D). Note that the AAV/CBA-Luc serum from rats injected into the brain reduced AAV/CBA-Luc expression (A) but not AAV/CBA-GFP expression (B). Incubation of serum from naive rats with AAV/CBA-Luc (C) or AAV/CBA-GFP (D) virus had no effect on the transgene expression. These results indicate the presence of AAV/CBA-Luc-specific antibodies in the serum of AAV/CBA-Luc brain-injected rats. Scale bar, 300 μm.
TABLE 1.
ELISA results showing serum anti-AAV titers of rats that received injections, to the braing
| Group of animals (n = 4) | Mean OD450 ± SD at day:
|
||
|---|---|---|---|
| 0 | 14 after first injection | 14 after second injection | |
| Different transgenesa | 0.071 ± 0.008 | 0.068 ± 0.010 | 0.083 ± 0.031 |
| 2-wk intervalb (same transgene) | 0.054 ± 0.011 | 0.069 ± 0.017 | 0.121 ± 0.092 |
| 4-wk intervalc (same transgene) | 0.067 ± 0.013 | 0.087 ± 0.036 | 0.089 ± 0.022 |
| Naived | 0.077 ± 0.012 | NAe | NA |
| Oral AAVd | NDf | ND | 0.473 ± 0.098 |
This group of rats received one brain injection of AAV/NSE-TH at day 0 and another at day 14 and one of AAV/NSE-Luc at day 28.
This group of rats received one brain injection of AAV/NSE-Luc at day 0 and another at day 14.
This group of rats received one brain injection of AAV/NSE-Luc at day 0 and another at day 28.
These control groups of rats received no viral injection (naive) or one oral AAV administration (oral AAV).
NA, not applicable
ND, not done
At a dilution of 1:80.
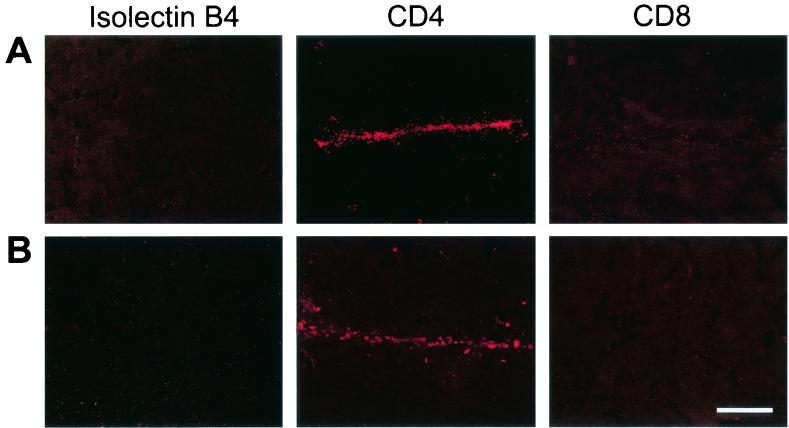
Additional immunohistochemistry was performed in order to exclude an inflammatory response or cytotoxic tissue damage looking for markers of isolectin B4, T-cell helper/macrophage CD-4, and suppressor or cytotoxic cells CD-8 showing no tissue damage in all groups (Fig. 6).
FIG. 6.
Lack of significant tissue damage and inflammatory responses after rAAV administrations. Representative sections from each group were immunohistochemically analyzed using antibodies against isolectin B4 (left column), T-cell helper/macrophage CD-4 (middle column), and suppressor or cytotoxic cell CD-8 (right column). Fluorescence microscopy images of sections showed no detectable cytotoxic tissue damage after repeated injections (row A) and no difference between the groups (row B) (control animals received a single injection of rAAV). Scale bar, 300 μm.
In summary, these quantitative in vivo results suggested that differences in the viral expression cassette result in differences in the outer viral capsid structure. The extent of these structural differences may modulate a humoral response to a first rAAV injection such that transgene expression mediated by a second rAAV—differing only slightly in its genome, e.g., promoter elements, but containing the same transgene—is not hindered in rat brain.
DISCUSSION
One of the biggest challenges in optimizing viral vectors for gene therapy relates to the immune response of the host. This mechanism functions at both the cellular level, by creating cytotoxic T cells, and at the humoral level, by generating antibodies to viral or virus-encoded proteins. Although cell-mediated immune responses to AAV-mediated transgene expression are unlikely, with few exceptions reported so far (4, 37), the potential of a humoral response against the virion to inhibit successful and efficient gene transfer is real (11, 30). AAV vectors do not seem to induce strong cytotoxic T-cell responses; however, antibodies to AAV-based vectors have been detected. One study reported that neutralizing antibodies to the viral capsid proteins of rAAV prevent transgene expression following readministration and suggested that transient immunosuppression could be a solution (37). Another study carried out in nonhuman primate muscle tissue reported that the humoral immunity to AAV2 prevented transduction by a second injection of the vector (11). Recent data derived from mouse lung or muscle indicate that this may be overcome by using another serotype of AAV (27) or possibly hybrid vectors (32).
Current studies on avoiding a host immune response by improving viral gene transfer techniques have reported for adenoviral vectors that an antibody response may prevent successful repeat administration (16, 40), but polymer-coated adenovirus could change virus tropism and interaction with the immune system (22). Another study used a hybrid herpes simplex virus/AAV amplicon vector and observed no immune response after a single injection into adult rat striatum (15). Future rAAV-mediated strategies aiming at avoiding immune responses could include transient immune modulation at the time of vector administration as recently reported (21). In addition, the question of preimmunity in the host remains and furthermore, the route of administration also has an influence on the immune response (50).
To date few published studies have thoroughly addressed the issue of a host immune response in mammalian brain after rAAV infection. Our work confirms a previous study in which an rAAV expressing β-galactosidase driven by the CMV promoter was injected into murine brain at 0 and 2 months; this study reported that antibodies to rAAV capsid protein and β-Gal appeared at low levels at 2 and 4 months (36). However, these antibodies did not prevent readministration of rAAV-β-Gal and were not associated with any substantial loss of transgene expression at these time points, indicating, together with our results, that readministration is feasible, despite the generation of antibodies to the transgene and AAV capsid antigen. An immediate local immune response develops to the transgene due to the mechanical injury of inoculation, having, however, little effect on transgene expression in brain. Our results now extend this study (36), suggesting that the viral expression cassette can be used to modulate a host immune response in the context of repeated rAAV delivery into rat CNS.
One reason for the detected low-level immune response in this study and the study of Lo et al. (36) could be that the blood-brain barrier interferes with antigen presentation, thus limiting an immune response against the transgene and/or AAV capsid proteins. In order to make use of the rAAV gene transfer technology for repeated delivery of therapeutic proteins to treat chronic brain diseases, such a limited immune reaction would be ideal.
This is the first study showing quantitative analysis of a potential host immune response after repeated delivery of different rAAV vectors into the rat brain. We extend the previous studies by showing that there is an optimum time window for repeated vector administration which is of direct clinical relevance. Interestingly, this study is the first to show the potential of the promoter and other regulatory and transgene sequences to shape the outer viral capsid structure. Our data suggest that rAAVs expressing the same transgene but driven by different promoters differ in their capsid structure and thus influence a potential host immune response after readministration. It was suggested recently that the promoter driving the transgene expression level may have a big influence on an immune response (14). Our data support this finding. However, few studies analyzing any effects of AAV capsid alternations on transduction and transgene expression are available and were mostly carried out in skeletal muscle (8). Shi et al. (45) showed recently that scaffolding sequences have a big impact on the surface structure of AAV particles. These recent studies together with our data may allow the design of gene therapy protocols using readministration of rAAV vectors. Moreover, promoter and other regulatory sequences may lead to altered vector tropism not simply via transcriptional control, but by altering the AAV capsid structure. This could modulate the affinity of the virus for cell membrane proteins and thereby facilitate or inhibit uptake into specific target cells.
In summary, our study has characterized several aspects of humoral immunity to rAAV in the adult rat brain relevant to studies involving viral vector readministration. Three major findings have emerged from this study: First, repeated administration of rAAV was efficient and did not create a host immune response with any decrease in transgene expression when the interval between repeated injections was 3 months. Second, and of most interest, is the provocative finding that the host immune response was variable depending on differences in the expression cassettes of the rAAV vectors used including the noncoding sequences. We could show that the host immune response could be avoided if the repeatedly injected rAAVs differed only in their promoter and/or cDNA sequence. Third, as previously reported for adenovirus-mediated gene transfer into the liver of baboons (40) and for murine liver- or muscle-directed rAAV-mediated gene transfer (9, 42, 44, 49), sequential delivery of different rAAV serotypes into the CNS gives additional possibilities in avoiding host immune responses as we showed by injecting rAAV5 vectors followed by rAAV2. Therefore, the host immune response after repeated rAAV delivery depends on several factors which can be modulated, thus opening new experimental designs for AAV gene transfer protocols. Our data show that rAAV technology has significant potential for repeated gene delivery to the CNS without a significant immune response together with long-term expression of the transgene. These data further emphasize the potential of rAAV for human gene therapy of neurological disorders.
Acknowledgments
Mihail Y. Mastakov and Kristin Baer contributed equally to this study.
We thank Alexandre Mouravlev, Ruian Xu, Michael Schmidt, and Richard H. Smith for generously providing plasmids and generating rAAV5 vector stocks. We thank Michael Kaplitt for helpful discussions.
This work was supported by the New Zealand Health Research Council, the Marsden Fund, the Jefferson Faculty Fund, and stipends of the Deutsche Forschungsgemeinschaft and the Schweizerischer Nationalfonds (K.B.).
REFERENCES
- 1.Anand, V., N. Chirmule, M. Fersh, A. M. Maguire, and J. Bennett. 2000. Additional transduction events after subretinal readministration of recombinant adeno-associated virus. Hum. Gene Ther. 11:449-457. [DOI] [PubMed] [Google Scholar]
- 2.Beck, S. E., L. A. Jones, K. Chesnut, S. M. Walsh, T. C. Reynolds, B. J. Carter, F. B. Askin, T. R. Flotte, and W. B. Guggino. 1999. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J. Virol. 73:9446-9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomer, U., L. Naldini, T. Kafri, D. Trono, I. M. Verma, and F. H. Gage. 1997. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 71:6641-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockstedt, D. G., G. M. Podsakoff, L. Fong, G. Kurtzman, W. Mueller-Ruchholtz, and E. G. Engleman. 1999. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immunol. 92:67-75. [DOI] [PubMed] [Google Scholar]
- 5.Bromberg, J. S., L. A. Debruyne, and L. Qin. 1998. Interactions between the immune system and gene therapy vectors: bidirectional regulation of response and expression. Adv. Immunol. 69:353-409. [PubMed] [Google Scholar]
- 6.Brooks, A. I., M. W. Halterman, C. A. Chadwick, B. L. Davidson, M. Haak-Frendscho, C. Radel, C. Porter, and H. J. Federoff. 1998. Reproducible and efficient murine CNS gene delivery using a microprocessor-controlled injector. J. Neurosci. Methods 80:137-147. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlin, N. L., B. Du, S. de Lacalle, and C. B. Saper. 1998. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 793:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao, H., Y. Liu, J. Rabinowitz, C. Li, R. J. Samulski, and C. E. Walsh. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2:619-623. [DOI] [PubMed] [Google Scholar]
- 9.Chiorini, J. A., F. Kim, L. Yang, and R. M. Kotin. 1999. Cloning and characterization of adeno-associated virus type 5. J. Virol. 73:1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 11.Chirmule, N., W. Xiao, A. Truneh, M. A. Schnell, J. V. Hughes, P. Zoltick, and J. M. Wilson. 2000. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J. Virol. 74:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark, K. R., X. Liu, J. P. McGrath, and P. R. Johnson. 1999. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum. Gene Ther. 10:1031-1039. [DOI] [PubMed] [Google Scholar]
- 13.Clark, K. R., T. J. Sferra, and P. R. Johnson. 1997. Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum. Gene Ther. 8:659-669. [DOI] [PubMed] [Google Scholar]
- 14.Cordier, L., G. P. Gao, A. A. Hack, E. M. McNally, J. M. Wilson, N. Chirmule, and H. L. Sweeney. 2001. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum. Gene Ther. 12:205-215. [DOI] [PubMed] [Google Scholar]
- 15.Costantini, L. C., D. R. Jacoby, S. Wang, C. Fraefel, X. O. Breakefield, and O. Isacson. 1999. Gene transfer to the nigrostriatal system by hybrid herpes simplex virus/adeno-associated virus amplicon vectors. Hum. Gene Ther. 10:2481-2494. [DOI] [PubMed] [Google Scholar]
- 16.Dai, Y., E. M. Schwarz, D. Gu, W. W. Zhang, N. Sarvetnick, and I. M. Verma. 1995. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc. Natl. Acad. Sci. USA 92:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson, B. L., C. S. Stein, J. A. Heth, I. Martins, R. M. Kotin, T. A. Derksen, J. Zabner, A. Ghodsi, and J. A. Chiorini. 2000. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 97:3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donello, J. E., J. E. Loeb, and T. J. Hope. 1998. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J. Virol. 72:5085-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.During, M. J., R. J. Samulski, J. D. Elsworth, M. G. Kaplitt, P. Leone, X. Xiao, J. Li, A. Freese, J. R. Taylor, R. H. Roth, J. R. Sladek, Jr., K. L. O'Malley, and D. E. Redmond, Jr. 1998. In vivo expression of therapeutic human genes for dopamine production in the caudates of MPTP-treated monkeys using an AAV vector. Gene Ther. 5:820-827. [DOI] [PubMed] [Google Scholar]
- 20.Erles, K., P. Sebokova, and J. R. Schlehofer. 1999. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J. Med. Virol. 59:406-411. [DOI] [PubMed] [Google Scholar]
- 21.Fields, P. A., V. R. Arruda, E. Armstrong, K. Chu, F. Mingozzi, J. N. Hagstrom, R. W. Herzog, and K. A. High. 2001. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of f9. Mol. Ther. 4:201-210. [DOI] [PubMed] [Google Scholar]
- 22.Fisher, K. D., Y. Stallwood, N. K. Green, K. Ulbrich, V. Mautner, and L. W. Seymour. 2001. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 8:341-348. [DOI] [PubMed] [Google Scholar]
- 23.Fisher, K. J., K. Jooss, J. Alston, Y. Yang, S. E. Haecker, K. High, R. Pathak, S. E. Raper, and J. M. Wilson. 1997. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 3:306-312. [DOI] [PubMed] [Google Scholar]
- 24.Flotte, T. R., S. A. Afione, C. Conrad, S. A. McGrath, R. Solow, H. Oka, P. L. Zeitlin, W. B. Guggino, and B. J. Carter. 1993. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. USA 90:10613-10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge, Y., S. Powell, M. Van Roey, and J. G. McArthur. 2001. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood. 97:3733-3737. [DOI] [PubMed] [Google Scholar]
- 26.Grimm, D., A. Kern, K. Rittner, and J. A. Kleinschmidt. 1998. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 9:2745-2760. [DOI] [PubMed] [Google Scholar]
- 27.Halbert, C. L., E. A. Rutledge, J. M. Allen, D. W. Russell, and A. D. Miller. 2000. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 74:1524-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halbert, C. L., T. A. Standaert, M. L. Aitken, I. E. Alexander, D. W. Russell, and A. D. Miller. 1997. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J. Virol. 71:5932-5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halbert, C. L., T. A. Standaert, C. B. Wilson, and A. D. Miller. 1998. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J. Virol. 72:9795-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez, Y. J., J. Wang, W. G. Kearns, S. Loiler, A. Poirier, and T. R. Flotte. 1999. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J. Virol. 73:8549-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herzog, R. W., J. N. Hagstrom, S. H. Kung, S. J. Tai, J. M. Wilson, K. J. Fisher, and K. A. High. 1997. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc. Natl. Acad. Sci. USA 94:5804-5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildinger, M., A. Auricchio, G. Gao, L. Wang, N. Chirmule, and J. M. Wilson. 2001. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J. Virol. 75:6199-6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jooss, K., Y. Yang, K. J. Fisher, and J. M. Wilson. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 72:4212-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung, S. C., I. P. Han, A. Limaye, R. Xu, M. P. Gelderman, P. Zerfas, K. Tirumalai, G. J. Murray, M. J. During, R. O. Brady, and P. Qasba. 2001. Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice. Proc. Natl. Acad. Sci. USA 98:2676-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplitt, M. G., P. Leone, R. J. Samulski, X. Xiao, D. W. Pfaff, K. L. O'Malley, and M. J. During. 1994. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 8:148-154. [DOI] [PubMed] [Google Scholar]
- 36.Lo, W. D., G. Qu, T. J. Sferra, R. Clark, R. Chen, and P. R. Johnson. 1999. Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum. Gene Ther. 10:201-213. [DOI] [PubMed] [Google Scholar]
- 37.Manning, W. C., S. Zhou, M. P. Bland, J. A. Escobedo, and V. Dwarki. 1998. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum. Gene Ther. 9:477-485. [DOI] [PubMed] [Google Scholar]
- 38.Mastakov, M. Y., K. Baer, R. Xu, H. Fitzsimons, and M. J. During. 2001. Combined injection of rAAV with mannitol enhances gene Expression in the rat brain. Mol. Ther. 3:225-232. [DOI] [PubMed] [Google Scholar]
- 39.McCown, T. J., X. Xiao, J. Li, G. R. Breese, and R. J. Samulski. 1996. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 713:99-107. [DOI] [PubMed] [Google Scholar]
- 40.Morral, N., W. O'Neal, K. Rice, M. Leland, J. Kaplan, P. A. Piedra, H. Zhou, R. J. Parks, R. Velji, E. Aguilar-Cordova, S. Wadsworth, F. L. Graham, S. Kochanek, K. D. Carey, and A. L. Beaudet. 1999. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. USA 96:12816-12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moskalenko, M., L. Chen, M. van Roey, B. A. Donahue, R. O. Snyder, J. G. McArthur, and S. D. Patel. 2000. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J. Virol. 74:1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muramatsu, S., H. Mizukami, N. S. Young, and K. E. Brown. 1996. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology 221:208-217. [DOI] [PubMed] [Google Scholar]
- 43.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 43a.Paxinos, G., and C. Watson. 1986. Rat brain atlas. Academic Press, Inc., San Diego, Calif.
- 44.Rutledge, E. A., C. L. Halbert, and D. W. Russell. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi, W., G. S. Arnold, and J. S. Bartlett. 2001. Insertional mutagenesis of the adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors targeted to alternative cell-surface receptors. Hum. Gene Ther. 12:1697-1711. [DOI] [PubMed] [Google Scholar]
- 46.Snyder, R. O., C. H. Miao, G. A. Patijn, S. K. Spratt, O. Danos, D. Nagy, A. M. Gown, B. Winther, L. Meuse, L. K. Cohen, A. R. Thompson, and M. A. Kay. 1997. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 16:270-276. [DOI] [PubMed] [Google Scholar]
- 47.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2001. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 12:839-846. [DOI] [PubMed] [Google Scholar]
- 48.Wobus, C. E., B. Hugle-Dorr, A. Girod, G. Petersen, M. Hallek, and J. A. Kleinschmidt. 2000. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J. Virol. 74:9281-9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao, W., N. Chirmule, S. C. Berta, B. McCullough, G. Gao, and J. M. Wilson. 1999. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 73:3994-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao, W., N. Chirmule, M. A. Schnell, J. Tazelaar, J. V. Hughes, and J. M. Wilson. 2000. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol. Ther. 1:323-329. [DOI] [PubMed] [Google Scholar]
- 51.Xiao, X., J. Li, and R. J. Samulski. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, R., C. G. Janson, M. Mastakov, P. Lawlor, D. Young, A. Mouravlev, H. Fitzsimons, K. L. Choi, H. Ma, M. Dragunow, P. Leone, Q. Chen, B. Dicker, and M. J. During. 2001. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Ther. 8:1323-1332. [DOI] [PubMed] [Google Scholar]
- 53.Young, D., P. A. Lawlor, P. Leone, M. Dragunow, and M. J. During. 1999. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat. Med. 5:448-453. [DOI] [PubMed] [Google Scholar]