Abstract
Extracellular signal-regulated kinase 1 (ERK1) and ERK2 (ERK1/2) dramatically enhance survival of cells exposed to heat shock. Using Cos-7 cells and primary human fibroblasts (IMR90 cells), we demonstrated that heat shock activates ERKs via two distinct mechanisms: stimulation of the ERK-activating kinases, MEK1/2, and inhibition of ERK dephosphorylation. Under milder heat shock conditions, activation of ERKs proceeded mainly through stimulation of MEK1/2, whereas under more severe heat shock MEK1/2 could no longer be activated and the inhibition of ERK phosphatases became critical. In Cos-7 cells, nontoxic heat shock caused rapid inactivation of the major ERK phosphatase, MKP-3, by promoting its aggregation, so that in cells exposed to 45°C for 20 min, 90% of MKP-3 became insoluble. MKP-3 aggregation was reversible and, 1 h after heat shock, MKP-3 partially resolubilized. The redistribution of MKP-3 correlated with an increased rate of ERK dephosphorylation. Similar heat-induced aggregation, followed by partial resolubilization, was found with a distinct dual-specificity phosphatase MKP-1 but not with MKP-2. Therefore, MKP-3 and MKP-1 appeared to be critical heat-labile phosphatases involved in the activation of ERKs by heat shock. Expression of the major heat shock protein Hsp72 inhibited activation of MEK1/2 and prevented inactivation of MKP-3 and MKP-1. Hsp72ΔEEVD mutant lacking a chaperone activity was unable to protect MKP-3 from heat inactivation but interfered with MEK1/2 activation similar to normal Hsp72. Hence, Hsp72 suppressed ERK activation by both protecting dual-specificity phosphatases, which was dependent on the chaperone activity, and suppressing MEK1/2, which was independent of the chaperone activity.
Extracellular signal-regulated kinase 1 (ERK1) and ERK2 are mitogen-activated protein (MAP) kinases, which are preferentially triggered by growth-promoting stimuli such as growth factors and oncogenes (16, 27). Activation of MAP kinases proceeds through phosphorylation of specific Tyr and Thr residues catalyzed by the MEK kinase family. MEK kinases, in turn, are activated via phosphorylation by members of the Raf kinase family (16).
Activation of the ERK pathway was shown to enhance cell resistance to a variety of stressful treatments, including heat shock (HS), growth factor withdrawal, hypoxia, chemotherapeutic agents, etc. (3, 5, 30, 35). Survival and proliferation of many types of tumor cells often rely on growth factor-stimulated ERK signaling, and many human tumors harbor hyperactivated components of ERK pathway (34). The exact mechanisms of ERK-mediated cell survival have not been established, but it is clear that ERKs may target different components of the death pathway. For example, it was shown that inhibition of ERK signaling sensitizes HeLa cells to apoptosis mediated by death receptors, such as Fas-R, TRAIL-R, or TNF-R (12, 31). In this system ERK seemed to control apoptosis at or upstream of caspase 8. It was also reported that the ERK signaling pathway is involved in the phosphorylation and the subsequent inactivation of the proapoptotic Bcl-2 family member, Bad (28). In another case, permanent activation of ERK by overexpression of B-Raf conferred on fibroblasts resistance to growth factor withdrawal. In this case, ERK intervened late in the apoptotic pathway, downstream of cytochrome c release (3).
Heat stress is a potent inducer of apoptosis and necrosis in many cell types (7). Heating of cells triggers numerous signaling events, which contribute to either cell death or survival. For example, HS causes activation of stress kinase c-Jun N-terminal kinase (JNK), which promotes cell death, and duration of JNK activity closely correlates with the extent of cell death (32, 33). Furthermore, inhibition of JNK pathway greatly increases survival of cells upon HS (33). Besides stimulating JNK, HS also stimulates ERKs and, whereas activation of JNK triggers apoptosis, activation of ERKs may provide protection of cells from HS-mediated cell death (35). Indeed, specific inhibition of HS-activated ERK dramatically enhanced sensitivity of NIH 3T3 cells or primary human fibroblasts, whereas overexpression of ERKs makes cells resistant to heat-induced death (8, 35).
Another survival mechanism triggered by HS is induction of HS proteins (Hsps) (25). Many Hsps, including the major inducible protein Hsp72, play a critical role in protection of cellular polypeptides from stress-induced denaturation and in refolding of damaged proteins (4, 11). In addition, Hsps directly interfere with several components of the apoptotic machinery, thus enhancing cell protection. For example, Hsp72 was shown to inhibit activation of JNK by various stressful treatments (19). These different signaling pathways have different thresholds and different rates of activation, which all affect the outcome of stressful treatments. Therefore, it seems that whether cells die or survive after HS depends upon an intricate balance of opposing signaling events.
The mechanism of JNK activation upon HS has recently been established. It was shown that HS activates JNK mostly through inhibition of JNK dephosphorylation, without significant stimulation of JNK-activating kinase cascade (19). In unstressed cells, the kinase cascade has certain background activity, and JNK phosphorylation is kept at a very low level by highly active JNK phosphatases. After heat treatment the rate of JNK dephosphorylation is dramatically reduced, thus leading to stabilization of phosphorylated forms of JNK and the enhanced activity (19). Interestingly, Hsp72 inhibits HS-mediated activation of JNK through stimulation of JNK dephosphorylation (19). It was reported that brain-specific JNK phosphatase M3/6 dissociates from JNK6 after HS and becomes inactive and insoluble (23). Inactivation and aggregation of M3/6 appears to be the major event in activation of JNK by HS and potentially other protein-damaging stresses. It should be noted that in tissues other than brain distinct JNK phosphatases must be involved in HS regulation.
In contrast to JNK, the mechanism of ERK activation upon HS is poorly understood. It was reported that in NIH 3T3 cells HS leads to activation of Raf1 through ceramide production (35). On the other hand, because of the homology of the JNK and ERK signaling pathway, we suggested that inhibition of ERK dephosphorylation might play a significant role in HS regulation. There are number of phosphatases that can dephosphorylate ERKs both in vitro and in vivo (26). For example, the serine/threonine-specific phosphatase PP2A can inactivate both MEK and ERK (1, 20). PP2A inhibition leads to hyperactivation of ERKs and cell transformation. Similarly, mutations in MEK, which lead to constitutive activation of ERK2, are accompanied by cellular transformation in fibroblasts (20). Another phosphatase involved in ERK signaling is MKP-1. MKP-1 is a dual specificity phosphatase induced by many stresses which activate ERKs (14). MKP-1 resides in the nucleus and is likely to be responsible for inactivation of ERKs upon their translocation into the nucleus. Yet another dual-specificity ERK phosphatase, MKP-3, is cytoplasmic and is expressed constitutively (10). MKP-3 binds ERK, and this binding renders MKP-3 catalytically active (2). In vivo MKP-3's specificity toward ERKs is very high, and MKP-3 was suggested to be the major phosphatase that keeps ERK activity at low levels in unstimulated cells. We investigated here the mechanisms of activation of ERKs by HS and the involvement of Hsp72and MKP-3 in this regulation.
MATERIALS AND METHODS
Cell cultures, materials, and stresses.
Human lung fibroblasts (IMR90 cells) that underwent 7 to 15 population doublings were grown in minimal essential medium supplemented with 15% fetal bovine serum and 2 mM glutamine. Cos-7 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. All cells were grown at 37°C in an atmosphere of 5% CO2 to 60 to 85% confluence.
Emetine (Sigma) was used at a final concentration of 10 μM. Menadione (Sigma) was prepared fresh each time as a 0.1 M stock solution in phosphate-buffered saline (PBS) and added to cells at a final concentration of 1 mM for 30 min. Phorbol 12,13-dibutyrate (PDB; Sigma) was made as a 2 mM stock solution in dimethyl sulfoxide and added to cells at a final concentration of 1 μM for 7 min. UV irradiation was performed in a UV Stratalinker 1800 (Stratagene, La Jolla, Calif.) with 400 J/m2. HS conditions are described in the text.
Plasmids and transfection.
Plasmid encoding MKP-3 with C-terminal myc tag was a gift of M. Keyse (Imperial Cancer Research Fund). Plasmid encoding MKP-3 phosphatase-dead mutant, MKP-3(C/S) was a gift of J. Denu (Oregon Health Sciences University). For transfection, cells were grown in 35-mm-diameter dishes to 50 to 70% confluence and then washed twice with Opti-MEM 1 (Life Technologies, Gaithersburg, Md.). Cos-7 cells were transfected with GenePORTER transfection reagent (Gene Therapy Systems, San Diego, Calif.) in accordance with the manufacturer's protocol by using 10 μl of the reagent and 2 μg of DNA per dish for 16 h. The dishes were then washed once and left in a regular medium for 24 to 32 h. When required, cells were infected with adenoviruses at 16 h posttransfection (see below).
Antibodies and Western blotting.
In the present study, we used antibodies to phospho-p42/44 MAP kinase (rabbit polyclonal; Cell Signaling), p42 kinase (rabbit polyclonal K-23; Santa Cruz), phospho-MEK1/2 (rabbit polyclonal; Cell Signaling), MEK1 (rabbit polyclonal; StressGen), MEK2 (rabbit polyclonal; StressGen), c-myc (mouse monoclonal 9E10; Zymed), MKP-1 (rabbit polyclonal M-18; Santa Cruz), γ-tubulin (mouse monoclonal B-7; Santa Cruz), MKP-2 (no. 610850BD; Transduction Laboratories), MKP-3 (goat polyclonal N-18; Santa Cruz), Hsp72 (mouse monoclonal, SPA-810; StressGen), and Hsp72ΔEEVD (mouse monoclonal, SPA-820; StressGen). A working dilution of 1:1,000 was used for all antibodies. After an immunoblotting step with the primary antibody, secondary antibodies conjugated with peroxidase were visualized with ECL substrates (Amersham, Arlington Heights, Ill.), and the resulting films were quantified by densitometry.
Adenovirus-based expression of Hsp72 and Hsp72ΔEEVD.
A recombinant adenovirus vector expressing Hsp72 or Hsp72ΔEEVD (AdTR5-DC/HSP70-GFP) was constructed by cloning a dicistronic transcription unit encoding human Hsp72 and Aequorea victoria green fluorescent protein (GFP) gene, separated by encephalomyocarditis virus internal ribosome entry site from pTR-DC/HSP70-GFP (21, 22), into an adenovirus transfer vector. Expression of this transcription unit is controlled by the tetracycline-regulated transactivator protein tTA (9) which we expressed from the separate recombinant adenovirus (AdCMV/tTA) (18). Recombinant adenoviruses were generated by standard techniques as detailed by Jani et al. (13). Inoculation of cells with 3 × 107 PFU of each virus per 35-mm dish was sufficient to infect almost 100% of the cells. At 36 h after infection the cells accumulated large amounts of Hsp72, as judged by immunoblotting of cytosol extracts (not shown). As a control, adenovirus-expressing GFP under the regulation of tTA was used. Adenoviruses were propagated in 293 cells, and high-titer stocks were obtained and purified by CsCl2 density gradient centrifugation.
Cytosol extract preparation and fractionation.
Cells were washed twice with PBS (unless they were depleted of ATP and hence already in PBS), aspirated, and lysed in 200 μl of lysis buffer per 35-mm dish (40 mM HEPES, pH 7.5; 50 mM KCl; 1% Triton X-100; 2 mM dithiothreitol; 1 mM Na3VO4; 50 mM β-glycerophosphate; 50 mM NaF; 5 mM EDTA; 5 mM EGTA; 1 mM phenylmethylsulfonyl fluoride; 1 mM benzamidine; 5 μg each of leupeptin, pepstatin A, and aprotinin/ml). The lysates were clarified by centrifugation in a microcentrifuge at 15,000 rpm for 5 min. The total protein concentration was measured in the supernatants, and then they were diluted with lysis buffer to achieve an equal protein concentration in all samples. All procedures were performed at 4°C. For the separation of soluble and insoluble fractions, the lysates were diluted with lysis buffer to achieve an equal protein concentration in all samples prior to centrifugation at 15,000 × g for 10 min. The pellets were washed once with the lysis buffer and resuspended in a volume of lysis buffer equal to the volume of the supernatant. All samples were supplemented with loading sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis buffer containing 2% SDS and boiled for 3 min before being subjected to immunoblotting. Boiling of samples in SDS buffer led to complete resolubilization of pellets.
Dephosphorylation assay.
Cells were washed two times with PBS and left in PBS supplemented with 5 μM rotenone and 10 mM 2-deoxyglucose for the indicated times as described earlier (19).
RESULTS
Heat shock activates ERKs via both stimulation of MEK1/2 and inhibition of ERK dephosphorylation.
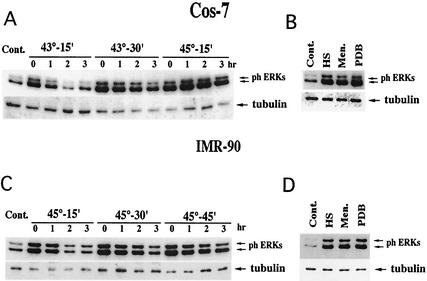
In order to investigate the mechanism of activation of MAP kinases ERK1 and ERK2 by HS, Cos-7 cells and primary human fibroblasts (IMR90 cells) were subjected to various HS conditions, and activation of ERKs was assayed by immunoblotting of cell lysates with anti-phospho-ERK antibody. Exposure of Cos-7 cells to various HS conditions (15 min at 43°C, 30 min at 43°C, or 15 min at 45°C) resulted in about 3.5-fold activation of ERKs (Fig. 1A). Of note, these HS conditions did not cause cell toxicity. Similar activation of ERKs (3.5-fold) was observed when Cos-7 cells were treated with ERK inducer PDB, a phorbol ester analogue, or menadione, an agent that causes an oxidative stress (Fig. 1B). Interestingly, although the degree of ERK activation by heat stresses of various severities was similar, the duration of ERK activities correlated well with the strength of the HS. Indeed, 1 h after Cos-7 cells were heated at 43°C for 15 min, ERK activity has almost returned to background levels. However, when Cos-7 cells were heated at 43°C for 30 min, ERK remained activated even at the 3-h time point. Furthermore, 3 h after cells were heated at 45°C for 15 min, ERK activity remained at its maximal level (Fig. 1A).
FIG. 1.
Duration and extent of ERK activation in cells subjected to various stresses. (A and C) Duration of ERK signal in cells subjected to various HS conditions. Cos-7 cells (A) or IMR90 cells (C) were exposed to the indicated HS conditions or left untreated (Cont.). Cells were harvested at the indicated time points after HS, and ERK activation was assayed by immunoblotting of cell lysates with anti-phospho-ERK antibody or tubulin to control for equal loading. (B and D) Activation of ERKs in cells subjected to various stresses. Cos-7 cells (B) or IMR90 cells (D) were subjected to HS (Cos-7 cells, 43°C for 30 min; IMR90 cells, 45°C for 30 min) or treated with 1 mM menadione for 30 min or 1 μM PDB for 7 min. ERK activation was assayed as in panels A and C.
As with Cos-7 cells, exposure of IMR90 cells to various nontoxic HS conditions (i.e., 45°C for 15, 30, or 45 min) led to a similar (ca. 10-fold) activation of ERKs (Fig. 1C). Although increasing the severity of HS conditions did not affect the extent of ERK activation, it resulted in an increased duration of ERK signaling. Thus, in cells heated at 45°C for 15, 30, or 45 min, ERK remained active for 1, 2, or >3 h, respectively (Fig. 1C). Similar 10-fold activation of ERKs was seen when IMR90 cells were treated with either PDB or menadione (Fig. 1D). Stronger ERK activation in IMR90 cells, compared to Cos-7 cells, can probably be explained by the fact that the background level of ERK activity was much higher in Cos-7 cells than in the fibroblasts.
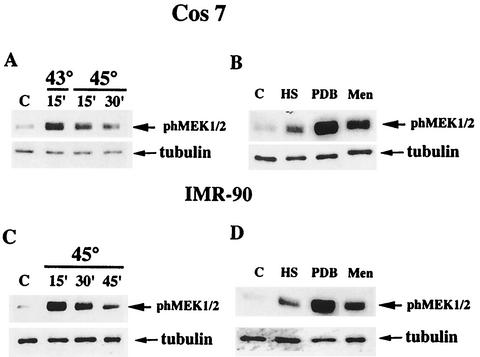
To elucidate the mechanism of ERK activation upon HS, we tested whether ERK activating kinase MEK1/2 was stimulated upon HS. Since MEK1/2 is activated by phosphorylation by kinases of Raf family, the phosphorylation state of MEK1/2 reflects its level of activity. Therefore, lysates of Cos-7 cells exposed to HS were immunoblotted with anti-phospho-MEK1/2 antibody. When Cos-7 cells were heated at 43°C for 15 min, the level of MEK1/2 phosphorylation increased by about sixfold. Surprisingly, when stronger HS conditions were applied (i.e., 43°C for 30 min or 45°C for 15 min), the increase of MEK1/2 phosphorylation was less: ∼2.5- and ∼1.5-fold, respectively (Fig. 2A) (see Discussion). Treatment of cells with PDB resulted in an almost 20-fold increase of MEK1/2 phosphorylation, and treatment with menadione led to a 10-fold increase (Fig. 2B). With IMR90 fibroblasts, heating of cells at 45°C for 15 min resulted in a sixfold increase in the MEK1/2 phosphorylation, whereas both PDB and menadione caused much stronger MEK1/2 phosphorylation (27- or 10-fold, respectively) (Fig. 2D).
FIG. 2.
MEK1/2 activation in cells subjected to various stresses. (A and C) Activation of MEK1/2 in cells subjected to various HS conditions. Cos-7 cells (A) or IMR90 cells (C) were exposed to the indicated HS conditions or left untreated (lanes C). Cells were harvested, and MEK1/2 activation was assayed by immunoblotting of cell lysates with anti-phospho-MEK1/2 antibody. The same membranes were reblotted with anti-tubulin antibody to control for equal loading. (B and D) Activation of MEK1/2 in cells subjected to various stresses. Cos-7 cells (B) or IMR90 cells (D) were subjected to HS (Cos-7 cells, 43°C for 30 min; IMR90 cells, 45°C for 30 min) or treated with 1 mM menadione for 30 min or 1 μM PDB for 7 min. MEK1/2 activation was assayed as in panels A and C.
As with Cos-7 cells, stronger HS conditions dramatically reduced the extent of MEK1/2 phosphorylation in IMR90 cells. So when IMR90 cells were subjected to HS at 45°C for 30 min, only a threefold activation of MEK1/2 was observed, and almost no MEK1/2 activation was detected when cells were heated at 45°C for 45 min (Fig. 2C). It is noteworthy that the level of total MEK1/2 under all heat-shocked conditions remained the same (not shown).
Since ERK activation was similar in cells treated with either HS, PDB, or menadione but HS activated MEK1/2 to a much lesser extent than did PDB or menadione, we sought to determine whether some other factor(s) besides MEK1/2 contributes to ERK activation. Recently, we have shown that stress kinase JNK is activated by HS mainly through inhibition of its dephosphorylation and not through the stimulation of upstream kinase SEK1 (19). Therefore, we investigated the effect of HS on the rate of ERK dephosphorylation by using an assay recently developed in our lab (19). Cells were subjected to various stresses to stimulate ERK phosphorylation, and further phosphorylation was prevented by rapid ATP depletion. The extent of ERK phosphorylation was assayed at different time points after ATP depletion by immunoblotting of cellular lysates with anti-phospho-ERK antibody. It is of note that similar results were obtained when the ERK-activating kinase cascade was inhibited by staurosporine (not shown).
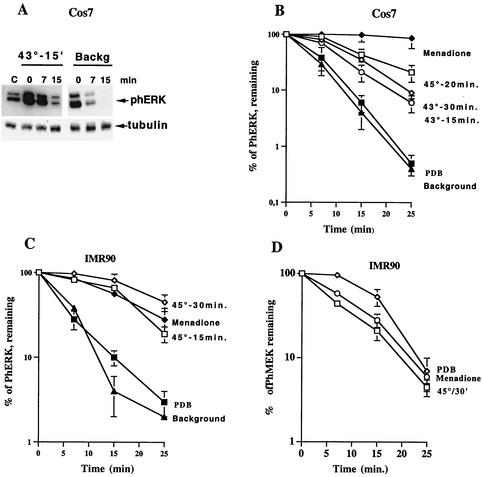
Using this approach, we determined the rate of ERK dephosphorylation in unstressed cells and hence the activity of ERK-specific phosphatases. As shown in Fig. 3A and B, dephosphorylation of ERKs in unstressed Cos-7 cells was extremely fast. When cells were subjected to HS, ERK dephosphorylation significantly slowed down. The level of inhibition of ERK dephosphorylation depended upon the severity of HS, since stronger HS led to stronger inhibition of ERK dephosphorylation (Fig. 3B). Therefore, stronger inhibition of ERK dephosphorylation after severe HS appeared to compensate for the weaker activation of MEK1/2, resulting in similar levels of ERK activities at various HS conditions.
FIG. 3.
Dephosphorylation of ERKs and MEK in cell subjected to various stresses. (A) Dephosphorylation of ERKs in unstimulated cells and in cells heated at 43°C for 15 min. Cos-7 cells were either left untreated (Backg) or heated at 43°C for 15 min. Further phosphorylation was prevented, as described in Materials and Methods. The extent of ERK phosphorylation was assayed at the indicated time points after ATP depletion as in Fig. 1A and C. The same membrane was reblotted with antitubulin antibody to control for equal loading. (B and C). Quantification of the rates of ERK dephosphorylation in cells subjected to different stresses. Cos-7 cells (B) and IMR90 cells (C) were either left untreated (Background), exposed to indicated HS conditions, or treated with 1 mM menadione for 30 min or 1 μM PDB for 7 min. ERK dephosphorylation was assayed as in panel A. Each curve represents the average of at least three experiments. (D) Quantification of the rates of phMEK1/2 dephosphorylation in IMR90 cells subjected to different stresses. IMR90 cells were exposed to HS at 45°C for 15 min or treated with 1 mM menadione for 30 min or 1 μM PDB for 7 min. The extent of MEK1/2 phosphorylation was assayed at the indicated time points after ATP depletion. Each curve represents the average of at least three experiments.
Even very mild HS at 43° for 15 min slowed down ERK dephosphorylation. In contrast, when ERK was stimulated with PDB, ERK dephosphorylation proceeded with the same speed as observed in unstressed cells (Fig. 3B). Interestingly, menadione slowed down ERK dephosphorylation even more strongly than did HS (Fig. 3B). Similar results were obtained with IMR90 fibroblasts, i.e., the rate of ERK dephosphorylation in cells treated with PDB was very fast and close to that seen in unstimulated cells, whereas HS strongly reduced the rate of ERK dephosphorylation (Fig. 3C). These experiments indicated that HS activates ERK both by stimulating MEK1/2, which then phosphorylates ERK, and also by preventing ERK dephosphorylation. Of note, HS did not affect the rate of MEK1/2 dephosphorylation (Fig. 3D). These results suggested that HS activated MEK1/2 not so much by inhibiting MEK1/2 phosphatase but rather at the level of Raf1 kinase or upstream. Therefore, it appears that HS specifically targeted ERK phosphatase.
HS inactivates MKP-3.
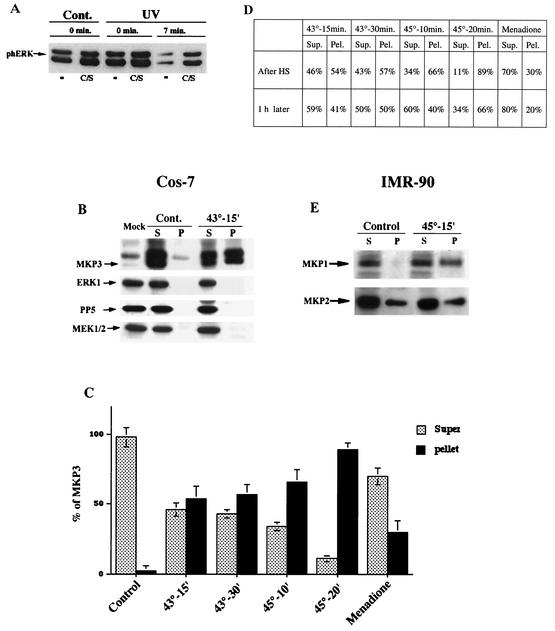
A dual-specificity phosphatase, MKP-3, was suggested to play a critical role in ERK dephosphorylation. This phosphatase has a high specificity for ERKs and is constitutively expressed in the cytoplasm of many cell types. Accordingly, transfection of a phosphatase-dead mutant, MKP-3(C/S), in Cos-7 cells, caused an approximately twofold increase of the background level of phosphorylated ERK1/2 and also reduced the rate of ERK1/2 dephosphorylation in UV-irradiated cells by about twofold (Fig. 4A). These data indicate that MKP-3 contributes significantly to ERK dephosphorylation in Cos-7 cells, but other phosphatases also play a role in this process.
FIG. 4.
MKP-3 shifts from soluble to insoluble fraction upon HS of Cos-7 cells. (A) Effect of MKP-3(C/S) on dephosphorylation of ERK in control and UV-irradiated cells. Cos-7 cells were transfected with the plasmid expressing MKP-3(C/S) or vector alone. Transfected cells were either left untreated (Cont.) or UV irradiated at 400 J/m2 and left for 15 min. The extent of ERK phosphorylation was assayed at the indicated time points after ATP depletion as in Fig. 1A and C. The same membrane was reblotted with anti-MKP-3 antibody to control for MKP-3(C/S) expression or with anti-tubulin antibody to control for equal loading. (B) Redistribution of MKP-3 from soluble to insoluble fraction in cells heated at 43°C for 15 min. Cos-7 cells were transfected with either the plasmid expressing myc-tagged MKP-3 or empty vector (Mock). At 48 h after transfection, untreated cells or cells heated at 43°C for 15 min were harvested and lysed. Lysates were fractionated into soluble and insoluble fractions as described in Materials and Methods and then assayed for the presence of MKP-3 by immunoblotting with anti-myc antibody. The same samples were also immunoblotted with anti-ERK1, anti-PP5, and anti-MEK1/2 antibodies. S, supernatant; P, pellet. (C and D) Redistribution of MKP-3 from soluble to insoluble fractions in cells exposed to various stresses. Cos-7 cells transfected same as for panel B were subjected to the indicated HS conditions or were treated with 1 mM menadione for 30 min. Either right after the treatments or 1 h later the cells were harvested, and cell lysates were assayed as for panel B. Each bar on the graph in panel C represents average of at least three experiments. In panel D, the data represent a typical experiment. Sup., supernatant; Pel., pellet. (E) Redistribution of MKP-1 and MKP-2 from soluble to insoluble fractions in IMR90 cells heated at 45°C for 15 min. IMR90 cells were subjected to the indicated HS. Lysates were fractionated into soluble and insoluble fractions as described in Materials and Methods and assayed for the presence of MKP-1 and MKP-2 by immunoblotting with the corresponding antibody. S, supernatant; P, pellet.
Since HS specifically inhibited ERK dephosphorylation, we investigated how this treatment affected MKP-3. Cos-7 cells were transiently transfected with the plasmid expressing myc-tagged MKP-3. At 48 h after transfection, cells were either left untreated or subjected to HS at 43°C for 15 or 30 min, and 1 h later cell lysates were prepared. To investigate the association of ERKs with MKP-3, we immunoprecipitated MKP-3 by using anti-myc antibody and tested for the presence of ERK in the precipitate by immunoblotting with anti-ERK1 antibody. However, the efficiency of coprecipitation was very low, probably due to the unstable nature of this complex in vivo (not shown). Surprisingly, much less MKP-3 precipitated from lysates prepared from heat-shocked cells compared to untreated cells. These data suggested that following HS MKP-3 could aggregate or bind to large structures, became insoluble, and therefore could be retained in the cell debris fraction.
To check this possibility directly, MKP-3-transfected Cos-7 cells were exposed to HS and left to recover for 1 h to allow aggregation to proceed. Cell lysates were then prepared in the presence of Triton X-100, fractionated to soluble and insoluble fractions by centrifugation at 15,000 × g (see Materials and Methods), and immunoblotted with anti-myc antibody to assay the distribution of MKP-3. As shown in Fig. 4B, MKP-3 was found in unstressed cells in the soluble fraction only. However, even mild HS at 43°C for 15 min caused a large fraction (40%) of MKP-3 to become insoluble (Fig. 4B to D). Upon stronger HS conditions, more MKP-3 became insoluble; thus, when Cos-7 cells were heated at 45°C for 20 min, ca. 66% of MKP-3 shifted into the insoluble fraction (Fig. 4C and D). It is worth noting that under all assayed HS conditions, the ERKs and MEK1/2 remained soluble (Fig. 4B and data not shown). Also, another serine/threonine phosphatase, PP5, remained in the soluble fraction (Fig. 4B). These data suggested that HS could inactivate MKP-3 phosphatase, most likely by promoting MKP-3 aggregation, leading to ERK activation. Treatment of cells with PDB, which did not affect ERK dephosphorylation, had no effect on MKP-3 redistribution (results not shown). Interestingly, menadione, which was also shown to inhibit ERK dephosphorylation, had very little effect on MKP-3 aggregation. After menadione treatment, 80% of the MKP-3 remained soluble, suggesting that menadione inactivated ERK phosphatase not by promoting its aggregation but by some other mechanism (Fig. 4C and D) (e.g., by oxidizing critical SH groups in its active center).
In the MKP-3 inactivation experiments described above, cells were harvested 1 h after HS to allow aggregation to proceed. To check whether aggregation is in fact a slow process, we also fractionated lysates prepared from cells harvested right after HS. To our surprise, even more MKP-3 was found in the insoluble fraction immediately after HS than 1 h later. In fact, in cells subjected to very mild HS, i.e., at 43°C for 15 min, 54% of the MKP-3 was found in the insoluble fraction (Fig. 4B to D), and in cells heated at 45°C for 20 min 90% of MKP-3 became insoluble (Fig. 4C and D). Therefore, aggregation of MKP-3 upon HS is a fast and reversible process.
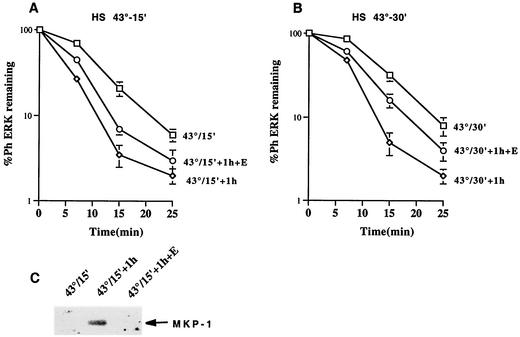
If aggregation of MKP-3 were responsible for the inhibition of ERK dephosphorylation upon HS, then ERK dephosphorylation right after HS should occur more slowly than 1 h later, since during this period MKP-3 partially redistributed back to soluble fraction (see above). In fact, as seen in Fig. 5A and B, ERK dephosphorylation 1 h after HS was faster than that assayed right after HS. On the other hand, the increase in the speed of ERK dephosphorylation observed 1 h after HS can also be caused by an induction of alternative dual-specificity phosphatases (15), which can dephosphorylate ERKs. To see whether induction of new phosphatases played a role in the increased rate of ERK dephosphorylation observed 1 h after HS, Cos-7 cells were heated at 43°C for 15 or 30 min and incubated in the presence of a potent protein synthesis inhibitor, emetine, to prevent accumulation of phosphatases. As shown in Fig. 5A and B, the rate of ERK dephosphorylation in emetine-treated cells was intermediate between that seen in cells recovered in the absence of emetine and those harvested right after HS, indicating that induction of other phosphatase(s) could contribute to the increased rate of ERK dephosphorylation. In fact, we found that one of the stress-inducible phosphatases, MKP-1, accumulated in Cos-7 cells heated at 43°C for 15 during a 1-h recovery period, and this accumulation was completely blocked when cells were incubated in the presence of emetine (Fig. 5C). Therefore, we concluded that both de novo induction of phosphatases such as MKP-1, and the recovery of MKP-3 from the insoluble fraction contributed to the increase in the rate of ERK dephosphorylation.
FIG. 5.
Effect of MKP-3 resolubilization and de novo synthesis of MKP-1 on the rate of ERK dephosphorylation during recovery from HS. (A and B) Dephosphorylation of ERKs in cells heated at 43°C for 15 min (A) or 30 min (B). Cos-7 cells were heated at 43°C for 15 min. The rate of ERK dephosphorylation (measured as described for Fig. 3A) was assay either immediately after HS (□) or after 1 h of recovery at 37°C with (○) or without (⋄) the protein synthesis inhibitor emetine. Each curve represents the average of at least three experiments. (C) Induction of MKP-1 in cells heated at 43°C for 15 min. Cos-7 cells were heated at 43°C for 15 min, and the cells were either immediately harvested or incubated with or without emetine. The induction of MKP-1 was assayed in cell lysates by immunoblotting with anti-MKP-1 antibody.
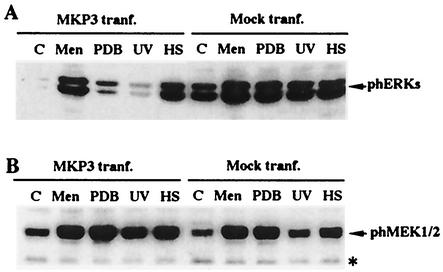
Next, we checked the effect of HS on MKP-3 activity. For this purpose, we assayed ERK activation by HS and menadione compared to the activation iduced by PDB and UV in cells transfected with plasmid expressing either myc-tagged MKP-3 or empty vector. In cells transfected with empty vector, the background activity of ERKs was rather high, and HS, UV, PDB, and menadione treatment further stimulated ERK phosphorylation (Fig. 6A). Interestingly, in cells expressing MKP-3 the background level of ERK activity was much lower, indicating that MKP-3 phosphatase, when expressed in transfected cells, effectively dephosphorylated ERKs. Little activation of ERKs by either UV or PDB treatment was detected (Fig. 6A). Therefore, MEK1/2-dependent phosphorylation of ERK after either the UV or the PDB treatment could not overcome the effects of MKP-3 expression. In contrast, HS and menadione were able to overwhelm the effects of MKP-3 and strongly activated ERKs in MKP-3-transfected cells. This experiment implied that HS and menadione led to inactivation of MKP-3, thus bringing about ERK activation (Fig. 6A). Importantly, the expression of MKP-3 did not affect either the background level of MEK1/2 phosphorylation or phosphorylation of MEK1/2 triggered by any agent (Fig. 6B).
FIG. 6.
Activation of ERKs and MEK1/2 by various stresses in cells expressing MKP-3. (A) Activation of ERKs by various stresses in cells transfected (tranf.) with either MKP-3-expressing plasmid or vector alone (Mock tranf.). Cos-7 cells transfected as described for Fig. 4A were treated with menadione (Men; 1 mM, 30 min) or PDB (1 μM, 7 min), UV irradiated (400 J/m2), or subjected to HS at 43°C for 30 min. Immediately after these treatments, cells were harvested and ERK activation was assayed. (B) Activation of MEK1/2 by various stresses in cells transfected with either MKP-3-expressing plasmid or vector alone. This experiment was carried out as described for panel A, but the samples were reblotted with anti-phospho-MEK1/2 antibody. The band marked with an asterisk is a cross-reacting protein that provides an internal control for equal loading. Lanes C, untreated controls.
As mentioned above, unstressed Cos-7 cells had negligible levels of MKP-1, a phosphatase that can dephosphorylate ERKs, as well as JNK and p38, and heat treatment rapidly induced this phosphatase. In contrast, in IMR90 cells MKP-1 was expressed constitutively, thus allowing us to investigate the effect of HS on this alternative dual-specificity phosphatase. IMR90 cells were exposed to HS at 45°C for 15 min, cell lysates were fractionated by centrifugation into soluble and insoluble fraction as described above, and the levels of endogenous MKP-1 in both fractions were monitored by immunoblotting with the corresponding antibodies. In control cells, MKP-1 was almost entirely soluble, whereas HS shifted almost 50% of the MKP-1 into the particulate fraction (Fig. 4E). A 1-h recovery period led to partial resolubilization of MKP-1 (data not shown). Therefore, both MKP-3 and MKP-1 appeared to be inactivated and to aggregate in response to nontoxic heat treatment.
MKP-2 is another dual-specificity phosphatase that can inactivate JNK and ERK kinases. We found that in both IMR90 and Cos-7 cells MKP-2 was expressed constitutively and was not further induced by HS. In contrast to MKP-1 and MKP-3, ca. 20% of MKP-2 was found in the particulate fractions in unstressed IMR90 or Cos-7 cells (Fig. 4 E), and HS of the cells did not trigger further MKP-2 aggregation. Therefore, aggregation in response to nontoxic HS is a specific property of only a subset of dual-specificity phosphatases.
Dual effect of Hsp72 on ERK pathway.
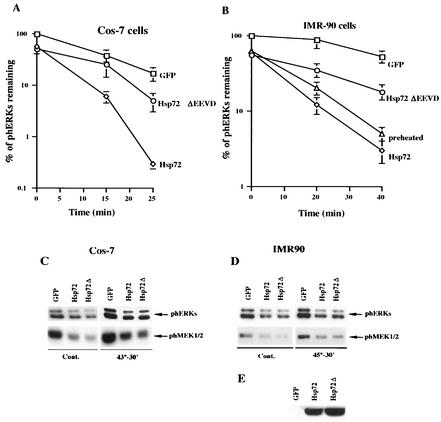
Recently, we have shown that the major inducible member of Hsp70 family, Hsp72, can protect JNK dephosphorylation from inactivation by HS. Therefore, we investigated whether Hsp72 can also prevent inactivation of ERK phosphatases by HS. For these experiments we used bicistronic adenovirus vector expressing Hsp72, along with GFP, under the control of a tetracycline-regulated promoter (see Materials and Methods). As a control we used adenovirus-expressing GFP only under the control of the same promoter. Cos-7 cells were infected with either Hsp72- or GFP-expressing adenoviruses, and the corresponding proteins were induced for 36 h. Almost 100% of cells were infected with virus under these conditions, as judged by the expression of GFP, which was monitored by fluorescence microscopy. It should be noted that without HS Cos-7 cells have very low levels of endogenous Hsp72, and its induction after HS takes at least 2 to 3 h. The cells were exposed to HS, and the rate of ERK dephosphorylation was assayed immediately after the treatment. As shown in Fig. 7A, adenoviral expression of recombinant Hsp72 increased the rate of ERK dephosphorylation approximately twofold. Moreover, Hsp72 also suppressed ERK activation by HS about twofold (Fig. 7A and C).
FIG. 7.
Hsp72 and Hsp72ΔEEVD suppress HS mediated activation of ERKs and MEK1/2. (A and B) Quantification of the rates of ERK dephosphorylation in cells overexpressing the normal or mutant form of Hsp72 or preheated at 45°C for 30 min. Cos-7 cells (A) or IMR90 cells (B) were infected with adenovirus expressing either GFP (□), Hsp72 (⋄), or Hsp72ΔEEVD (○). At 36 h postinfection, cells were heated for 30 min at 43°C (A) or 45°C (B), and the rate of ERK dephosphorylation was assayed. The percentage of ERK activity was normalized to GFP-expressing cells. Each curve represents the average of at least three experiments. Alternatively, in panel B IMR90 cells were subjected to HS at 45°C for 30 min, recovered at 37°C for 18 h, and subjected to a second HS at 45°C for 30 min (preheated). (C and D) Hsp72 and Hsp72ΔEEVD suppress background and HS-induced ERKs and MEK1/2 activity. Cos-7 cells (C) and IMR90 cells (D) were infected as described for panels A and B and either left untreated (Cont.) or heated at 43°C (A) or 45°C (B). The extent of ERK and MEK1/2 activation was assayed by immunoblotting cell lysates with anti-phospho-ERK or anti-phosph-MEK1/2 antibody. (E) Expression of Hsp72 or Hsp72ΔEEVD in IMR90 cells infected with the corresponding adenovirus. IMR90 cells were infected with GFP, Hsp72, or Hsp72ΔEEVD as described above. Cell lysates were immunoblotted with SPA820 antibody, which recognizes both normal and mutant Hsp72 proteins.
Similar results were obtained with IMR90 cells. Naive IMR90 cells do not express endogenous Hsp72 (Fig. 7E, GFP lane), and induction of Hsp72 after HS takes 3 to 4 h. Overexpression of exogenous Hsp72 in IMR90 cells, followed by exposure to HS at 45°C for 30 min, led to an approximately twofold increase in the rate of ERK dephosphorylation compared to control GFP-expressing cells (Fig. 7B). Activation of ERKs by HS was suppressed in Hsp72-expressing IMR90 cells by about twofold (Fig. 7B and D). These experiments suggested that Hsp72 could, at least partially, protect ERK phosphatase from HS-mediated inactivation, a result similar to the effect of Hsp72 on JNK dephosphorylation.
To investigate the effects of endogenous Hsp72 on ERK dephosphorylation, we induced Hsp72, together with other Hsps, by subjecting IMR90 cells to mild HS at 45°C for 30 min and then allowing the cells to recover at 37°C for 18 h. By that time the levels of Hsp72 increased dramatically, whereas the levels of MKP-1 and MKP-2 were similar to that seen in naive control cells (data not shown). Both naive and HS-pretreated cells were exposed to a second HS at 45°C for 30 min, and the rates of ERK dephosphorylation were monitored as described above. Figure 7B shows that physiological induction of Hsps by mild HS increased ERK dephosphorylation twofold compared to control cells. Similar results were obtained in Cos-7 cells (data not shown).
Next, we investigated whether the chaperone activity of Hsp72 is required for its ability to alleviate HS-mediated inhibition of ERK phosphatases. For these experiments, we used adenovirus expressing a mutant form of Hsp72, which has a deletion of the C-terminal four amino acids EEVD (Hsp72ΔEEVD). This deletion prevents Hsp72 from interaction with its substrates, therefore abolishing chaperone activity of Hsp72. Hsp72ΔEEVD was expressed under the control of a tetracycline-regulated promoter. Cos-7 and IMR90 cells were infected with Hsp72ΔEEVD-expressing virus; 36 h later the cells were exposed to various HS conditions, and the rate of ERK dephosphorylation was assayed. In contrast to normal Hsp72, Hsp72ΔEEVD failed to speed up ERK dephosphorylation in either Cos-7 cells, or IMR90 cells (Fig. 7A and B). (Normal Hsp72 and its mutant variant, Hsp72ΔEEVD, were expressed at similar levels [Fig. 7E].) Interestingly, in both cell types, Hsp72ΔEEVD suppressed ERK activation by HS to a similar extent as normal Hsp72 (Fig. 7C and D). Moreover, Hsp72ΔEEVD also reduced the background ERK activity in both IMR90 and Cos-7 cells (in Cos-7 cells the effect on ERK was even greater than the effect of normal Hsp72) (Fig. 7C and D). These experiments demonstrated that Hsp72 prevented inhibition of ERK phosphatases by HS in both Cos-7 and IMR90 cells. Moreover, although Hsp72 effect on HS-triggered ERK activation was independent of Hsp72 chaperone activity, the effect of Hsp72 on ERK dephosphorylation strictly relies on the chaperone activity of Hsp72.
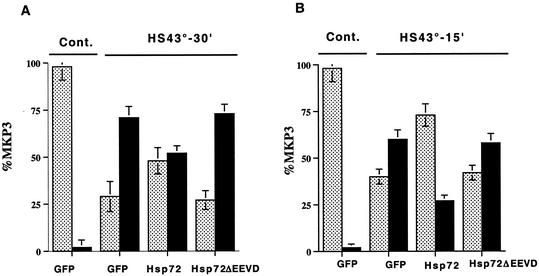
Next, we investigated the effect of Hsp72 on the solubility of MKP-3 phosphatase. Cos-7 cells were transfected with plasmid expressing myc-tagged MKP-3, incubated overnight, and 12 h later infected with adenovirus expressing either Hsp72 or GFP as a control. At 36 h postinfection (i.e., at 48 h posttransfection), the cells were subjected to HS. Cell lysates were then prepared, fractionated into soluble and insoluble fractions, and immunoblotted with anti-myc antibody to assay for MKP-3 solubility. When control GFP-expressing cells were subjected to HS at 43°C for 30 min and recovered for 1 h, only 30% of the MKP-3 remained soluble, whereas in Hsp72-expressing cells at least 50% of the MKP-3 was still soluble (Fig. 8A). In milder HS conditions (43°C for 15 min), 40% of the MKP-3 remained soluble in control cells, whereas in Hsp72-expressing cells ca. 70% of MKP-3 remained in a soluble fraction (Fig. 8B). In contrast to normal Hsp72, Hsp72ΔEEVD, which did not affect ERK dephosphorylation, could not protect MKP-3 from aggregation (Fig. 8A and B).
FIG. 8.
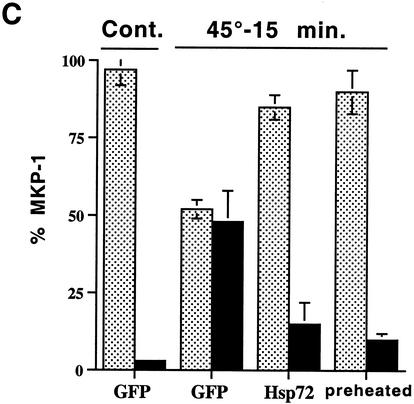
Hsp72 but not Hsp72ΔEEVD prevents aggregation of MKP-3 in cells exposed to HS. (A and B) Cos-7 cells were transfected with the plasmid expressing myc-tagged MKP-3, and 12 h later infected with adenovirus expressing GFP, Hsp72, or Hsp72ΔEEVD. At 36 h postinfection, the cells were heated at 43°C for 30 (A) or 15 min (B), and MKP-3 redistribution from soluble to insoluble fraction was assayed as described for Fig. 4A. Each bar on the graph represents the average of at least three experiments. (C) IMR90 cells were infected with adenovirus expressing GFP, Hsp72, or Hsp72ΔEEVD. At 36 h postinfection, cells were heated at 45° for 15 min, and MKP-1 redistribution from soluble to insoluble fraction was assayed as described for Fig. 4A. Alternatively, IMR90 cells were subjected to HS at 45°C for 30 min, recovered at 37°C for 18 h, and subjected to a second HS at 45°C for 15 min (preheated). Each bar on the graph represents the average of at least three experiments. Bars: , supernatant; ▪, pellet.
Another dual-specificity phosphatase, MKP-1, also readily aggregated upon HS (see above). Accordingly, in IMR90 cells subjected to HS at 45°C for 15 min 40% of the MKP-1 went into the particulate fraction. In Hsp72-expessing cells, aggregation of MKP-1 was completely prevented (Fig. 8C). A similar result was seen with cells preexposed to mild HS, followed by recovery to express endogenous Hsps (Fig. 8C). As we observed with MKP-3, Hsp72ΔEEVD mutant did not protect MKP-1 from aggregation (data not shown).
Interestingly, as mentioned above, Hsp72ΔEEVD, being unable to regulate ERK dephosphorylation, still potently suppressed ERK activation by HS in both Cos-7 and IMR90 cells. Furthermore, Hsp72ΔEEVD, as well as normal Hsp72, reduced ERK background activity in both cell types. These results suggested that in the ERK activation pathway Hsp72 has an additional target other than ERK phosphatase located upstream of ERK. Accordingly, we investigated the effect of Hsp72 and Hsp72ΔEEVD on the levels of MEK1/2 phosphorylation. In both IMR90 and Cos-7 cells, the rate of MEK1/2 dephosphorylation after HS was fast, and neither wild-type Hsp72 nor Hsp72ΔEEVD had any significant effect on the rate of MEK1/2 dephosphorylation (data not shown). In Cos-7 cells, both Hsp72 and Hsp72ΔEEVD reduced the levels of MEK1/2 phosphorylation after HS (Fig. 7C). Also, Hsp72ΔEEVD, similar to wild-type Hsp72, strongly suppressed the background levels of MEK1/2 activity (Fig. 7C). In IMR90 cells, as in Cos-7 cells, both Hsp72 and Hsp72ΔEEVD suppressed the phosphorylation of MEK1/2 after HS at 45°C for 30 min (Fig. 7D). In cells subjected to milder HS conditions, i.e., 45°C for 15 min, when the activation of MEK1/2 was stronger, the effect of both normal Hsp72 and Hsp72ΔEEVD was even more pronounced (data not shown). Normal Hsp72 and Hsp72ΔEEVD also suppressed the background MEK1/2 activation (Fig. 7D). These results suggest that Hsp72 could suppress both background and HS-induced ERK activation upstream of MEK1/2.
DISCUSSION
HS activates cell death signaling pathways, including JNK (32), and survival pathways, including ERKs (35), and also induces Hsps (25). The mechanism of JNK activation by HS appeared to be primarily linked to inactivation of JNK dephosphorylation (25). Here we investigated the mechanisms of activation of a survival kinase ERK by HS.
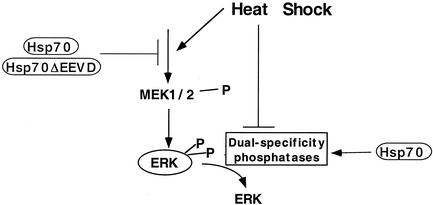
We demonstrated that even relatively mild heat treatment rapidly reduced the rate of ERK dephosphorylation due to inactivation of the ERK phosphatases, e.g., MKP-3 and MKP-1. In addition, as shown previously, HS stimulated the ERK-activating kinases, MEK1/2, which probably proceeds through a ceramide-dependent mechanism (35). Unexpectedly, more severe HS conditions evoked weaker activation of MEK1/2 and simultaneously led to a stronger inhibition of ERK phosphatases. Since both activation of MEK1/2 and inactivation of ERK phosphatases contribute to ERK activity, various HS treatments culminated in ERK activation to a similar extent. Therefore, ERK activation in heat-shocked cells is precisely tuned by two counteracting processes (Fig. 9). It appears that under milder HS conditions the activation of ERKs proceeds mainly through stimulation of MEK1/2, whereas inder more-severe HS the inhibition of ERK phosphatases becomes most important. Although the levels of ERK activation by heat were similar in a wide range of conditions, the duration of ERK activity closely correlated with the strength of the heat treatments. With JNK it was shown that the duration of JNK activity is the major factor that determines whether a cell death program is to be initiated. The importance of the duration of ERK activation is yet to be determined.
FIG. 9.
Effects of HS and Hsp72 on activation of ERKs.
HS triggered MKP-3 and MKP-1 inactivation by promoting their aggregation, since significant fractions of MKP-3 and MKP-1 became insoluble even under mild HS conditions. It is possible that the shift of these phosphatases into the particulate fraction after HS was due to association with large cellular structures. This possibility, however, does not affect our model, since ERKs remained soluble after HS. As reported before, HS also stimulates the aggregation of the JNK phosphatase M3/6 (23). Therefore, it seems that MKP-3, MKP-1, and M3/6, as well as potentially some other dual-specificity phosphatases, are extremely heat labile, and their inactivation serves as a general mechanism of MAP kinase activation upon HS. Importantly, a distinct phosphatase that can dephosphorylate ERK, MKP-2, did not aggregate in response to these HS conditions. Therefore, the effects of HS on MKP-3, MKP-1, and M3/6 appeared to be very specific.
Interestingly, since hyperthermia was developed as a method of anticancer therapy, investigators tried to identify a putative heat-labile protein whose inactivation leads to cell death. It appears that certain dual-specificity phosphatases may represent these critical labile proteins, since their heat inactivation leads to activation of various death and survival programs, ultimately defining the outcome of the treatment.
The aggregation of MKP-3 and MKP-1 was a very fast event, since they shifted into the insoluble fraction almost immediately after heat treatment. Surprisingly, the aggregation was not an irreversible event, since 1 h after HS some fractions of MKP-3 and MKP-1 resolubilized. Accordingly, the rate of ERK dephosphorylation increased by 1 h after HS. This increase in the speed of ERK dephosphorylation activity was not solely dependent on the resolubilization of aggregated phosphatases. Heat treatment of cells induced de novo synthesis of dual-specificity phosphatases, which also contributed to enhancement of ERK dephosphorylation, since inhibition of protein synthesis with emetine reduced the speedup of ERK dephosphorylation by about 50%. Therefore, resolubilization of aggregated phosphatases after HS, along with the synthesis of phosphatases de novo, serves to limit ERK signaling defining the time window of ERK activation.
Whereas kinases are activated rapidly in response to HS (within a few minutes), the accumulation of Hsps proceeds much more slowly (within hours). Hsps, beside their well-established role in assisting refolding and degradation of heat-damaged proteins, are also involved in the regulation of stress kinases. Indeed, in the JNK pathway Hsp72 inhibits JNK activation through protection of JNK phosphatases (6) and inhibition of an upstream component of JNK signaling cascade, Ask1 (24). We report here that Hsp72 also counteracts heat-mediated inactivation of ERK phosphatase. Importantly, Hsp72ΔEEVD mutant, which has no chaperone activity, failed to protect ERK dephosphorylation and to prevent aggregation of phosphatases. Therefore, Hsp72-mediated protection of ERK phosphatases relies on the chaperone activity of Hsp72. Surprisingly, Hsp72ΔEEVD was able to reduce background and heat-induced levels of ERK activation to the same extent as normal Hsp72, suggesting that Hsp72ΔEEVD interferes with the ERK-activating kinase cascade. Indeed, we observed a dramatic reduction in both background and heat-induced levels of MEK1/2 activity upon Hsp72ΔEEVD expression. A similar level of suppression of MEK1/2 was seen with normal Hsp72. These results indicate that in the ERK signaling pathway Hsp72 can both inhibit the kinase cascade upstream of MEK1/2 and protect ERK phosphatase from inactivation (Fig. 9). Furthermore, whereas suppression of the kinase cascade does not require the chaperone activity of Hsp72, protection of the phosphatase does (Fig. 9).
The effect of Hsp72 on the kinase cascade could involve an Hsp72 cofactor, Bag1. As reported previously, Bag1 binds and stimulates Raf1, and Hsp72, by titrating Bag1 from its complex with Raf1, abolishes Raf1 signaling (29). Hsp72ΔEEVD has an intact ATPase domain, and therefore it should retain its ability to interact with Bag1 and thus its ability to inhibit Raf1 and subsequently ERKs.
ERK activation is implicated in increased resistance of cells to various stresses. Therefore, suppression of ERKs by Hsp72 could have an adverse effect on cell survival. In fact, unlike many examples of Hsp72-mediated cell protection, which is often associated with the suppression of JNK, it was reported that the overexpression of Hsp72 in Jurkat cells makes them more susceptible to Fas-dependent apoptosis (17). Interestingly, these authors also noticed that cells overexpressing Hsp72 have increased phosphatase activities, which could contribute to the suppression of ERKs. Suppression of ERKs by Hsp72 could serve to eliminate cells damaged by severe stress, whose survival can put at risk the organism as a whole. The reduction of MEK1/2 activation under severe HS conditions may serve the same purpose, i.e., the avoidance of an excessive activation of the survival pathway.
In conclusion, it appears that HS activates both the death-promoting kinase JNK and the survival-promoting kinase ERK as well as induces the synthesis of Hsp72, which can turn off both kinases. Activation of both MAP kinases involves inhibition of the corresponding phosphatases. Therefore, dual-specificity phosphatases emerge as critical heat-labile proteins whose activities determine the cell fate under HS.
Acknowledgments
This work was supported by an NIH grant.
REFERENCES
- 1.Alessi, D. R., N. Gomez, G. Moorhead, T. Lewis, S. M. Keyse, and P. Cohen. 1995. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 5:283-295. [DOI] [PubMed] [Google Scholar]
- 2.Camps, M., A. Nichols, C. Gillieron, B. Antonsson, M. Muda, C. Chabert, U. Boschert, and S. Arkinstall. 1998. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280:1262-1265. [DOI] [PubMed] [Google Scholar]
- 3.Erhardt, P., E. J. Schremser, and G. M. Cooper. 1999. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol. Cell. Biol. 19:5308-5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feige, U., R. Morimoto, I. Yahara, and B. Polla. 1996. Stress-inducible cellular responses. Birkhauser Verlag, Basel, Switzerland.
- 5.Gabai, V. L., A. B. Meriin, J. A. Yaglom, J. Y. Wei, D. D. Mosser, and M. Y. Sherman. 2000. Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stress-induced inhibition of JNK dephosphorylation. J. Biol. Chem. 275:38088-38094. [DOI] [PubMed] [Google Scholar]
- 6.Gabai, V. L., A. B. Meriin, D. D. Mosser, A. W. Caron, S. Rits, V. I. Shifrin, and M. Y. Sherman. 1997. HSP70 prevent activation of stress kinases: a novel pathway of cellular thermotolerance. J. Biol. Chem. 272:18033-18037. [DOI] [PubMed] [Google Scholar]
- 7.Gabai, V. L., and M. Y. Sherman. 2002. Invited review: interplay between molecular chaperones and signaling pathways in survival of heat shock. J. Appl. Physiol. 92:1743-1748. [DOI] [PubMed] [Google Scholar]
- 8.Gabai, V. L., J. A. Yaglom, V. Volloch, A. B. Meriin, T. Force, M. Koutroumanis, B. Massie, D. D. Mosser, and M. Y. Sherman. 2000. Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol. Cell. Biol. 20:6826-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groom, L. A., A. A. Sneddon, D. R. Alessi, S. Dowd, and S. M. Keyse. 1996. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 15:3621-3632. [PMC free article] [PubMed] [Google Scholar]
- 11.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-579. [DOI] [PubMed] [Google Scholar]
- 12.Holmstrom, T. H., S. E. Tran, V. L. Johnson, N. G. Ahn, S. C. Chow, and J. E. Eriksson. 1999. Inhibition of mitogen-activated kinase signaling sensitizes HeLa cells to Fas receptor-mediated apoptosis. Mol. Cell. Biol. 19:5991-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jani, A., H. Lochmuller, G. Acsadi, M. Simoneau, J. Huard, A. Garnier, G. Karpati, and B. Massie. 1997. Generation, validation, and large scale production of adenoviral recombinants with large size inserts such as a 6.3-kb human dystrophin cDNA. J. Virol. Methods 64:111-124. [DOI] [PubMed] [Google Scholar]
- 14.Keyse, S. M. 1998. Protein phosphatases and the regulation of MAP kinase activity. Semin. Cell. Dev. Biol. 9:143-152. [DOI] [PubMed] [Google Scholar]
- 15.Keyse, S. M., and E. A. Emslie. 1992. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature 359:644-647. [DOI] [PubMed] [Google Scholar]
- 16.Kyriakis, J. M. 1999. Making the connection: coupling of stress-activated ERK/MAPK (extracellular-signal-regulated kinase/mitogen-activated protein kinase) core signalling modules to extracellular stimuli and biological responses. Biochem. Soc. Symp. 64:29-48. [PubMed] [Google Scholar]
- 17.Liossis, S.-N. C., X. Z. Ding, J. G. Kiang, and G. C. Tsokos. 1997. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J. Immunol. 158:5668-5675. [PubMed] [Google Scholar]
- 18.Massie, B., F. Couture, L. Lamoureux, D. D. Mosser, C. Guilbault, P. Jolicoeur, F. Belanger, and Y. Langelier. 1998. Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette. J. Virol. 72:2289-2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meriin, A. B., J. A. Yaglom, V. L. Gabai, D. D. Mosser, L. Zon, and M. Y. Sherman. 1999. Protein damaging stresses activate JNK via inhibition of its phosphatase: a novel pathway controlled by Hsp72. Mol. Cell. Biol. 19:2547-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millward, T. A., S. Zolnierowicz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24:186-191. [DOI] [PubMed] [Google Scholar]
- 21.Mosser, D. D., A. W. Caron, L. Bourget, C. Denis-Larose, and B. Massie. 1997. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17:5317-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosser, D. D., A. W. Caron, L. Bourget, P. Jolicoeur, and B. Massie. 1997. Use of a dicistronic expression cassette encoding the green fluorescent protein for the screening and selection of cells expressing inducible gene products. BioTechniques 22:150-161. [DOI] [PubMed] [Google Scholar]
- 23.Palacios, C., M. K. Collins, and G. R. Perkins. 2001. The JNK phosphatase M3/6 is inhibited by protein-damaging stress. Curr. Biol. 11:1439-1443. [DOI] [PubMed] [Google Scholar]
- 24.Park, H. S., S. G. Cho, C. K. Kim, H. S. Hwang, K. T. Noh, M. S. Kim, S. H. Huh, M. J. Kim, K. Ryoo, E. K. Kim, W. J. Kang, J. S. Lee, J. S. Seo, Y. G. Ko, S. Kim, and E. J. Choi. 2002. Heat shock protein hsp72 is a negative regulator of apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 22:7721-7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirkkala, L., P. Nykanen, and L. Sistonen. 2001. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15:1118-1131. [DOI] [PubMed] [Google Scholar]
- 26.Saxena, M., and T. Mustelin. 2000. Extracellular signals and scores of phosphatases: all roads lead to MAP kinase. Semin. Immunol. 12:387-396. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer, H. J., A. D. Catling, S. T. Eblen, L. S. Collier, A. Krauss, and M. J. Weber. 1998. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668-1671. [DOI] [PubMed] [Google Scholar]
- 28.Scheid, M. P., and V. Duronio. 1998. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/Akt: involvement of MEK upstream of Bad phosphorylation. Proc. Natl. Acad. Sci. USA 95:7439-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song, J., M. Takeda, and R. I. Morimoto. 2001. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat. Cell Biol. 3:276-282. [DOI] [PubMed] [Google Scholar]
- 30.Strasser, A., and R. L. Anderson. 1995. Bcl-2 and thermotolerance cooperate in cell survival. Cell Growth Differ. 6:799-805. [PubMed] [Google Scholar]
- 31.Tran, S. E., T. H. Holmstrom, M. Ahonen, V. M. Kahari, and J. E. Eriksson. 2001. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J. Biol. Chem. 276:16484-16490. [DOI] [PubMed] [Google Scholar]
- 32.Verheij, M., R. Bose, X. H. Lin, B. Yao, W. D. Jarvis, S. Grant, M. J. Birrer, E. Szabo, L. I. Zon, J. M. Kyriakis, A. Haimovitz-Friedman, Z. Fuks, and R. N. Kolesnick. 1996. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 380:75-79. [DOI] [PubMed] [Google Scholar]
- 33.Volloch, V. Z., V. L. Gabai, S. Ritz, and M. Y. Sherman. 2000. Hsp72 can protect cells from heat-induced apoptosis by accelerating the inactivation of stress kinase JNK. Cell Stress Chaperones 5:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein-Oppenheimer, C. R., W. L. Blalock, L. S. Steelman, F. Chang, and J. A. McCubrey. 2000. The Raf signal transduction cascade as a target for chemotherapeutic intervention in growth factor-responsive tumors. Pharmacol. Ther. 88:229-279. [DOI] [PubMed] [Google Scholar]
- 35.Woessmann, W., Y. H. Meng, and N. F. Mivechi. 1999. An essential role for mitogen-activated protein kinases, ERKs, in preventing heat-induced cell death. J. Cell Biochem. 74:648-662. [DOI] [PubMed] [Google Scholar]