Abstract
p27Kip1 (p27) is often inappropriately downregulated in aggressive human cancers. Although p27 can inhibit cyclin-dependent kinases (CDKs), low p27 does not always correlate with increased CDK activity. Furthermore, cells derived from p27−/− mice respond to antimitogens, maintain restriction point control, and do not deregulate CDKs. Thus, disruption of a p27 function other than CDK inhibition may contribute to the disease state. A yeast two-hybrid screen identified growth factor receptor-bound protein 2 (GRB2) as a p27 binding partner. We now demonstrate that p27 can inhibit GRB2 function by blocking its association with the guanine nucleotide exchange factor SOS. Endogenous p27 is rapidly exported from the nucleus to the cytoplasm in response to mitogen stimulation, where it binds GRB2 concomitant with a decrease in GRB2-associated SOS. As predicted, mitogen-stimulated p27−/− cells maintained their GRB2-SOS complexes for significantly longer. The Ras/mitogen-activated protein kinase pathway does not appear to be deregulated in cells lacking p27 despite excess GRB2-SOS, suggesting that additional control mechanisms are present. A transient-transfection approach was employed to show that p27 can inhibit Ras activation by targeting GRB2 and further revealed that the CDK and GRB2 inhibitory functions of p27 are separable and distinct. Thus, p27 downregulation may compromise control of Ras, one of the most common oncogenic events in human cancer.
During G1 phase cells respond to extracellular signals and decide whether to proliferate or adopt an alternate fate (23). Extracellular information is transmitted through signaling pathways intimately connected to the G1 cell cycle machinery, helping ensure proper implementation of cell fate decisions (20, 25). Deregulation of the decision-making process—either by direct alteration of cell cycle components or by inappropriate activation of signal transduction cascades—contributes to the hyperproliferation characteristic of cancer cells (11, 26).
Cyclin-dependent kinases (CDKs) catalyze progression through the G1 phase of the cell cycle and are tightly controlled by both positive and negative regulatory events (43). Two families of CDK inhibitory proteins (CKIs) have been identified: The INK4 proteins (p15, p16, and p19) specifically inhibit cyclin D-CDK4/6, while the Cip/Kip proteins (p21, p27, and p57) are traditionally considered more broad-spectrum inhibitors of cyclin D, E, and A CDK complexes (61). Accumulating evidence from diverse experimental systems has revealed significant limitations of these simple designations, especially with respect to p27 (62). The relationship between cyclin-CDKs and p27 is actually quite complex and, depending on context, can result in diametrically opposed outcomes. For instance, p27 can inhibit cyclin D-CDK4 or act as an assembly factor, and it can inhibit cyclin E-CDK2 or be phosphorylated and targeted for ubiquitin-dependent degradation (9, 35, 45, 49, 60, 75). These alternative outcomes suggest p27 might have important functions in addition to CDK inhibition, which in turn could influence physiological processes other than proliferation. In fact, p27 has been implicated in cellular adhesion, apoptotic control, and senescence; it is unclear whether CDK regulation is solely responsible for these phenotypic effects (59).
The consequences of ablating p27 in mice further highlight limitations of the CDK inhibitory model (21, 33, 46). These animals are viable and develop normally, yet are 33% larger than p27+/+ littermates due to an increased number of cells. The mice do not accumulate aggressive tumors in multiple tissues or organs but rather a hyperplasia confined to the intermediary lobe of the pituitary. Given the putative role of p27 as an essential CDK inhibitor regulating G1 progression, it is remarkable that p27−/− cells maintain normal cell cycle distribution, contain similar amounts of CDK activity, and still respond appropriately to both mitogenic and antimitogenic signals (13). Thus, while the knockout mouse confirms that p27 is a tumor suppressor, it raises the possibility that this ability is not manifested via CDK inhibition.
We performed a yeast two-hybrid screen to identify novel p27 targets, using as bait a p27 mutant that cannot tightly bind cyclin-CDKs, p27(CDK−) (75). One of the positive clones encoded the C-terminal SH3 domain of GRB2 (growth factor receptor-bound protein 2), a highly conserved adaptor protein which helps activate the Ras/mitogen-activated protein (MAP) kinase signal transduction pathway in response to extracellular signals (19, 41, 54, 64). While our characterization of this binding interaction was in progress, Sugiyama et al. reported similar findings, and they concluded that GRB2 regulates p27 by promoting its degradation in the cytoplasm (67). This conclusion was surprising for two reasons: GRB2 is well characterized yet not previously implicated in protein degradation, and there are conflicting data regarding whether p27 is degraded in the nucleus or cytoplasm (53).
Because we suspected that p27's tumor-suppressing ability resides in a function other than CDK inhibition, we hypothesized that it might bind and regulate GRB2 rather than vice versa. This idea was particularly attractive because GRB2 plays a key role by activating Ras, one of the most commonly mutated oncogenes in human cancer (2). This role is initiated when mitogen stimulation results in phosphorylation of receptor tyrosine kinases, generating a binding site for the SH2 domain of GRB2 (39). The guanine nucleotide exchange factor SOS then interacts with GRB2 SH3 domains, and the resulting GRB2-SOS complex recruits the proto-oncogene Ras and converts it from the inactive GDP-bound form to the active GTP-bound state (7, 48).
p27 contains a proline-rich region similar to those used by SOS to bind the SH3 domain of GRB2 (67). We now show, with purified proteins and by transient transfection, that p27 competes with SOS for binding GRB2. This competition appears to be physiologically relevant because mitogen stimulation of quiescent fibroblasts drives rapid formation of p27-GRB2 complexes and a concomitant decrease in SOS associated with GRB2. These novel regulatory events require mitogenic activation of the Ras/MAP kinase pathway and p27 export to the cytoplasm, implying that p27 is part of a negative feedback loop regulating GRB2 function in response to mitogen stimulation. Consistent with this hypothesis, mitogen-stimulated p27−/− mouse embryonic fibroblasts (MEFs) maintain their GRB2-SOS complexes for significantly longer than their p27+/+ counterparts. Endogenous Ras was not significantly deregulated in p27−/− cells despite their excess GRB2-SOS, suggesting that additional control mechanisms prevent its inappropriate activation. We therefore used a transient transfection approach to directly demonstrate that p27 can inhibit Ras activation by targeting GRB2. Inhibition of Ras activation does not require the cyclin-CDK binding domain of p27, indicating that the GRB2 and CDK inhibitory functions are separable and distinct.
Based on these results, we propose that a previously unrecognized function of p27 is to regulate cytoplasmic GRB2 in response to mitogenic stimulation. Thus, p27 deregulation in human cancers may contribute to tumorigenesis via disrupted GRB2-SOS complex formation and inappropriate Ras activation, rather than or in addition to alleviation of CDK inhibition.
MATERIALS AND METHODS
Cell culture, plasmids, and transfections.
All cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS). p27+/+ and p27−/− immortalized MEFs were a gift of Matthew Fero (Fred Hutchinson Cancer Research Center). Cells were serum-starved by growing to confluency and incubating in 0.1% fetal bovine serum for an additional 36 to 48 h. For transient transfections, the human embryonic kidney cell line 293 (HEK293) was plated in 60-mm dishes and transfected with the indicated concentration of plasmid DNA by the calcium phosphate method, as described previously (60). Protein expression was driven by the cytomegalovirus promoter, and total DNA concentration per plate was normalized by adding the appropriate empty vector.
Bacterial expression of tagged proteins.
Full-length human GRB2 was PCR cloned in-frame with a glutathione S-transferase (GST) tag in the plasmid pGEX with BamHI and EcoRI restriction sites. Histidine-tagged versions of proteins were generated by PCR cloning into the pET16B plasmid (Novagen) with EcoRI and BamHI sites. All constructs were confirmed by sequencing. Plasmids were transformed into the BL21(DE3) Escherichia coli strain and grown in 50 ml of Luria-Bertani medium to an optical density of 0.6 at 598 nm. Protein expression was then induced for 4 h at 37°C with 0.4 mM isopropylthiogalactopyranoside (IPTG), and the cells were collected by centrifugation. The GST-fused Raf-1 Ras-binding domain (RBD; amino acids 1 to 149) was provided by Daniel Mueller (University of Minnesota, Minneapolis) and expressed and purified as described previously (70).
In vitro binding assays.
Bacterially expressed histidine-tagged p27 (His-tagged p27) and histidine-tagged SOS(1133-1337) were purified with Ni-nitrilotriacetic acid-agarose beads according to the manufacturer's protocol (Qiagen). GST-fused GRB2 was purified with GST-agarose beads (Novagen). For in vitro binding assays, His-tagged p27 was bound to nickel beads and then incubated at 4°C for 20 min with GST-fused GRB2 in 250 μl of binding buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40). Protein complexes were washed three times with 1 ml of the same buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. The SOSN10 blocking peptide (Calbiochem) was resuspended in water and used at concentrations of 20 and 50 μM.
Raf-1 Ras-binding domain affinity precipitation.
GST-fused RBD was purified from E. coli with GST-agarose beads and used to preferentially precipitate the active form of Ras (Ras-GTP) from cell extracts as described previously (70). Active Ras precipitating with GST-fused RBD was visualized by SDS-PAGE and Western blotting with a monoclonal Ras antibody (Transduction Labs).
Western blotting and immunoprecipitation.
Samples to be analyzed by Western blotting were resolved by SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes. Cells were lysed in cold lysis buffer containing 20 mM HEPES (pH 7.2), 100 mM NaCl, 10% glycerol, and 0.5% Triton X-100 with protease and phosphatase inhibitors as described previously (60). Extracts were sonicated for 15 s and centrifuged to remove insoluble material. Protein concentration was determined by the Bradford assay, and for Western blotting equal amounts of protein (20 to 100 μg) were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and visualized by immunoblotting with the indicated antibody.
To detect protein-protein interactions, 0.5 to 2 mg of cell lysate was incubated with primary antibody for either 1 h (GRB2-SOS) or overnight (GRB2-p27). Protein A-Sepharose (Immobilon) was then added, and the incubation was continued for an additional 1 h. Immune complexes were collected by centrifugation and washed three times with lysis buffer. Precipitating proteins were resolved by SDS-PAGE and visualized by immunoblotting with the relevant antibody, as indicated. The following antibodies were used: monoclonal GRB2, SOS, p27, and Ras were all from Transduction Laboratories; polyclonal ERK2 and monoclonal anti-phosphorylated ERK1 and ERK2 (phospho-ERK1/2) antibodies were from Santa Cruz; β-tubulin antibody was from Tucker LeBien, University of Minnesota; and the 9e10 anti-Myc tag antibody was from Jim Roberts, Fred Hutchinson Cancer Research Center. Primary antibodies were detected with horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G (Amersham Pharmacia Biotech) and visualized by the enhanced chemiluminescence detection system (Santa Cruz).
Preparation of nuclear and cytoplasmic extracts.
Cells from a 100-mm plate were washed twice with ice-cold phosphate-buffered saline, detached from the plate with trypsin-EDTA, and pelleted by brief centrifugation. Cells were lysed by resuspending in 100 to 200 μl of hypotonic buffer for 5 min (20 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM Na3VO4, 1 mM EDTA, 10% glycerol, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg each of aprotinin, pepstatin, and leupeptin per ml, 1 mM dithiothreitol, and 0.2% NP-40). Cells were then gently pipetted up and down for 1 min on ice and centrifuged (13,000 rpm, 4°C) for 10 s. Supernatant was collected as the cytoplasmic extract. Nuclear extracts were prepared by resuspension of the pellet (crude nuclei) in high-salt buffer (hypotonic buffer with 20% glycerol and 420 mM NaCl) at 4°C for 30 min, and the supernatants were collected after centrifugation (13,000 rpm, 4°C) for 5 min.
Inhibitors.
The MAP kinase kinase (MKK) inhibitor PD98059 (50 μg/ml final concentration; Calbiochem) was added to 150-mm plates of serum-starved p27+/+ and p27−/− fibroblasts 1 h before refeeding. Dimethyl sulfoxide was used as a solvent control. The CRM1 inhibitor leptomycin B (2 ng/ml final concentration; Sigma) was added to 150-mm plates of serum-starved p27+/+ and p27−/− fibroblasts 30 min before refeeding; 70% methanol was used as a solvent control. The antimitogen transforming growth factor beta (Invitrogen) was added to proliferating p27+/+ and p27−/− cells at 2 ng/ml for 15 h before harvest.
RESULTS
Purified p27 binds GRB2.
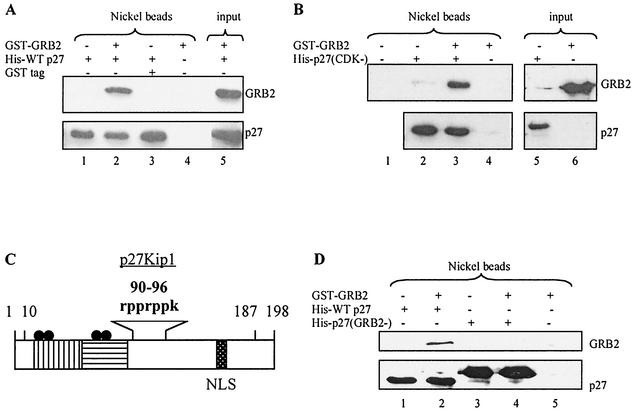
A yeast two-hybrid screen with a p27 mutant that cannot bind cyclin-CDKs, p27(CDK−), as bait identified the C-terminal SH3 domain of GRB2, similar to the results of Sugiyama et al. (67, 75). Direct binding between wild-type p27 and GRB2 was confirmed with purified histidine-tagged p27 (His-tagged p27) to precipitate GRB2 (Fig. 1A). p27(CDK−) precipitated GRB2 as effectively as wild-type p27-consistent with the two-hybrid result (Fig. 1B)-and indicated that the interaction does not require p27's ability to tightly bind cyclin-CDKs. The region of p27 required for binding GRB2 was identified by noting that GRB2 SH3 domains bind proline-rich sequences, and p27 contains a proline-rich region at amino acids 91 to 95 (Fig. 1C) (77). We therefore generated a His-tagged p27 mutant in which all four prolines were converted to alanine. This mutant failed to precipitate GRB2 and hence was termed p27(GRB2−), in agreement with the observations of Sugiyama et al. (Fig. 1D) (67). As will be discussed below, p27(GRB2−) still tightly binds cyclin-CDKs, further evidence that the CDK and GRB2 interaction domains are separable and distinct.
FIG. 1.
p27 directly binds GRB2. (A) His-tagged p27 precipitates GRB2. Wild-type His-tagged p27 (His-WTp27) was bound to nickel beads and incubated with purified GST-fused GRB2. The beads were then washed, and protein complexes were analyzed by SDS-PAGE and Western blotting. GRB2 precipitated in the presence of p27 (lane 2) but not in its absence (lane 4). Input represents a fraction of His-p27 and GST-GRB2 loaded directly on the gel. Nickel beads represents samples subjected to affinity purification of His-tagged protein with nickel beads. (B) A p27 mutant that cannot tightly bind cyclin-CDKs precipitates GRB2. His-tagged p27(CDK−) was bound to nickel beads and incubated with purified GST-fused GRB2. The beads were then washed, and protein complexes were analyzed by SDS-PAGE and Western blotting. GRB2 precipitated in the presence of p27(CDK−) (lane 3), but not in its absence (lane 4). (C) p27 functional domains. The cyclin binding domain (vertical lines) and the CDK binding domain (horizontal lines) are distinct from the proline-rich region (amino acids 90 to 96). Also shown are the nuclear localization signal (NLS; amino acids 152 to 154), MAP kinase and CDK2 phosphorylation sites (S10 and T187, respectively), and the point mutations in p27(CDK−) (four black dots at R30A, L32A, F62A, and F64A). (D) A p27 mutant lacking prolines does not precipitate GRB2. His-tagged wild-type p27 or a His-tagged p27 mutant lacking prolines, p27(GRB2−), was bound to nickel beads and incubated with purified GST-fused GRB2. The beads were then washed, and protein complexes were analyzed by SDS-PAGE and Western blotting. GRB2 precipitated with wild-type p27 (lane 2), but not the mutant lacking prolines (lane 4). p27(GRB2−) ran slightly higher than wild-type p27 due to the mutations and the presence of additional histidines.
p27 competes with SOS for binding GRB2.
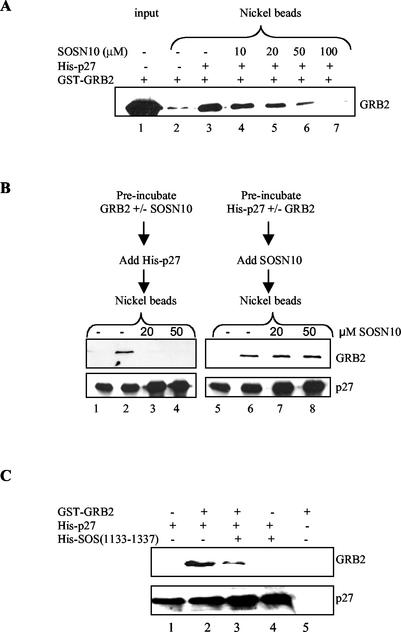
Upon mitogenic stimulation, GRB2 recruits the guanine nucleotide exchange factor SOS to the plasma membrane, where it activates Ras by catalyzing exchange of GDP for GTP (7, 54, 64). Both p27 and one of the SOS proline-rich regions bind the C-terminal SH3 domain of GRB2, raising the possibility that SOS and p27 might compete for binding (39, 77). We first determined whether a peptide derived from the SOS proline-rich domain (SOSN10) could block the interaction between p27 and GRB2 (39). While this peptide has a high affinity for the N-terminal SH3 domain GRB2, it likely interacts with the C-terminal SH3 domain as well (39). Indeed, high concentrations of SOSN10 blocked the ability of His-tagged p27 to precipitate GRB2 (Fig. 2A). In contrast, the peptide had no effect when added after incubating p27 and GRB2 together (Fig. 2B). These results suggest that p27 occupation of the GRB2 SH3 binding domain might prevent its association with SOS.
FIG. 2.
p27 competes with SOS for binding GRB2. (A) The peptide SOSN10 competes with p27 for binding GRB2. Purified His-tagged p27 was incubated with GST-fused GRB2 in the presence of increasing concentrations of SOSN10 (amino acids 1149 to 1158), followed by affinity precipitation with nickel beads and Western blotting for GRB2. Lane 1 shows input GRB2, and lane 2 is a negative control showing GRB2 background in the absence of His-tagged p27. The amount of GRB2 binding p27 is shown in lane 3, and increasing amounts of SOSN10 decreased the GRB2 precipitating with p27 (lanes 4 to 7). (B) The peptide SOSN10 cannot dissociate preformed GRB2-p27 complexes. In the left panels, GRB2 was incubated with nothing or the indicated concentrations of SOSN10 peptide and added to His-tagged p27, and the complexes were affinity precipitated with nickel beads. Lane 1 shows p27 precipitation in the absence of GRB2. Lane 2 depicts the amount of GRB2 bound to p27 in the absence of SOSN10, while lanes 3 and 4 show that preincubating GRB2 and SOSN10 blocked p27 binding. In the right panel, His-tagged p27 was incubated with nothing or GRB2 before addition of SOSN10 and then precipitated as above. Lane 5 shows p27 precipitation in the absence of GRB2, while lane 6 depicts the amount of GRB2 bound to p27 in the absence of SOSN10. Lanes 7 and 8 show that SOSN10 was unable to displace bound p27 from GRB2. (C) p27 competes with SOS(1133-1337) for binding GRB2. His-tagged p27 was bound to a polyclonal p27 antibody on protein A-Sepharose beads and then incubated with GST-fused GRB2 in the absence (lane 2) or presence (lane 3) of His-tagged SOS(1133-1337). The beads were then washed, and protein complexes were analyzed by SDS-PAGE and Western blotting. The presence of SOS(1133-1337) decreased the amount of GRB2 precipitating with p27. Lane 1 shows p27 precipitation in the absence of GRB2, while lane 5 is a negative control showing that GRB2 did not precipitate in the absence of p27.
To test this idea more directly, we attempted to examine competition between p27 and full-length SOS protein for binding GRB2. Unfortunately, soluble full-length SOS could not be obtained from E. coli, but we were able to express and purify a His-tagged SOS deletion mutant (amino acids 1133 to 1337) containing all four proline regions. As expected, the His-tagged SOS mutant precipitated GRB2, and this interaction was blocked by the SOS peptide (data not shown). We next determined whether it blocked association of p27 and GRB2. Because both p27 and SOS were His tagged, p27 was precipitated with a p27-specific antibody followed by Western blotting for associated GRB2. The SOS deletion mutant blocked p27 association with GRB2, further evidence that their binding is mutually exclusive (Fig. 2C).
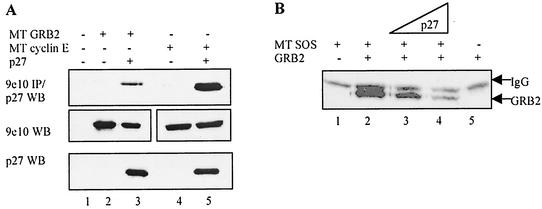
If this competition is physiologically relevant, we would expect p27 and GRB2 to associate in cells. Because p27 is located primarily in the nucleus while GRB2 resides in the cytoplasm, it was unclear whether the two proteins would bind (3, 78). We therefore transiently transfected HEK293 cells with wild-type p27 and Myc-tagged GRB2 under control of the cytomegalovirus promoter. As a positive control, p27 was also transfected with the known binding partner, Myc-tagged cyclin E. Extracts were prepared from transfected cells, followed by immunoprecipitation of Myc-tagged proteins and Western blotting for associated p27 (Fig. 3A). Much less p27 bound GRB2 compared to the cyclin E control even though protein expression levels were similar (compare lane 3 to lane 5), consistent with the idea that p27 and GRB2 might be located in different cellular compartments.
FIG. 3.
p27 competes with SOS for binding GRB2 in cells. (A) GRB2 and p27 bind when cotransfected in mammalian cells. HEK293 cells were transfected with 2.5 μg of cDNAs encoding Myc-tagged (MT) GRB2 or Myc-tagged cyclin E and p27 under control of the cytomegalovirus promoter. Cell extracts were Western blotted (WB) for protein expression levels (lower two panels) and immunoprecipitated with the 9e10 antibody directed against the Myc tag, followed by p27 Western blotting (top panel). Significantly less p27 bound GRB2 than cyclin E even though both were expressed at equal levels (compare lanes 3 and 5). Myc-tagged GRB2 and Myc-tagged cyclin E are shown side by side for illustrative purposes and ran at their expected molecular weights. (B) Competition between SOS and p27 for binding GRB2 in cells. HEK293 cells were transiently transfected with 2.5 μg of plasmids encoding Myc-tagged SOS and untagged GRB2 in the presence of increasing amounts of p27 plasmid (2.5 to 10 μg). After 36 h, cell extracts were harvested and immunoprecipitated with the 9e10 antibody and then Western blotted for associated GRB2, which ran just below the immunoglobulin light chain (shown in the lane 1 control). Increasing p27 decreased the amount of GRB2 precipitating with SOS (lanes 3 and 4). Lane 5 is a negative control showing that GRB2 did not precipitate in the absence of SOS. Western blotting confirmed equal expression of all transfected proteins (data not shown).
Nevertheless, the observed interaction allowed us to determine if p27 and SOS could compete for binding GRB2 in cells. HEK293 cells were transiently transfected with Myc-tagged SOS and untagged GRB2 in the presence and absence of cotransfected p27 (Fig. 3B). Increasing concentrations of p27 blocked GRB2 association with Myc-tagged SOS (compare lane 2 to lanes 3 and 4), similar to results obtained with purified proteins.
p27-GRB2 complex formation is enhanced by mitogen stimulation.
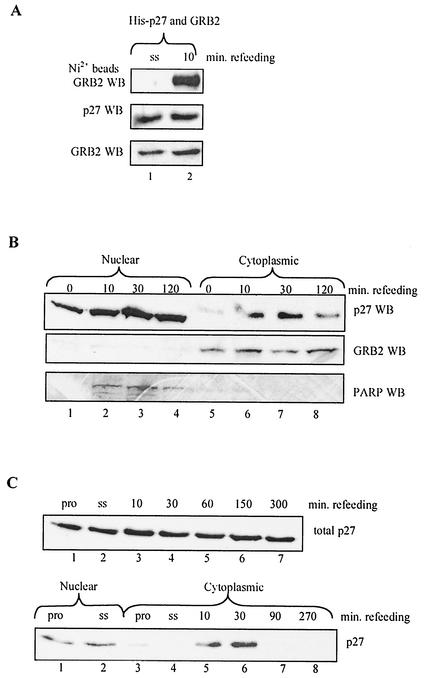
Because cotransfected p27 and GRB2 associated inefficiently, we used this assay to search for conditions promoting GRB2-p27 complex formation. Recent evidence indicates that p27 undergoes regulated nuclear export (albeit for reasons that are unclear) in a variety of situations, including 6 to 10 h after mitogen stimulation of quiescent cells (30, 53, 66, 71). We therefore examined the effects of mitogen stimulation on GRB2-p27 complex formation. HEK293 cells were transiently transfected with cDNAs encoding p27 and Myc-tagged GRB2 for 12 h, then washed, and serum-starved for an additional 12 h. At this point cells were stimulated with 10% fetal bovine serum (FBS) for various times and analyzed for GRB2-p27 complex formation (Fig. 4A). Refeeding rapidly and significantly increased p27 binding to GRB2 while having a minimal effect on steady-state expression levels, which may reflect colocalization of the two proteins in response to mitogen stimulation.
FIG. 4.
Mitogen stimulation promotes p27-GRB2 complex formation and rapid p27 export to the cytoplasm. (A) Mitogen stimulation enhances binding of cotransfected GRB2 and p27. HEK293 cells were transfected with 2.5 μg of GRB2 and His-tagged p27 for 12 h, serum-starved (ss) in 0.1% FBS for 24 h, and then refed with 10% FBS for 10 min. Cell extracts were Western blotted for protein expression levels (lower panels) and precipitated with nickel beads, followed by GRB2 Western blotting (WB, upper panel). Refeeding promoted p27-GRB2 complex formation without affecting overall expression levels. (B) Mitogen-dependent export of endogenous p27 to the cytoplasm. p27+/+ MEFs were serum starved in 0.1% FBS for 48 h and refed with 10% FBS for the indicated times. Cell extracts were fractionated into nuclear and cytoplasmic components as described in Materials and Methods and then Western blotted for p27. While nuclear levels of p27 remained relatively constant (upper panel, lanes 1 to 4), the amount of cytoplasmic p27 increased rapidly in response to refeeding (upper panel, lanes 5 to 8). In contrast, GRB2 was located exclusively in the cytoplasm (middle panel), while poly(ADP-ribose) polymerase (PARP) served as a control that was located only in the nuclear fraction (lower panel). (C) Rapid mitogen-dependent export of endogenous p27 to the cytoplasm in NIH 3T3 cells. NIH 3T3 cells were serum starved (ss) in 0.1% FBS for 48 h and refed with 10% FBS for the indicated times. Cell extracts were blotted for total p27 expression (top panel), or cells were fractionated into nuclear and cytoplasmic components as described in Materials and Methods (lower panel). As was observed in panel B, p27 was rapidly and transiently exported to the cytoplasm within 10 min of mitogen stimulation. p27 expression in proliferating cells (pro) was included as a control.
Because these results indicated that p27 association with GRB2 was likely a regulated event occurring in response to mitogen stimulation of quiescent cells, we initiated studies of the endogenous proteins to further investigate the possible connection between p27 and GRB2. The mitogen effect on binding of transfected p27 and GRB2 occurred substantially earlier than in previous work showing p27 export 6 to 10 h after exposure, so we first examined localization of endogenous p27 immediately after refeeding quiescent cells (30, 53). MEFs were serum-starved for 24 h and stimulated with 10% FBS for short times. Cells were then separated into nuclear and cytoplasmic fractions and Western blotted for p27 and control proteins (Fig. 4B) (29). While endogenous p27 was mainly nuclear in quiescent cells, a fraction rapidly and transiently accumulated in the cytoplasm upon mitogen stimulation (upper panel). As controls for the fractionation procedure, we used GRB2 (located exclusively in the cytoplasmic fraction; middle panel) and poly(ADP)-ribose polymerase (located exclusively in the nuclear fraction; lower panel). Similar results were obtained in the NIH 3T3 cell line, suggesting that rapid p27 export in response to mitogens is a general phenomenon (Fig. 4C).
Cytoplasmic relocalization of p27 occurs without any change in total protein levels, suggesting that export is not facilitating p27 degradation. It also seems unlikely that p27 is localized to the cytoplasm to allow activation of nuclear cyclin-CDKs, because export occurs well before cyclin-CDKs are synthesized and activated in response to mitogens (20, 25, 43). Instead, rapid mitogen-mediated p27 export might facilitate targeting of GRB2, consistent with the enhanced GRB2-p27 complex formation observed after refeeding transiently transfected cells (Fig. 4A).
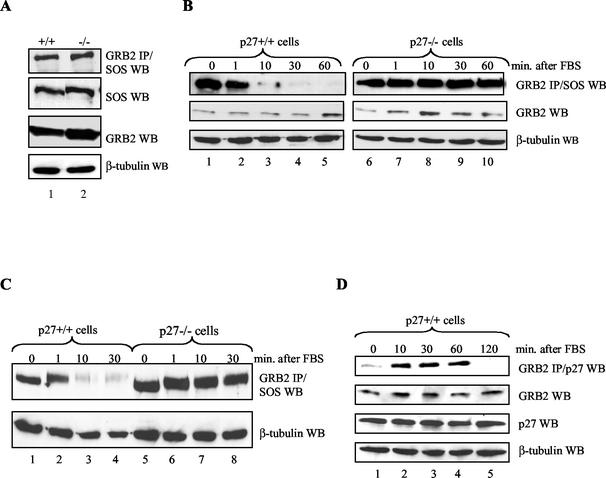
GRB2-SOS complexes are deregulated in p27−/− cells.
If competition between p27 and SOS for binding GRB2 is physiologically relevant, then at some point in cells endogenous p27 should target GRB2 and prevent its association with SOS. Conversely, under similar conditions cells lacking p27 should contain more GRB2-SOS complexes. We therefore compared GRB2 association with SOS in p27−/− and p27+/+ MEFs derived from matched littermates. Under proliferating conditions both cell types expressed similar amounts of GRB2 and SOS and contained similar amounts of GRB2-SOS complexes (Fig. 5A). Since the transient assay suggested that mitogen stimulation of serum-starved cells drives p27-GRB2 complex formation, we examined GRB2 association with SOS under these conditions. p27+/+ and p27−/− cells were serum-starved in 0.1% fetal calf serum for 48 h and refed with 20% fetal calf serum for the indicated times (Fig. 5B). Serum-starved p27+/+ cells contained high levels of GRB2-SOS that rapidly disappeared upon refeeding (Fig. 5B, lanes 1 to 5) (76). Serum-starved p27−/− cells contained similarly high levels of GRB2-SOS (Fig. 5B, lane 6), but upon refeeding these complexes failed to disappear and instead were maintained throughout the time course (Fig. 5B, lanes 7 to 10). Thus, p27 absence correlates with deregulation of GRB2-SOS complexes in response to mitogen stimulation.
FIG. 5.
Deregulation of GRB2-SOS complexes in p27−/− MEFs. (A) Proliferating p27+/+ and p27−/− cells contain similar amounts of GRB2-SOS complexes. Equal amounts of whole-cell extract from proliferating p27+/+ and p27−/− MEFs derived from matched littermates were Western blotted (WB) for SOS, GRB2, and β-tubulin as indicated (lower panels). The upper panel shows that similar amounts of SOS immunoprecipitated with GRB2 from both cell types. (B) GRB2-SOS complexes are deregulated in mitogen-stimulated p27−/− cells. p27+/+ and p27−/− MEFs derived from matched littermates were serum starved in 0.1% FBS for 48 h and then refed with 20% FBS for 1, 10, 30, or 60 min. GRB2-SOS complexes were analyzed by immunoprecipitating GRB2 from equal amounts of cell extract and Western blotting for associated SOS (top panel). GRB2 and β-tubulin expression levels are shown as controls (lower panels). While GRB2-SOS complexes rapidly disappeared in p27+/+ cells (lanes 1 to 5), they were maintained in cells lacking p27 (lanes 6 to 10). (C) Deregulation of GRB2-SOS complex formation in different p27−/− cells. GRB2-SOS complexes were analyzed as described for panel B with p27+/+ and p27−/− cells independently derived from a different set of matched littermates. Methods were as described for panel B. (D) Mitogen stimulation of quiescent p27+/+ cells causes rapid p27-GRB2 complex formation. p27+/+ and p27−/− MEFs were serum starved in 0.1% FBS for 48 h and then refed with 20% FBS for 10, 30, 60, or 120 min. GRB2-p27 complexes were analyzed by immunoprecipitating GRB2 from equal amounts of cell extract and Western blotting for associated p27 (top panel). Upon mitogen stimulation, GRB2-p27 complexes were rapidly and transiently formed. p27, GRB2, and β-tubulin expression levels are shown as controls (lower panels).
Both cell types expressed similar amounts of GRB2 and SOS, indicating that the excess complexes were not due to increased protein expression in p27−/− cells (Fig. 5B). Furthermore, GRB2 deregulation was not confined to a particular p27−/− cell line because independently generated p27+/+ and p27−/− lines showed similar results (Fig. 5C). The observed effect on GRB2-SOS complexes appeared to be quite specific for the period immediately after mitogen stimulation of quiescent cells. In addition to similar amounts of GRB2-SOS in proliferating cells (Fig. 5A), we also observed no differences during a serum starvation time course (up to 48 h) or after treating cells with the antimitogen transforming growth factor beta (data not shown).
If deregulated GRB2-SOS in p27−/− cells is a consequence of p27's failing to target GRB2, then p27-GRB2 complexes should form with similar kinetics in p27+/+ cells. Figure 5D shows that p27-GRB2 complexes were maximally formed 10 to 60 min after mitogen stimulation of serum-starved p27+/+ cells, correlating with the time course of p27 export (Fig. 4B) and disappearance of GRB2-SOS complexes (Fig. 5B and C).
p27 regulation of GRB2 occurs in response to specific mitogenic stimuli.
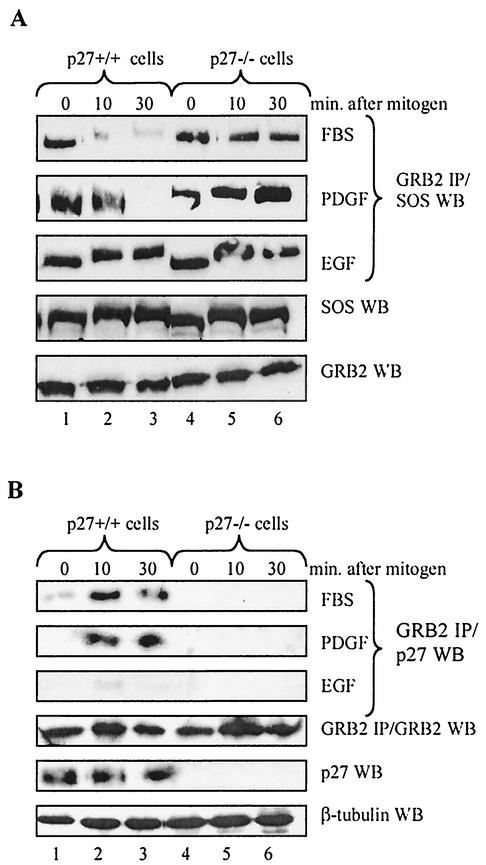
The rapidity of p27 export and GRB2 binding in response to mitogen stimulation suggests the involvement of a mitogen-activated signal transduction cascade. Because refeeding with FBS has pleiotropic effects on multiple signal transduction pathways, we sought to further define the events leading to p27 regulation of GRB2 by treating serum-starved p27+/+ and p27−/− cells with individual growth factors. Figure 6A compares GRB2-SOS complexes in response to stimulation with FBS, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF). GRB2-SOS dissociated in response to FBS and PDGF, but these complexes were maintained in the presence of EGF.
FIG. 6.
p27 regulation of GRB2-SOS complexes is mitogen specific. (A) Stimulation with FBS and PDGF but not EGF destabilizes GRB2-SOS complexes. p27+/+ and p27−/− MEFs were serum starved in 0.1% FBS for 48 h, refed with either 20% FBS, 20 ng of PDGF per ml, or 50 ng of EGF per ml, and harvested at the indicated time points. GRB2 was immunoprecipitated (IP) from equal amounts of extract and Western blotted (WB) for SOS. While FBS and PDGF caused the disappearance of GRB2-SOS in p27+/+ cells (top two panels), these complexes were maintained in the presence of EGF (third panel). The lower two panels show a representative Western blot for GRB2 and SOS from cells stimulated with FBS. Similar expression levels were observed upon stimulation with individual growth factors (not shown). (B) Stimulation with FBS and PDGF but not EGF drives p27-GRB2 complex formation. p27+/+ and p27−/− MEFs were serum starved in 0.1% FBS for 48 h, refed with either 20% FBS, 20 ng of PDGF per ml, or 50 ng of EGF per ml, and harvested at the indicated time points. GRB2 was immunoprecipitated from equal amounts of extract and Western blotted for p27 (top three panels). p27-GRB2 complexes were only apparent upon stimulation with FBS and PDGF (top two panels), consistent with the kinetics of GRB2-SOS disappearance observed in panel A. The lower three panels are controls showing protein expression levels from cells stimulated with FBS. Similar expression levels were observed upon stimulation with individual growth factors (not shown).
If p27 helps regulate GRB2 association with SOS, then p27-GRB2 complexes should form only when quiescent cells are treated with FBS and PDGF. Figure 6B shows that this was indeed the case, further evidence that p27 contributes to regulation of GRB2-SOS complexes in response to specific mitogenic signals. The fact that p27-GRB2 complexes did not form in the presence of EGF suggests that this mitogen fails to initiate a downstream event required for this interaction.
p27 regulation of GRB2 requires activation of the Ras/MAP kinase cascade.
PDGF is a well-known activator of the Ras/MAP kinase pathway, so its ability to initiate p27 regulation of GRB2 suggested that the MAP kinase pathway might be involved in this process. More specifically, we suspected that MAP kinase might help facilitate p27 export because this pathway is rapidly activated upon mitogen stimulation, and MAP kinase is known to phosphorylate p27 in vitro on a site (S10) required for export to the cytoplasm (30, 31, 53).
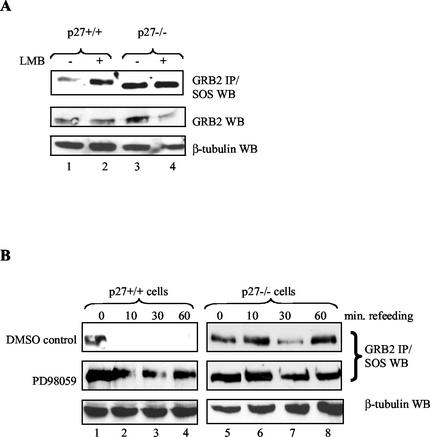
To test this idea, we first confirmed that p27 export is required for regulation of GRB2-SOS complex formation with the export inhibitor leptomycin B (Fig. 7A) (30, 71). Quiescent p27+/+ cells were treated with leptomycin B and then stimulated with mitogens. GRB2-SOS complexes now persisted in the presence of the drug (compare lanes 1 and 2), suggesting p27 export is necessary to target GRB2 and prevent its association with SOS. Importantly, leptomycin B did not alter GRB2-SOS complexes in p27−/− cells (lanes 3 and 4), indicating that its effect is dependent on the presence of p27.
FIG. 7.
Inhibiting p27 export stabilizes GRB2-SOS complexes. (A) The export inhibitor leptomycin B (LMB) prevents mitogen-mediated GRB2-SOS disappearance. p27+/+ and p27−/− MEFs were serum starved in 0.1% FBS for 48 h, treated with the export inhibitor leptomycin B (2 ng/ml; Sigma) for 30 min, and then refed with 20% FBS for an additional 30 min. Extracts were prepared and analyzed for GRB2-SOS complex formation by immunoprecipitating (IP) GRB2 and Western blotting (WB) for SOS (upper panel). Leptomycin B stabilized GRB2-SOS complexes in p27+/+ cells (compare lanes 1 and 2) but had no effect on cells lacking p27 (lanes 3 and 4). Lower panels show GRB2 and β-tubulin expression as controls. (B) The MKK inhibitor PD98059 preferentially blocks GRB2-SOS disappearance in p27+/+ cells. p27+/+ and p27−/− cells were serum starved for 48 h, treated with 50 μM PD98059 for 1 h, and then refed with 20% FBS for 10, 30, or 60 min. GRB2-SOS complex formation was analyzed by immunoprecipitating GRB2 from equal amounts of cell extract and Western blotting for associated SOS. The upper panels show treatment with the dimethyl sulfoxide (DMSO) control, while the middle panels show the effect of PD98059 on GRB2-SOS complexes. Although PD98059 had a modest effect on GRB2-SOS in p27−/− cells (lanes 5 to 8), it had a much greater effect on these complexes in p27+/+ cells (lanes 1 to 4). The lower panels are a β-tubulin control to show equal protein loading.
We next used the MKK inhibitor PD98059 to determine if MAP kinase activation is required for p27 regulation of GRB2 (1, 18). p27+/+ and p27−/− cells were serum-starved, treated with PD98059 or dimethyl sulfoxide carrier, and then refed to initiate p27 regulation of GRB2 (Fig. 7B). PD98059 had a modest stabilizing effect on GRB2-SOS complexes in p27−/− cells (compare top two right panels; lanes 5 to 8), as expected because previously published results indicate that MAP kinase may regulate SOS directly (5). In contrast, PD98059 had a much greater effect on GRB2-SOS stability in p27+/+ cells (compare top two left panels, lanes 1 to 4), suggesting that MAP kinase makes an additional p27-dependent contribution to regulation of GRB2-SOS complexes.
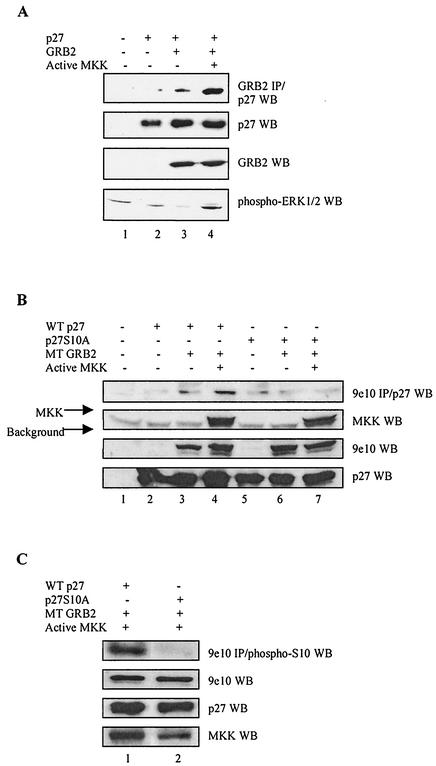
As a more direct test of whether MAP kinase can promote p27-GRB2 complex formation, we returned to the transient transfection assay in which inefficient binding between cotransfected p27 and GRB2 can be enhanced by serum starvation/mitogen stimulation (Fig. 4A). We now sought to determine whether this effect was mediated by active MAP kinase. HEK293 cells were transiently transfected with cDNAs encoding p27 and GRB2 in the presence or absence of constitutively active MKK under control of the cytomegalovirus promoter (42). Endogenous MAP kinase was activated in the presence of exogenous MKK and formation of GRB2-p27 complexes increased, consistent with the idea that MAP kinase activity can promote p27 association with GRB2 (Fig. 8A).
FIG. 8.
Activating MAP kinase promotes p27 binding to GRB2. (A) Active MKK drives GRB2-p27 complex formation. HEK293 cells were cotransfected with 2.5 μg of cDNAs encoding constitutively active MKK, p27, and GRB2. Cell extracts were Western blotted (WB) for expression levels of transfected proteins (second and third panels), activated endogenous ERK1/2 MAP kinase (bottom panel), and p27 coimmunoprecipitating with GRB2 (top panel). Active MKK increased the amount of active endogenous MAP kinase and greatly enhanced p27 binding to GRB2 (lane 4). (B) MKK stimulation of p27-GRB2 complex formation requires serine 10 on p27. HEK293 cells were cotransfected with cDNAs encoding constitutively active MKK, p27, p27S10A, and Myc-tagged (MT) GRB2. Cells extracts were Western blotted for expression levels of transfected proteins (bottom three panels) and p27 or p27S10A coimmunoprecipitating with Myc-tagged GRB2 (top panel). As in panel A, active MKK drove wild-type (WT) p27 binding to GRB2 (upper panel, compare lanes 3 and 4). In contrast, p27S10A failed to bind GRB2 and was not stimulated by active MKK (lanes 6 and 7). (C) p27 bound to GRB2 is phosphorylated on serine 10. HEK293 cells were cotransfected with cDNAs encoding constitutively active MKK, p27, p27S10A, and Myc-tagged GRB2. Cell extracts were Western blotted for expression levels of transfected proteins (bottom three panels). Myc-tagged GRB2 was immunoprecipitated from cell extracts with the 9e10 antibody and Western blotted with an antibody recognizing p27 phosphorylated at serine 10 (Santa Cruz).
Since MAP kinase can phosphorylate p27 at serine 10—a site known to be involved in p27 export—it seemed likely that S10 phosphorylation might also be required for rapid p27 export in response to mitogen stimulation. We tested this idea by determining whether the S10 site was required for mitogen-dependent p27-GRB2 complex formation. HEK293 cells were transfected with either wild-type p27 or the p27S10A mutant in the presence of GRB2 and active MKK. Figure 8B shows that while cotransfected MKK drove the formation of wild-type p27-GRB2 complexes, p27S10A failed to associate with GRB2 in the presence or absence of active MKK (top panel). As a complementary approach, the experiment was repeated and GRB2 immunoprecipitates were probed with an antibody directed against p27 phosphorylated on serine 10 (Fig. 8C). Wild-type p27 bound to GRB2 was recognized by this antibody, further evidence that phosphorylation at serine 10 plays a role in p27 regulation of GRB2. Since unphosphorylated p27 and GRB2 expressed in E. coli bind, these results are consistent with the idea that S10 phosphorylation facilitates p27 export to the cytoplasm so it can target GRB2.
Regulation of Ras activation by p27.
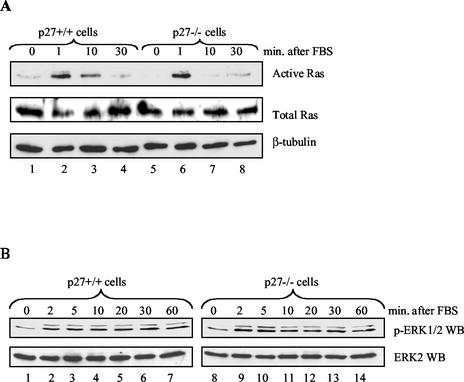
The ability of p27 to inhibit GRB2-SOS complex formation implies that it might contribute to regulation of Ras activation. We therefore compared the amounts of active Ras in serum-starved, mitogen-stimulated p27+/+ and p27−/− cells by precipitating GTP-bound Ras with a GST-fused Raf-1 RBD (45, 70). The amount of active Ras in p27−/− cells did not increase despite dramatically more GRB2-SOS complexes (Fig. 9A). We also examined activation of MAP kinase because it represents a more sensitive measure of Ras activity, but again failed to see significant differences between the p27+/+ and p27−/− cells (Fig. 9B). Similar data were obtained when cells were stimulated with the individual growth factors PDGF and EGF (data not shown). Given that Ras activation is highly regulated by multiple mechanisms, it seems likely that other regulatory components are keeping Ras under control despite the excess GRB2-SOS complexes.
FIG. 9.
Ras/MAP kinase pathway is not deregulated in p27−/− cells. (A) Ras activation in p27+/+ and p27−/− MEFs. p27+/+ and p27−/− MEFs were serum starved in 0.1% FBS for 48 h, refed with 20% FBS, and harvested at the indicated time points. Active Ras was affinity precipitated from equal amounts of extract with purified GST-fused Raf-1 RBD, as described in Materials and Methods. Precipitates were separated by SDS-PAGE and Western blotting for active Ras (upper panel). Total Ras and the β-tubulin control are shown in the middle and lower panels, respectively. (B) MAP kinase activation in p27+/+ and p27−/− MEFs. MEFs were serum starved in 0.1% FBS for 48 h, refed with 20% FBS, and harvested at the indicated time points. Equal amounts of extract were analyzed by SDS-PAGE and Western blotted for active MAP kinase (ERK1/2) with a phosphorylation-specific antibody (upper panel) or for total MAP kinase (ERK2) (lower panel).
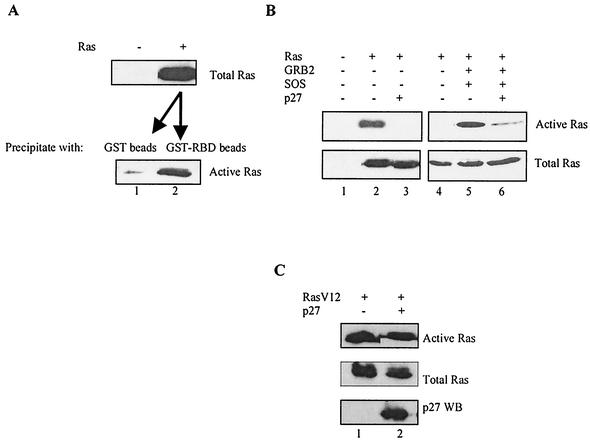
We therefore hypothesized that it might be possible to isolate p27 regulation of Ras by recapitulating these events by transient transfection, especially because regulated binding of p27 to GRB2 was successfully examined with this approach. We first demonstrated that wild-type Ras can be transfected into HEK293 cells and the amount of active form can be determined with the GST-Raf1 RBD pulldown assay (Fig. 10A). Next we determined if cotransfecting p27 could inhibit Ras activation. In the two left panels of Fig. 10B, we show that cotransfecting p27 inhibited Ras activation without affecting levels of the Ras protein. This effect does not appear to be an indirect consequence of altering the GTP-bound form of the protein, since cotransfected p27 had no effect on a constitutively active RasV12 mutant that does not require recruitment by GRB2-SOS (Fig. 10C) (32).
FIG. 10.
p27 inhibition of Ras activation. (A) Measuring activation of transfected Ras. A plasmid (2 μg) containing wild-type Ras under control of the cytomegalovirus promoter (pCMV/WT Ras) was transiently transfected into HEK293 cells, and extracts were prepared 36 h later. The top panel shows expression levels of the transfected Ras (lane 2) relative to the untransfected control (lane 1). Equal amounts of the Ras-containing extract from lane 2 were then precipitated with either GST beads alone as a control (bottom panel, lane 1) or GST-fused RBD (bottom panel, lane 2) and then Western blotted to determine the amount of active Ras. (B) Activation of transfected Ras is inhibited by cotransfected p27. In the left two panels, HEK293 cells were transiently transfected with 2 μg of Ras (pCMV/WT Ras) in the presence or absence of 5 μg of cotransfected p27. The upper panel depicts active Ras, as determined by affinity precipitation with GST-fused Raf-1 RBD followed by Western blotting, and shows that p27 inhibited Ras activation (compare lanes 2 and 3). The lower panel shows that total Ras expression levels were not decreased in the presence of p27. The right two panels show that cotransfecting GRB2 and SOS greatly stimulated Ras activation (compare lanes 4 and 5). This is a much lighter exposure than that for the panels on the left, which accounts for the apparent lack of active Ras in lane 4. Upon longer exposure, the extent of activation with Ras alone was similar to that observed in lane 2. Cotransfecting 5 μg of p27 inhibited GRB2/SOS-stimulated Ras activation without affecting expression levels of total Ras (lane 6). (C) Constitutively active RasV12 is not inhibited by cotransfected p27. HEK293 cells were transiently transfected with 2 μg of a plasmid expressing an active form of Ras (pCMV/RasV12) in the presence and absence of 5 μg of p27 as indicated. p27 had no effect on the amount of active Ras (upper panel) or total Ras (middle panel). Methods were as described for panel B.
To further investigate whether this inhibition involved targeting GRB2, we established conditions in which Ras activation was massively stimulated by cotransfecting SOS and GRB2 (Fig. 10B, right panels; compare lanes 4 and 5). These panels represent a much shorter exposure than those on the left due to the extent to which SOS and GRB2 enhance Ras activation. Cotransfecting wild-type p27 again decreased the amount of active Ras without affecting the steady-state levels of the protein, consistent with the idea that p27 is inhibiting GRB2-SOS-dependent activation of Ras (Fig. 10B, lanes 5 and 6).
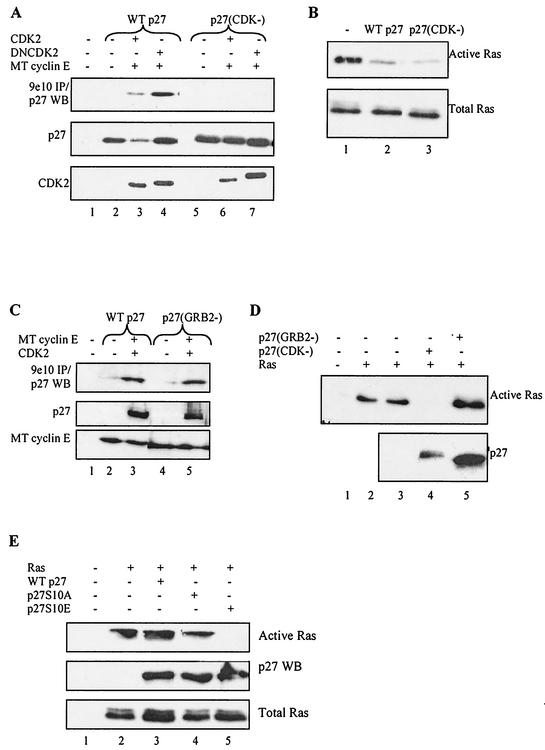
These results suggested that p27 inhibits Ras activation by targeting GRB2. Alternatively, however, they could be an indirect effect of inhibiting cyclin-CDKs and/or cell cycle progression. To distinguish between these possibilities, we examined the effect of p27(CDK−)—which cannot bind cyclin-CDKs tightly and fails to arrest the cell cycle—on Ras activation (75). We first confirmed that p27(CDK−) does not immunoprecipitate with cyclin-CDKs in our transient transfection assay (Fig. 11A). Next we cotransfected p27(CDK−) with Ras and found that it inhibited Ras activation as well as wild-type p27, indicating that the CDK inhibitory function is not required (Fig. 11B).
FIG. 11.
CDK and GRB2 inhibitory functions of p27 are separable and distinct. (A) p27(CDK−) does not tightly bind cyclin-CDK complexes. HEK293 cells were transfected with either wild-type (WT) p27 or a p27 mutant that cannot tightly bind cyclin-CDKs, p27(CDK−). Myc-tagged (MT) cyclin E and CDK2 or dominant negative CDK2 (DNCDK2) were cotransfected as indicated. After 36 h, cell extracts were prepared and Western blotted (WB) for p27 expression (middle panel), CDK2 (bottom panel), and p27 immunoprecipitating (IP) with the Myc-tagged cyclin E-CDK2 complex by using the 9e10 antibody (upper panel). Cyclin E was expressed equally in all lanes (not shown). Lane 1 is an untransfected control, while lanes 2 and 5 show that p27 did not precipitate with the 9e10 antibody in the absence of Myc-tagged cyclin E. Wild-type p27 precipitated with CDK (lanes 3 and 4), while p27(CDK−) did not (lanes 6 and 7). p27 levels were lower in lane 3, and less precipitated with active cyclin E-CDK2 because it was phosphorylated and directed to the proteasome under these conditions. (B) Activation of transfected Ras is inhibited by cotransfected p27(CDK−). HEK293 cells were transiently transfected with the Ras expression vector in the presence or absence of wild-type p27 or p27(CDK−) as indicated. Active Ras was affinity precipitated with purified GST-fused RBD as described in the text (upper panel). Lane 1 shows the amount of active Ras in the absence of p27, while the lower panel shows that total Ras expression levels were unaffected. (C) p27(GRB2−) binds cyclin-CDK complexes. HEK293 cells were transiently transfected with wild-type p27 or the p27(GRB2−) mutant, which lacks prolines and hence cannot bind GRB2. Myc-tagged cyclin E and CDK2 were cotransfected as indicated. Cell extracts were prepared after 36 h and Western blotted for Myc-tagged cyclin E expression (bottom panel), p27 (middle panel), and p27 immunoprecipitating with Myc-tagged cyclin E-CDK2 with the 9e10 antibody (upper panel). Lane 1 is an untransfected control, while lanes 2 and 4 show that p27 did not precipitate with the 9e10 antibody in the absence of Myc-tagged cyclin E. Lanes 3 and 5 show that wild-type p27 and p27(GRB2−) bound cyclin E-CDK2 equally well. (D) Activation of transfected Ras is not inhibited by cotransfected p27(GRB2−). HEK293 cells were transiently transfected with the Ras expression vector in the presence or absence of cotransfected p27(CDK−) or p27(GRB2−) as indicated. Active Ras was affinity precipitated with purified GST-fused Raf-1 RBD (upper panel). Lanes 2 and 3 are duplicates showing the amount of active Ras in the absence of p27. p27(CDK−) inhibited Ras activation while p27(GRB2−) did not, despite the fact that in this particular experiment p27(GRB2−) was expressed at much higher levels (lower panel). p27(CDK−) runs slightly higher than p27(GRB2−) due to the different mutations. Equal amounts of Ras were expressed in each lane (not shown). (E) p27S10E is a more potent inhibitor of transfected Ras. HEK293 cells were transiently transfected with 2 μg of Ras and 1 μg of wild-type p27, p27S10A, or p27S10E, as indicated. With these lowered ratios neither wild-type p27 nor p27S10A inhibited Ras activation (upper panel, compare lane 2 with lanes 3 and 4). In contrast, p27S10E was expressed at similar levels yet dramatically inhibited Ras activation (lane 5). Methods were as described for panel B.
If p27 inhibition of Ras activation involves targeting GRB2, then the p27 mutant that cannot bind GRB2 should fail to inhibit Ras. We first confirmed that p27(GRB2−) still binds cyclin-CDKs in the transient transfection assay, ruling out the possibility that ablating prolines generated a globally misfolded or inactive p27 mutant (Fig. 11C). Next we cotransfected p27(GRB2−) with Ras and found that it did not inhibit Ras activation even though it was expressed at very high levels, indicating that the GRB2 binding region is required (Fig. 11D). Thus, p27 inhibition of Ras activation requires the ability to bind GRB2 and is not an indirect effect of inhibiting cyclin-CDKs. These results provide further evidence that the CDK and GRB2 binding domains and inhibitory functions are separable and distinct.
Our previous data suggested that phosphorylation of p27S10 is required for binding GRB2 in cells. To determine whether modification of this site is required for inhibiting Ras activation, we generated a p27 mutant containing glutamic acid in place of serine at position 10 (p27S10E) in order to mimic the phosphorylated state. HEK293 cells were transfected with low levels of wild-type p27 that were insufficient to inhibit activation of cotransfected Ras (Fig. 11E). Likewise, p27S10A also failed to inhibit Ras under these conditions. However, p27S10E dramatically blocked Ras activation, even though it was expressed at levels similar to wild-type p27 and p27S10A. These results are consistent with previous data indicating that phosphorylation of S10 helps mediate p27 targeting of GRB2, most likely by facilitating its export to the cytoplasm.
p27 inhibition of endogenous Ras activation.
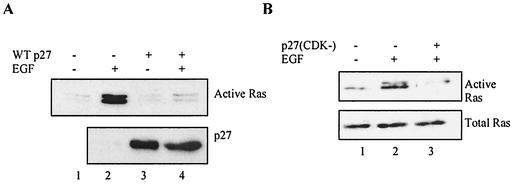
A central result in the initial characterization of p27 was that it tightly bound and inhibited endogenous cyclin-CDK complexes when overexpressed in tissue culture cells (28, 51, 72). We took advantage of the fact that HEK293 cells are transfected with >80% efficiency to determine if p27 overexpression could also inhibit activation of endogenous Ras. Transfecting proliferating cells with wild-type p27 had no effect on the amount of active Ras, suggesting that p27 does not target GRB2 under these conditions (not shown). We therefore repeated the experiment but serum starved the transfected cells for 12 h and then stimulated them with EGF to activate endogenous Ras (Fig. 12A) (57).
FIG. 12.
Transfected p27 inhibits mitogen-dependent activation of endogenous Ras. (A) Wild-type p27 inhibits EGF-mediated activation of endogenous Ras. HEK293 cells were transfected with either vector alone or wild-type p27 as indicated. Because these cells are transfected to >80% efficiency, it was possible to analyze p27's effects on endogenous Ras. After 12 h the cells were washed, serum starved in 0.1% serum for 24 h, and then treated with 50 ng of EGF per ml for 15 min as indicated. Active Ras as determined by GST-fused Raf-1 RBD affinity precipitation is shown in the top panel, while p27 expression is shown in the lower. Transfected p27 had no effect on residual Ras activity in the absence of EGF (lanes 1 and 3), but significantly inhibited EGF-mediated Ras activation (lanes 2 and 4). (B) The CDK inhibitory function of p27 is not required to inhibit EGF-mediated activation of Ras. HEK293 cells were transfected with either vector alone or p27(CDK−) as indicated. After 12 h the cells were washed, serum starved in 0.1% serum for 24 h, and then treated with 50 ng of EGF per ml for 15 min as indicated. Active Ras was determined as described above. p27(CDK−) inhibited EGF-mediated Ras activation (upper panel) but did not affect expression levels of the Ras protein (lower panel).
Transfected p27 had no effect on the low levels of active Ras in serum-starved cells (compare lanes 1 and 3), consistent with previous results indicating that p27 does not target GRB2 under these conditions (Fig. 5A and B). However, transfected p27 inhibited EGF-dependent Ras activation (Fig. 12A, compare lanes 2 and 4). This inhibition did not require the CDK inhibitory function because p27(CDK−) also blocked EGF-mediated activation of Ras (Fig. 12B). These results indicate that p27 overexpression can specifically regulate endogenous Ras activation during mitogen stimulation of quiescent cells and provide further evidence that the CDK and GRB2 binding domains and inhibitory functions of p27 are separate and distinct. These results are somewhat in contrast to the failure of EGF stimulation to induce endogenous p27 regulation of GRB2 in p27+/+ cells. This difference could be attributable to cell type, or more likely p27 overexpression simply bypasses a step that cannot be accomplished by EGF stimulation.
DISCUSSION
p27's CDK inhibitory function may not define its tumor-suppressing ability.
p27 inhibition of cyclin-CDKs is well documented, as is its deregulation in human cancers (59, 61, 73). Less evident, however, is whether p27 disruption contributes to tumorigenesis solely via inappropriate CDK activity. There are clues that the tumor-suppressing ability of p27 may reside in some function other than CDK inhibition. The p27 gene is seldom deleted or mutated even though this seems the most straightforward way to overcome its tumor-suppressing function and enhance CDK activity (40). In fact, p27 levels are actually higher than normal in some instances, an unexpected occurrence if the goal of disrupting p27 is to alleviate CDK inhibition (24, 55). More commonly, p27 protein levels are inappropriately downregulated in the disease state, yet a convincing correlation between low p27 and excess CDK activity is lacking (33, 59, 68). Even when such correlations exist they are difficult to interpret, since a priori increased CDK activity could be responsible for low p27 levels rather than vice versa (45, 49, 60, 75). Finally, analogous disruptions that would be expected to enhance CDK activity (e.g., disruption of p27 family members and cyclin upregulation) appear to be much less frequent than p27 deregulation (11, 16, 73).
Generation of mice lacking the p27 gene has clarified some of these issues and supports a role for p27 in tumor suppression (21, 33, 46). However, in our view characterization of these animals has not supported the assumption that p27's CDK inhibitory function mediates tumor suppression. The first surprise was that p27−/− mice are viable despite lacking a putative critical negative regulator of G1 progression. Second, p27−/− mice are relatively normal and do not develop tumors in various tissues or organs. Third, cells derived from these animals display normal CDK activity, cell cycle progression, and response to mitogenic and antimitogenic stimuli (13). Together these observations lend further credence to the idea that p27's tumor-suppressing ability might reside in a function other than CDK inhibition (59).
Expanding roles for p27.
The relationship between p27 and various cyclin-CDKs is actually quite complex and entails more than simple CDK inhibition (63). Accumulating data indicate that p27 mediates a physiologically relevant association between cyclin D and CDK4 to generate an active complex (9, 35). Paradoxically, depending upon context, p27 association with cyclin E-CDK2 can result in either CDK inhibition or p27 phosphorylation and elimination by the ubiquitin/proteasome system (49, 60). These mechanistic observations—along with p27's involvement in diverse cellular processes such as adhesion, anergy, apoptotic control, and cellular senescence-hint that p27 might perform functions other than inhibiting CDKs and regulating G1 progression (59).
Novel role for p27: regulating GRB2 association with SOS.
We now present evidence that p27 is part of a negative feedback loop controlling GRB2 association with SOS. We show that p27 is exported from the nucleus to the cytoplasm within 10 min of stimulating quiescent cells with mitogens, well before p27 is degraded and cyclin-CDKs are synthesized or activated (11, 36, 62). These results suggest that p27 export is not alleviating CDK inhibition in the nucleus or facilitating its degradation in the cytoplasm and instead point towards a novel cytoplasmic function distinct from regulation of cyclin-CDKs.
We found that exported p27 binds GRB2, raising the possibility that it might regulate GRB2 function. Consistent with this idea p27 and SOS both use proline-rich regions to bind GRB2, and p27 can block GRB2-SOS complex formation with purified proteins and in a transient transfection assay. This competition appears to be physiologically relevant because GRB2-SOS complexes persist in mitogen-stimulated p27−/− cells but rapidly dissociate in cells containing p27. While p27 might be responsible for GRB2-SOS dissociation, we consider this explanation unlikely because in vitro purified p27 failed to dissociate preformed GRB2-SOS complexes (data not shown). It seems more probable that mitogens facilitate GRB2-SOS dissociation by a p27-independent mechanism, whereupon cytoplasmic p27 binds free GRB2 and prevents its reassociation with SOS (10, 14, 76).
An alternative explanation is that loss of CDK inhibitory function in mitogen-stimulated p27−/− cells is indirectly responsible for increased GRB2-SOS. In other words, p27 inhibition of CDKs might indirectly block GRB2-SOS complex formation. We do not think this explanation is likely because p27-GRB2 complexes formed in p27+/+ cells concomitant with the disappearance of GRB2-SOS, consistent with a competition between p27 and SOS for binding GRB2. Furthermore, the CDK and GRB2 binding domains are separable and the inhibitory functions are distinct. Deregulation of GRB2-SOS complex formation was observed immediately after mitogen stimulation of quiescent p27−/− cells, significantly before the well-characterized mitogen-dependent induction and activation of cyclin-CDKs (11, 36). Finally, the export inhibitor leptomycin B stabilized GRB2-SOS complexes in p27+/+ cells. If p27 inhibition of CDKs indirectly blocked SOS association with GRB2, then retaining p27 in the nucleus and increasing CDK inhibition should have prevented—not enhanced—GRB2-SOS complex formation.
p27 targeting of GRB2 appears to be a highly regulated event, as GRB2-SOS complexes were not deregulated in proliferating p27−/− cells, in response to serum withdrawal, or after exposure to transforming growth factor beta. Intriguingly, PDGF was able to initiate p27 regulation of GRB2 while EGF was not. Since the targets of these respective pathways have been well characterized, this specificity may shed light on downstream events required for establishing the negative feedback loop. One possibility is that the inability of EGF to initiate p27 regulation of GRB2 results from a failure to export p27 to the cytoplasm. We do not think this is the case, however, because both PDGF and EGF activate MAP kinase to similar extents in p27+/+ cells (data not shown). A more likely possibility is that EGF does not cause dissociation of the GRB2-SOS complexes present in quiescent cells, thereby preventing p27 access to GRB2.
Both MAP kinase activity and p27 export appear to be required for targeting GRB2. These two criteria may be connected because MAP kinase can phosphorylate p27S10 (at least in vitro), a site known to be involved in p27 export (30, 53). In support of this model we found that exogenously expressed active MKK can drive p27-GRB2 complex formation. However, there is some indication that p27S10 is already phosphorylated in quiescent cells, in which case MAP kinase may facilitate p27 export by other means (e.g., phosphorylating another p27 site or activating export machinery) (31, 53). We are currently distinguishing between these possibilities by mapping p27 phosphorylation sites and identifying new binding partners resulting from mitogen stimulation.
p27 can regulate Ras independently of its CDK inhibitory function.
Because GRB2-SOS plays a critical role in activating Ras, it seemed likely that p27 regulation of these complexes might help establish the initial burst of Ras activation upon mitogen stimulation of quiescent cells (37). However, Ras activity was not significantly altered in p27−/− cells despite dramatic deregulation of GRB2-SOS complexes. These results are perhaps not unexpected, given that the p27−/− mice are viable, develop normally, and do not acquire aggressive tumors in many tissues and organs. Previous work has shown that Ras activation is tightly controlled by multiple and sometimes redundant mechanisms, and there is evidence that the extent of Ras activation does not directly correlate with the amount of GRB2-SOS (5, 37). One possibility is that the excess GRB2-SOS complexes in mitogen-stimulated p27−/− cells are inactive; alternatively, these complexes may be active, but downstream events are inhibited. We are currently searching for additional disruptions that, in conjunction with p27 absence, lead to hyperactivation of endogenous Ras in response to mitogen stimulation. However, at this point we cannot rule out the possibility that p27 is targeting GRB2 for some purpose other than regulating Ras.
In order to directly investigate whether p27 can regulate Ras, we isolated the relevant events from their physiological context with the transient transfection assay. Wild-type p27 inhibited activation of transfected Ras and more importantly had the same effect when Ras activation was stimulated by cotransfected SOS and GRB2. In addition, p27(CDK−) inhibited activation of transfected Ras, while p27(GRB2−) did not, indicating that the ability to bind GRB2 but not cyclin-CDKs is required to inhibit Ras. These results further indicate that the CDK and GRB2 inhibitory domains and functions are separable and distinct. We took advantage of these mutants and the high transfectability of HEK293 cells to demonstrate that exogenously expressed wild-type p27 and p27(CDK−) also inhibited EGF-mediated activation of endogenous Ras. The fact that transfected but not endogenous p27 responded to EGF stimulation probably reflects the fact that additional steps are required to complete this regulatory network under more physiological conditions. Regardless, these results indicate that p27 can regulate Ras activation by targeting GRB2 rather than cyclin-CDKs and support our hypothesis that endogenous p27 might make a similar but subtler contribution upon mitogen stimulation of quiescent cells.
Does loss of GRB2 inhibitory function provide a more cogent explanation of the p27−/− phenotype?
Regulating GRB2/Ras has the potential to influence fundamental cell fate decisions, which might provide a better explanation for p27's involvement in disparate biological processes. One of the most compelling examples occurs during establishment of T-cell anergy, when T cells receive insufficient mitogenic signals and thus enter a nonproliferative and nonresponsive state (58). Ras is transiently activated by these insufficient signals but then shuts off via unknown mechanisms (38). Intriguingly, p27 has been described as an “anergy factor” necessary for establishing the anergic state, although the precise mechanism involved remains unknown (4, 47). We propose that insufficient mitogen signals initiate p27 export to the cytoplasm and GRB2 binding, thus contributing to the transient nature of Ras activation.
A similar analysis can be made with respect to the curious observation that p27−/− mice are 33% larger than wild-type mice due to an increased number of normal cells (22, 33, 46). This phenotype is difficult to rationalize as a consequence of increased CDK activity and deregulated cell cycle control, especially since neither CDKs nor cell cycle progression appears to be disrupted in p27−/− cells (13). Furthermore, while it is difficult to envision how loss of cell cycle control might result in excess normal cells, strict control of Ras is essential to proper cell fate determination (23, 63). Although we did not observe obvious changes in Ras activity in isolated p27−/− MEFs, subtle Ras deregulation could occur within the more complex environment of mouse development. Generation of a mouse expressing the p27(CDK−) mutant rather than wild-type p27 would go a long way towards answering this question.
Our results point towards disrupted GRB2 regulation occurring when quiescent cells respond to mitogens. However, given the profile of p27 expression during progression from G0 to S phase, it is tempting to speculate that p27 might help regulate Ras at other points in the cell cycle. After mitogen exposure and the initial rapid burst of activity, Ras activation is required again later in G1 for progression through the restriction point and into S phase (17, 69). Intriguingly, p27 is also exported 6 to 10 h after mitogen stimulation as cells approach the restriction point (39, 53). While the rationale for this export is unclear, it is generally thought to alleviate CDK inhibition in the nucleus and facilitate p27 degradation in the cytoplasm. However, given the complex relationship between p27 and cyclin-CDKs and the controversy surrounding the location of p27 degradation, we are investigating the possibility that p27 is contributing to regulation of Ras activation during this period (36, 53, 62, 71). Indeed, generation of active cyclin E-CDK2 could initiate the second burst of Ras activity by targeting p27 for elimination as cells approach the restriction point (12, 51, 69).
Alternative explanation for p27's tumor-suppressing function.
The ability of p27 to inhibit GRB2 provides an intriguing explanation for its widespread deregulation in aggressive human cancers. Ras is one of the most commonly activated oncogenes, and hence the Ras/MAP kinase pathway is exquisitely controlled to ensure proper development of the organism and prevent tumorigenesis (2). GRB2 gene dosage is rate limiting for tumorigenesis, and while GRB2 overexpression alone is not transforming, it does amplify Ras/MAP kinase activity (8, 15, 74). In similar fashion, abnormally low p27 might increase GRB2-SOS complex formation and the probability of inappropriately activating Ras. Maintaining proper p27 levels is clearly essential for tumor-suppressing function, as loss of a single p27 allele (without disruption of the other copy) predisposes to tumorigenesis (22). Paradoxically, however, complete loss of p27 expression via deletion or mutation might be selected against (at least in human cancers) if it causes enough Ras activity to induce an apoptotic response. In this view maintaining p27—or abnormally high p27 levels—in the cancer cell might actually contribute to tumorigenesis by buffering excessive Ras activity and preventing apoptosis. This possibility might also help explain the observations of cytoplasmic p27 in diseases such as chronic myelogenous leukemia and tuberous sclerosis, which may reflect a failed attempt to regulate GRB2/Ras rather than mislocalization of p27 to activate nuclear CDKs (59, 65). These ideas predict that p27 downregulation might not correlate with mutational activation of Ras, since both result in inappropriate Ras activity. While further work is required to definitively answer this question, there is one report from a lung cancer study indicating that p27 downregulation and Ras mutations are not found in the same tumors (6).
Acknowledgments
This work was supported by National Institutes of Health grant CA-87267 from the National Cancer Institute.
REFERENCES
- 1.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD098059 is a specific inhibitor of the activation of MAP kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 2.Barbacid, M. 1990. Ras oncogenes: their role in neoplasia. Eur. J. Clin. Investig. 20:225-235. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Sagi, D., D. Rotin, A. Batzer, V. Mandiyan, and J. Schlessinger. 1993. SH3 domains direct cellular localization of signaling molecules. Cell 74:83-91. [DOI] [PubMed] [Google Scholar]
- 4.Boussiotis, V. A., G. J. Freeman, P. A. Taylor, A. Berezovskaya, I. Grass, B. R. Blazar, and L. M. Nadler. 2000. P27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat. Med. 6:290-296. [DOI] [PubMed] [Google Scholar]
- 5.Buday, L., P. H. Warne, and J. Downward. 1995. Downregulation of the Ras activation pathway by MAP kinase phosphorylation of Sos. Oncogene 11:1327-1331. [PubMed] [Google Scholar]
- 6.Catzavelos, C., M. Tsao, G. DeBoer, N. Bhattacharya, F. A. Shephard, and J. M. Slingerland. 1999. Reduced expression of the cell cycle inhibitor p27Kip1 in non-small cell lung carcinoma: a prognostic factor independent of Ras. Cancer Res. 59:684-688. [PubMed] [Google Scholar]
- 7.Chardin, P., J. H. Camonis, N. W. Gale, L. Van Aelst, J. Schlessinger, M. H. Wigler, and D. Bar-Sagi. 1993. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science 260:1338-1343. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, A. M., T. M. Saxton, R. Sakai, S. Kulkarni, G. Mbamalu, W. Vogel, C. G. Tortorice, R. D. Cardiff, J. C. Cross, W. J. Muller, and T. Pawson. 1998. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell 95:793-803. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21Cip1 and p27Kip1 CDK ‘inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherniak, A. D., J. K. Klarlund, B. R. Conway, and M. P. Czech. 1995. Disassembly of Son-of-sevenless proteins from Grb2 during p21ras desensitization. J. Biol. Chem. 270:1485-1488. [PubMed] [Google Scholar]
- 11.Clurman, B., and J. M. Roberts. 1998. Cell cycle control: an overview, p. 175-193. In B. Vogelstein and K. W. Kinzler (ed.), The genetic basis of human cancer, 1st ed. McGraw-Hill, New York, N.Y.
- 12.Coats, S., W. M. Flannagan, J. Nourse, and J. Roberts. 1996. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 272:877-880. [DOI] [PubMed] [Google Scholar]
- 13.Coats, S., P. Whyte, M. L. Fero, S. Lacy, G. Chung, E. Randel, E. Firpo, and J. M. Roberts. 1999. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27Kip1-deficient cells. Curr. Biol. 9:163-173. [DOI] [PubMed] [Google Scholar]
- 14.Corbolan-Garcia, S., S.-S. Yang, K. R. Degenhardt, and D. Bar-Sagi. 1996. Identification of the MAP kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol. Cell. Biol. 10:5674-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daly, R. J., M. D. Binder, and R. L. Sutherland. 1994. Overexpression of the Grb2 gene in human breast cancer cell lines. Oncogene 9:2723-2727. [PubMed] [Google Scholar]
- 16.Deng, C., P. Zhang, J. W. Harper, S. J. Elledge, and P. Leder. 1995. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82:675-684. [DOI] [PubMed] [Google Scholar]
- 17.Dobrowolski, S., M. Harter, and D. W. Stacey. 1994. Cellular ras activity is required for passage through multiple points of the G0/G1 phase in BALB/c 3T3 cells. Mol. Cell. Biol. 14:5441-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the MAP kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan, S. E., B. W. Giddings, M. W. Brooks, L. Buday, A. M. Sizeland, and R. A. Weinberg. 1993. Association of SOS Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature 363:45-51. [DOI] [PubMed] [Google Scholar]
- 20.Ewen, M. E. 2000. Relationship between Ras pathways and cell cycle control. Progress Cell Cycle Res. 4:1-17. [DOI] [PubMed] [Google Scholar]
- 21.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 85:733-744. [DOI] [PubMed] [Google Scholar]
- 22.Fero, M. L., E. Randel, K. E. Gurley, J. M. Roberts, and C. J. Kemp. 1998. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396:177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frame, S., and A. Balmain. 2000. Integration of positive and negative growth signals during ras pathway activation in vivo. Curr. Opin. Genet. Dev. 10:106-113. [DOI] [PubMed] [Google Scholar]
- 24.Fredersdorf, S., J. Burns, A. M. Milne, G. Packham, L. Fallis, C. E. Gillett, J. A. Royds, D. Peston, P. A. Hall, A. M. Hanby, D. M. Barnes, S. Shousha, M. J. O'Hare, and X. Lu. 1997. High level expression of p27kip1 and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27kip1 and degree of malignancy in human breast and colorectal cancers. Proc. Natl. Acad. Sci. USA 94:6380-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gille, H., and J. Downward. 1999. Multiple Ras effector pathways contribute to G1 cell cycle progression. J. Biol. Chem. 274:22033-22038. [DOI] [PubMed]
- 26.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 27.Heldin, C., and B. Westermark. 1999. Mechanism of action and in vivo role of PDGF. Phys. Rev. 79:1283-1316. [DOI] [PubMed] [Google Scholar]
- 28.Hengst, L., V. Dulic, J. M. Slingerland, E. Lees, and S. I. Reed. 1994. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA 91:5291-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell, K. E., E. Devaney, and J. Gruenberg. 1989. Subcellular fractionation of tissue culture cells. Trends Biol. Sci. 14:44-47. [DOI] [PubMed] [Google Scholar]
- 30.Ishida, N., T. Hara, T. Kamura, M. Yoshida, K. Nakayama, and K. I. Nakayama. 2002. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277:14355-14358. [DOI] [PubMed] [Google Scholar]
- 31.Ishida, N., M. Kitagawa, S. Hatakeyama, and K. Nakayama. 2000. Phosphorylation at serine 10, a major phosphorylation site of p27Kip1, increases its protein stability. J. Biol. Chem. 275:25146-25154. [DOI] [PubMed] [Google Scholar]
- 32.Katz, M. E., and F. McCormick. 1997. Signal transduction from multiple Ras effectors. Curr. Opin. Genet. Dev. 7:75-79. [DOI] [PubMed] [Google Scholar]
- 33.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell 85:721-732. [DOI] [PubMed] [Google Scholar]
- 34.Koff, A., M. Ohtsuki, K. Polyack, J. Roberts, and J. Massague. 1993. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by transforming growth factor beta. Science 260:536-539. [DOI] [PubMed] [Google Scholar]
- 35.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 36.Ladha, M. H., K. Y. Lee, T. M. Upton, M. F. Reed, and M. E. Ewen. 1998. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol. Cell. Biol. 18:6605-6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langlois, W. J., T. Sasaoka, A. R. Saltiel, and J. M. Olefsky. 1995. Negative feedback regulation and desensitization of insulin- and EGF-stimulated p21ras activation. J. Biol. Chem. 270:25320-25323. [DOI] [PubMed] [Google Scholar]
- 38.Li, W., C. D. Whaley, A. Mondino, and D. L. Mueller. 1996. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science 271:1272-1276. [DOI] [PubMed] [Google Scholar]
- 39.Li, N., A. Batzer, R. Daly, V. Yajkik, E. Skolnik, P. Chardin, D. Bar-Sagi, B. Margolis, and J. Schlessinger. 1993. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature 363:85-88. [DOI] [PubMed] [Google Scholar]
- 40.Lloyd, R. V., L. A. Erickson, L. Jin, E. Kulig, X. Qian, J. C. Cheville, and B. W. Scheithauer. 1999. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am. J. Pathol. 154:313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowenstein, E. J., R. J. Daly, A. G. Batzer, W. Li, B. Margolis, R. Lammers, A. Ullrich, E. Y. Skolnik, D. Bar-Sagi, and J. Schlessinger. 1992. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell 70:431-442. [DOI] [PubMed] [Google Scholar]
- 42.Mansour, S. J., W. T. Matten, A. S. Hermann, J. M. Candia, S. Rong, K. Fukasawa, G. F. VandeWoude, and N. G. Ahn. 1994. Transformation of mammalian cells by consitutively active MAP kinase kinase. Science 265:966-970. [DOI] [PubMed] [Google Scholar]
- 43.Morgan, D. O. 1997. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13:261-291. [DOI] [PubMed] [Google Scholar]
- 44.Mulcahy, L. S., M. R. Smith, and D. W. Stacey. 1985. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature 313:241-243. [DOI] [PubMed] [Google Scholar]
- 45.Muller, D., C. Bouchard, B. Rudolph, P. Steiner, I. Stuckmann, R. Saffrich, W. Ansorge, W. Huttner, and M. Eilers. 1997. CDK2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclinE/cdk2 complexes. Oncogene 15:2561-2576. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, D. Y. Loh, and K. Nakayama. 1996. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707-720. [DOI] [PubMed] [Google Scholar]
- 47.Nourse, J., E. Firpo, M. Flanagan, M. Meyerson, K. Polyak, M. H. Lee, J. Massague, G. Crabtree, and J. Roberts. 1994. IL-2 mediated elimination of the p27kip1 cyclin-CDK kinase inhibitor prevented by rapamycin. Nature 372:570-573. [DOI] [PubMed] [Google Scholar]
- 48.Olivier, J. P., T. Raabe, M. Henkenmeyer, B. Dickson, G. Mbamalu, B. Margolis, J. Schlessinger, E. Hafen, and T. Pawson. 1993. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of ras guanine nucleotide exchange, Sos. Cell 73:179-191. [DOI] [PubMed] [Google Scholar]
- 49.Pagano, M., S. W. Tam, A. M. Theodoras, P. Beer-Romero, G. Del Sal, V. Chau, P. R. Yew, G. F. Draetta, and M. Rolfe. 1995. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682-685. [DOI] [PubMed] [Google Scholar]
- 50.Peeper, D. S., T. M. Upton, M. H. Ladha, E. Neuman, J. Zalvide, R. Bernards, J. A. DeCaprio, and M. E. Ewen. 1997. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature 386:177-181. [DOI] [PubMed] [Google Scholar]
- 51.Polyak, K., M. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massague. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59-66. [DOI] [PubMed] [Google Scholar]
- 52.Polyak, K., J. Kato, M. J. Solomon, C. J. Sherr, J. Massague, J. M. Roberts, and A. Koff. 1994. p27kip1, a cyclin-CDK inhibitor, links transforming growth factor beta and contact inhibition to cell cycle arrest. Genes Dev. 8:9-22. [DOI] [PubMed] [Google Scholar]
- 53.Rodier, G., A. Montagnoli, L. Di Marcotullio, P. Coulombe, G. F. Draetta, M. Pagano, and S. Meloche. 2001. p27 cytoplasmic localization is regulated by phosphorylation on Ser 10 and is not a prerequisite for its proteolysis. EMBO J. 20:6672-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rozakis-Adcock, M., J. McGlade, G. Mbamalu, G. Pelicci, R. Daly, W. Li, A. Batzer, S. Thomas, J. Brugge, P. G. Pelicci, J. Schlessinger, and T. Pawson. 1992. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature 360:689-692. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Beato, M., F. I. Camacho, J. C. Martinez-Montero, A. I. Saez, R. Villuendas, L. Sanchez-Verde, J. F. Garcia, and M. A. Piris. 1999. Anomalous high p27/KIP1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27/KIP1-cyclin D3 colocalization in tumor cells. Blood 94:765-772. [PubMed] [Google Scholar]
- 56.Satoh, T., M. Endo, M. Nakafuku, S. Nakamura, and Y. Kaziro. 1990. Platelet-derived growth factor stimulates formation of active p21ras-GTP complex in Swiss mouse 3T3 cells. Proc. Natl. Acad. Sci. USA 87:5993-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satoh, T., M. Endo, M. Nakafuku, T. Akiyama, T. Yamamoto, and Y. Kaziro. 1990. Accumulation of p21ras-GTP in response to stimulation with EGF and oncogene products with tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 87:7926-7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz, R. H. 1997. T cell clonal anergy. Curr. Opin. Immunol. 9:351-357. [DOI] [PubMed] [Google Scholar]
- 59.Sgambato, A., A. Cittadini, B. Faraglia, and I. B. Weinstein. 2000. Multiple functions of p27Kip1 and its alterations in tumor cells: a review. J. Cell. Phys. 183:18-27. [DOI] [PubMed] [Google Scholar]
- 60.Sheaff, R. J., M. Groudine, M. Gordon, J. M. Roberts, and B. E. Clurman. 1997. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11:1464-1478. [DOI] [PubMed] [Google Scholar]
- 61.Sherr, C., and J. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1153. [DOI] [PubMed] [Google Scholar]
- 62.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 63.Shields, J. M., K. Pruitt, A. McFall, A. Shaub, and C. J. Der. 2000. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol. 10:147-154. [DOI] [PubMed] [Google Scholar]
- 64.Simon, M. A., G. S. Dodson, and G. M. Rubin. 1993. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos protein in vitro. Cell 73:169-177. [DOI] [PubMed] [Google Scholar]
- 65.Singh, S. P., J. Lipman, H. Goldman, F. H. Ellis, Jr, L. Aizenman, M. G. Cangi, S. Signoretti, D. S. Chiaur, M. Pagano, and M. Loda. 1998. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 58:1730-1735. [PubMed] [Google Scholar]
- 66.Smitherman, M., K. Lee, J. Swanger, R. Kapur, and B. E. Clurman. 2000. Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. Mol. Cell. Biol. 20:5631-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugiyama, Y., K. Tomoda, T. Tanaka, Y. Arata, N. Yoneda-Kato, and J. Kato. 2001. Direct binding of the signal-transducing adaptor Grb2 facilitates down-regulation of the cyclin-dependent kinase inhibitor p27Kip1. J. Biol. Chem. 276:12084-12090. [DOI] [PubMed] [Google Scholar]
- 68.Sweeney, K. J., A. Swarbrick, R. L. Sutherland, and E. A. Musgrove. 1998. Lack of relationship between CDK activity and G1 cyclin expression in breast cancer cells. Oncogene 16:2865-2878. [DOI] [PubMed] [Google Scholar]
- 69.Takuwa, N., and Y. Takuwa. 1997. Ras activity late in G1 phase required for p27Kip1 downregulation, passage through the restriction point, and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol. Cell. Biol. 17:5348-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor, S. J., and D. Shalloway. 1996. Cell cycle-dependent activation of Ras. Curr. Biol. 6:1621-1627. [DOI] [PubMed] [Google Scholar]
- 71.Tomoda, K., Y. Kubota, and J. Kato. 1999. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398:160-165. [DOI] [PubMed] [Google Scholar]
- 72.Toyoshima, H., and T. Hunter. 1994. p27, a novel inhibitor of G1-cyclin-CDK protein kinase activity, is related to p21. Cell 78:67-74. [DOI] [PubMed] [Google Scholar]
- 73.Tsihlias, J., L. Kapusta, and J. Slingerland. 1999. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu. Rev. Med. 50:401-423. [DOI] [PubMed] [Google Scholar]
- 74.Verbeek, B. S., S. S. Adriaansen-Slot, G. Rijksen, and T. M. Vroom. 1997. Grb2 overexpression in nuclei and cytoplasm of human breast cells: a histochemical and biochemical study of normal and neoplastic mammary tissue specimens. J. Pathol. 183:195-203. [DOI] [PubMed] [Google Scholar]
- 75.Vlach, J., S. Hennecke, and B. Amati. 1997. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 16:5334-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waters, S. B., K. Yamauchi, and J. E. Pessin. 1995. Insulin-stimulated dissociation of the Sos-Grb2 complex. Mol. Cell. Biol. 15:2791-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zarich, N., J. L. Oliva, R. Jorge, E. Santos, and J. M. Rojas. 2000. The isoform-specific stretch of hSos1 defines a new Grb2-binding domain. Oncogene 19:5872-5883. [DOI] [PubMed] [Google Scholar]
- 78.Zeng, Y., K. Hirano, M. Hirano, J. Nishimura, and H. Kanaide. 2000. Minimal requirements for the nuclear localization of p27Kip1, a cyclin-dependent kinase inhibitor. Biochem. Biophys. Res. Commun. 274:37-42. [DOI] [PubMed] [Google Scholar]