Abstract
Protein tyrosine phosphatase-1B (PTP-1B) attenuates insulin, PDGF, EGF, and IGF-I signaling by dephosphorylating tyrosine residues located in the tyrosine kinase domain of the corresponding receptors. More recently, PTP-1B was shown to modulate the action of cytokine signaling via the nonreceptor tyrosine kinase JAK2. Transmission of the growth hormone (GH) signal also depends on JAK2, raising the possibility that PTP-1B modulates GH action. Consistent with this hypothesis, GH increased the abundance of tyrosine-phosphorylated JAK2 associated with a catalytically inactive mutant of PTP-1B. GH-induced JAK2 phosphorylation was greater in knockout (KO) than in wild-type (WT) PTP-1B embryonic fibroblasts and resulted in increased tyrosine phosphorylation of STAT3 and STAT5, while overexpression of PTP-1B reduced the GH-mediated activation of the acid-labile subunit gene. To evaluate the in vivo relevance of these observations, mice were injected with GH under fed and fasted conditions. As expected, tyrosine phosphorylation of JAK2 and STAT5 occurred readily in the livers of fed WT mice and was almost completely abolished during fasting. In contrast, resistance to the action of GH was severely impaired in the livers of fasted KO mice. These results indicate that PTP-1B regulates GH signaling by reducing the extent of JAK2 phosphorylation and suggest that PTP-1B is essential for limiting the action of GH during metabolic stress such as fasting.
Postnatal growth is profoundly reduced in mice and humans suffering from a deficit in growth hormone (GH) signaling (6, 30). GH promotes growth by acting directly on tissues, such as liver and bone, and by stimulating the synthesis of IGF-I, a growth factor required for complete postnatal development (11, 34, 35). In addition, GH is a major metabolic hormone, with effects on glucose, protein, and lipid metabolism (30, 34). As a consequence, secondary disorders often occur in individuals suffering from abnormal GH secretion. For example, excess GH in plasma leads to the development of insulin resistance, whereas a deficit is associated with increased adiposity (30, 44). In mice, GH is involved in the development of complications associated with diabetes, such as retinal neovascularization and nephropathy (22, 30).
Molecular events involved in the transmission of the GH signal have received considerable attention in recent years. Primary events include homodimerization of the GH receptor (GHR), recruitment of the tyrosine kinase Janus kinase 2 (JAK2) to the cytoplasmic domain of the receptor, and activation of JAK2 by autophosphorylation (3, 22). A variety of signaling proteins are then recruited to high-affinity binding sites on tyrosine-phosphorylated JAK2 and GHR, leading to the activation of signal transducers and activators of transcription (STATs) 1, 3, and 5b; the Ras-MAP (mitogen-activated protein) kinase pathway; and the insulin receptor substrate 1 (IRS-1)/phosphatidylinositol 3′ kinase/AKT pathway (22, 34). Equally important, but less understood, are the mechanisms limiting and terminating GHR signaling. These include internalization and degradation of the GHR; inhibition of signaling by negative regulators, such as suppressors of cytokine signaling (SOCS) and protein inhibitor of activated STAT (PIAS); and inactivation of JAK2 and downstream signaling molecules by dephosphorylation (16, 18, 22).
Given the central role of JAK2 in initiating GH signaling, identification of the phosphatases involved in its deactivation is important. Candidates that have been shown to be involved include the cytosolic tyrosine phosphatases SHP-1 and SHP-2 (20, 28, 46) and the transmembrane tyrosine phosphatase CD45 (25). Recently, the ubiquitously expressed protein tyrosine phosphatase 1B (PTP-1B) was shown to bind phosphorylated JAK2 in leptin- and gamma interferon (IFN-γ)-treated cells (10, 38, 53). We now show that PTP-1B interacts with JAK2 in a GH-dependent manner and dephosphorylates tyrosine residues present in the activation loop of JAK2. The absence of PTP-1B results in GH-dependent hyperphosphorylation of JAK2 and enhanced activation of STAT3 and STAT5, while overexpression of PTP-1B reduced GH-mediated activation of a STAT5-dependent gene. PTP-1B modulation of GH signaling is physiologically relevant, as shown by loss of GH resistance in the livers of fasted null PTP-1B mice.
MATERIALS AND METHODS
Reagents.
Reagents were from Fisher Biotech unless otherwise indicated. Rabbit polyclonal antibodies against PTP-1B and 3E2 monoclonal antibodies against TC-PTP were described previously (12, 52). Antibodies against the extracellular domain of the rat GHR were a gift of W. Baumbach (American Cyanamid, Princeton, N.J.). Other antibodies were purchased from Upstate Biotechnology (JAK2), Biosource International (JAK2 pYpY1007/1008), Cell Signaling (STAT3, STAT3 pY705, and STAT5 pY694), and Santa Cruz Biotechnology (GST, JAK1, JAK3, and STAT5b). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were from Jackson Immunoresearch. Human GH (hGH) was provided by the National Hormone and Pituitary Program (Torrance, Calif.).
Substrate trapping.
293 cells stably expressing JAK2 (293LA) (33) were transfected with 5 μg of the empty glutathione S-transferase (GST) vector pEBG (GST) or with pEBG expressing wild-type (WT) PTP-1B (GST- PTP-1B WT) or D181A PTP-1B (GST- PTP-1B D181A). D181A is a mutant with normal binding capacity but reduced catalytic activity (described in reference 42). Transfections were performed using Lipofectamine (Invitrogen, Life Technologies). Thirty-six hours after transfection, the cells were starved overnight in serum-free medium and then stimulated with 1 μg of hGH/ml for 10 min. The cells were lysed in TNE buffer (150 mM NaCl, 50 mM Tris-HCl, and 1% NP-40) supplemented with an EDTA-free cocktail of protease inhibitor (Complete; Roche Molecular Biochemicals). The protein concentrations of the lysates were determined, and GST-tagged proteins were retrieved using 20 μl of glutathione-Sepharose beads (Pharmacia Biotech). The beads were washed in lysis buffer, boiled in 2× sodium dodecyl sulfate (SDS) sample buffer, resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and analyzed by immunoblotting.
Cells and culture conditions.
Spontaneously immortalized murine embryonic fibroblasts (MEFs) lacking PTP-1B or T-cell PTP (TC-PTP) were previously described (8, 24). For rescue experiments, PTP-1B knockout (KO) MEFs were infected with a retrovirus vector encoding Myc-tagged murine PTP-1B, and stable transfectants were selected with hygromycin. Primary WT and PTP-1B KO fibroblasts (PMEFs) were isolated from E14 embryos. H4-II-E cells were established from rat liver hepatomas (40). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen) and antibiotics (5 U of penicillin/ml and 5 μg of streptomycin/ml).
Cell stimulation, preparation of cell lysate, and immunoblotting.
To study the effect of GH, confluent cells were incubated overnight in serum-free medium. Thirty minutes prior to stimulation, the medium was changed to fresh serum-free medium, followed by treatment with 100 ng of hGH/ml. At appropriate times, the cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in M-RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 1% NP-40, 0.25% Na deoxycholate) supplemented with 1 mM sodium vanadate, 50 mM NaF, and Complete protease inhibitors. Cell lysates were cleared by centrifugation, and the protein concentrations of all lysates were measured by the Bradford method. After SDS-PAGE, the proteins were transferred to polyvinylidene difluoride membranes (Immobilon; Millipore). The membranes were blocked in 1% bovine serum albumin when anti-phosphotyrosine antibodies were used and in 5% milk for all other antibodies. Horseradish peroxidase-conjugated anti-mouse or anti-rabbit was used as a secondary antibody, and enhanced chemiluminescence was used to develop the blot. The signals were quantified by densitometric scanning and NIH Image software.
Cell fractionation.
WT and KO PTP-1B MEFs were treated with 100 ng of hGH/ml for 10 min. The cells were washed on ice with cold PBS, scraped in homogenization buffer (3 mM imidazole, 8.5% sucrose), and homogenized by extrusion though a 22-gauge needle. Nuclei and unbroken cells were removed by low-speed centrifugation. The resulting supernatant was ultracentrifuged (100,000 × g for 15 min) for the isolation of cytosolic and total membrane proteins.
Transfection of rat liver cells.
Transfections of H4-II-E cells were performed exactly as described previously (5). Briefly, each well of near-confluent monolayers was exposed to 100 μl of a DNA solution (0.5 mg of DEAE-dextran/ml, 0.7 μg of the firefly luciferase plasmid mALS703WT, 0.3 μg of the plasmid pRL-TK, and 1.5 μg of expression vector). m703ALSWT was constructed by inserting the GH-responsive mouse acid-labile subunit (ALS) promoter into the luciferase plasmid pGL3-basic. The expression vectors used were pcDNA 3.1 or expression vectors encoding mouse PTP-1B (pcDNA3.1/PTP-1B [J. Wagner and M. Tremblay, unpublished data]), mouse TC-PTP (pcDNA4/TC-PTP [D. Simoncic and M. Tremblay, unpublished data]), or mouse PTP-PEST (pACTAG-2-mPTP-PEST [7]). The plasmid pRL-TK (Promega) encodes Renilla luciferase and was used to correct for variations in transfection efficiency. The plasmids were purified by ion-exchange chromatography (Qiagen, Chatsworth, Calif.). After a 40-h recovery period in DMEM supplemented with 10% fetal calf serum, the media were changed to serum-free DMEM without or supplemented with 100 ng of bovine GH/ml. Twenty hours later, the cell lysates were assayed for firefly and Renilla luciferase by the Dual-Luciferase Reporter System (Promega).
Liver hGH stimulation.
Male WT or PTP-1B KO mice are a hybrid of 129S/v and BALB/c backgrounds (12) backcrossed into a BALB/c background for three generations. At 9 or 10 weeks of age, they were offered ad libidum levels of a standard diet or fasted for 48 h. After anesthesia, the mice were administered saline or hGH (0.5 μg/g of body weight via the vena cava). The liver was removed at various times after injection and homogenized in M-RIPA buffer. Equal amounts of lysate were resolved by SDS-PAGE and immublotting. For RNA extraction and Northern analysis, WT and KO mice fasted for 48 h and were administered saline or hGH (1 μg/g of body weight) intraperitoneally. The livers were collected before (zero time) and after (30, 60, and 90 min) injection.
Northern analysis.
Total RNA was prepared from rat liver cells by the acid guanidium thiocyanate phenol-chloroform method and quantified by absorbance at 260 nm (5). Total RNA (15 μg/lane) was electrophoresed on a 1.2% agarose-formaldehyde gel, blotted onto a nylon membrane, and hybridized to [α-32P]dCTP-labeled DNA probes. The probes used included the coding region of mouse SOCS2, SOCS3, and CIS cDNA (obtained from D. J. Hilton and R. Starr [45]). Staining with ethidium bromide confirmed that the rRNA was intact and that equal amounts of RNA were loaded in all lanes.
Statistical analysis.
Unless otherwise indicated, results are reported as the mean ± standard error of the mean of at least three separate experiments. Statistical analyses were performed using a two-tailed, unpaired Student's t test.
RESULTS
JAK2 is a substrate of PTP-1B upon GH stimulation.
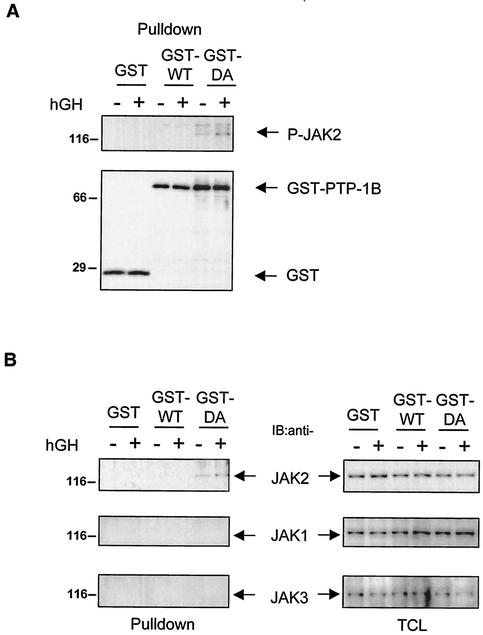
Leptin and IFN-γ have recently been shown to promote the association of JAK2 with PTP-1B (10, 38, 53). To determine whether GH could promote a similar interaction, substrate trapping was used (13). 293LA cells were transfected with expression vectors encoding WT PTP-1B (GST- PTP-1B WT), a catalytically inactive mutant (GST- PTP-1B D181A), or the corresponding empty vector (GST). After cell lysis, GST proteins were collected on glutathione beads, separated by SDS-PAGE, and immunoblotted with an antibody specific for phosphorylated tyrosine residues responsible for activating the kinase domain of JAK2 (anti-JAK2pYpY1007/1008). Under basal conditions, low levels of phosphorylated JAK2 were detected with GST- PTP-1B D181A, perhaps due to residual levels of phosphorylated JAK2 after serum starvation. More importantly, the amount of phosphorylated JAK2 associated with GST- PTP-1B D181A was increased about twofold when cells were incubated for 10 min in the presence of GH (Fig. 1A). A total of ∼5% phosphorylated JAK2 was found to be associated with PTP-1B D181A after GH stimulation. A smaller increase was also visible when the blot was reprobed with anti-JAK2 antibody (Fig. 1B). As expected, no JAK2 was detected with GST- PTP-1B WT, reflecting the transient nature of the interaction between catalytically active PTP-1B and its substrates. In selected cell systems, GH has also been shown to weakly activate JAK1 or JAK3 (27, 43). These kinases were also present in 293 cells but were not detected in materials precipitated with the PTP-1B mutant. Similarly, other components of the GH signaling cascade (GHR, STAT3, and STAT5) were also present in the cells but were not trapped by the PTP-1B D181A mutant (data not shown). Overall, these results confirm that phosphorylated JAK2 binds to PTP-1B and suggest a role for PTP-1B in GH signaling.
FIG. 1.
PTP-1B D181A binds JAK2 in hGH-stimulated cells. (A) 293LA cells were transiently transfected with GST vector (GST), GST- PTP-1B WT (GST-WT), and GST-PTP-1B D181A (GST-DA). The cells were incubated in the absence (−) or presence (+) of 1 μg of hGH/ml for 10 min. Proteins associated with glutathione-Sepharose (GST) beads were removed by centrifugation (Pulldown). The precipitated proteins were resolved by SDS-PAGE and immunoblotted with antibodies against pY1007/1008JAK2 (P-JAK2). Immunoblotting with the anti-GST antibody demonstrated that comparable amounts of GST-containing proteins were pulled down from cells transfected with GST-PTP-1B WT and GST- PTP-1B D181A. (B) Precipitated materials (Pulldown) and total cell lysates (TCL; 10 μg) were resolved by SDS-PAGE and immunoblotted (IB) with antibodies against JAK2, JAK1, and JAK3. The results shown are representative of three independent experiments.
GH-treated fibroblasts lacking PTP-1B have increased JAK2 phosphorylation.
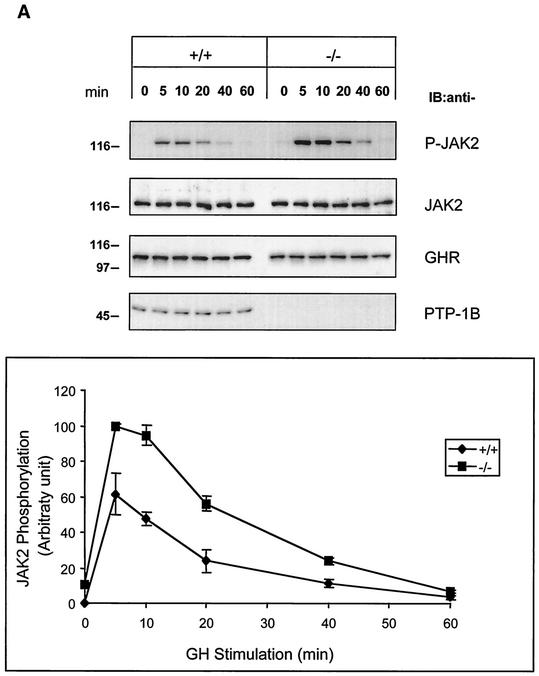
To determine whether JAK2 phosphorylation is altered in the absence of PTP-1B, we used spontaneously immortalized MEFs isolated from WT and PTP-1B KO mouse embryos. The cells were incubated with 100 ng of hGH/ml for various times, and the lysates were analyzed by SDS-PAGE and immunoblotting. As shown in Fig. 2A, JAK2 phosphorylation peaked within 5 min of the addition of hGH in both WT and KO PTP-1B MEFs. After 5 min of hGH exposure, however, JAK2 phosphorylation was 63% higher in KO than in WT MEFs and remained near maximal after 10 min. JAK2 phosphorylation returned to basal levels in both cell lines after 60 min. The abundance of JAK2 and GHR did not differ significantly at any point between WT and KO PTP-1B MEFs. These results suggest that PTP-1B attenuates the ability of GH to activate JAK2 but that JAK2 dephosphorylation is not solely dependent on PTP-1B.
FIG. 2.
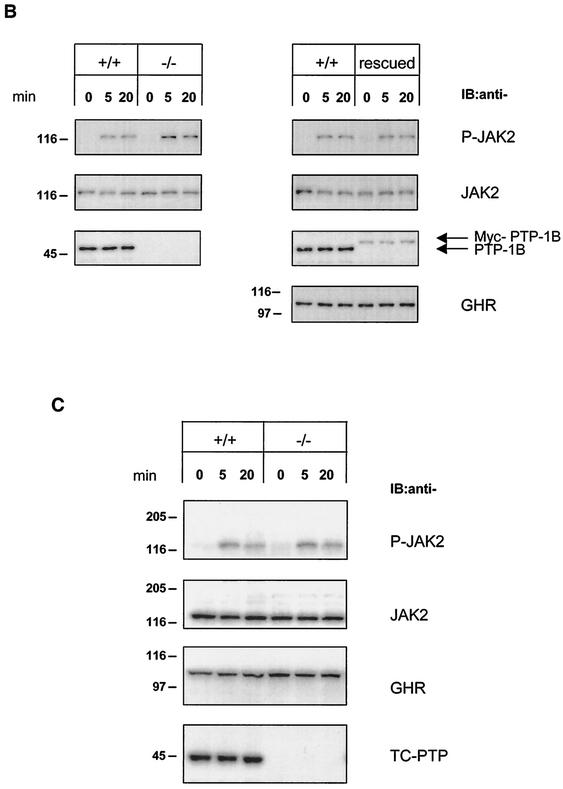
Hyperphosphorylation of JAK2 in PTP-1B-deficient fibroblasts stimulated with hGH. (A) WT (+/+) and PTP-1B KO (−/−) MEFs were stimulated with hGH (100 ng/ml) for the indicated times. Total cell lysates (20 μg) were subjected to SDS-PAGE and immunoblotted (IB) using antibodies specifically detecting total JAK2, GHR, and PTP-1B or tyrosine-phosphorylated JAK2 (P-JAK2). P-JAK2 signals were quantified by densitometry and are reported as the mean ± standard error of the mean of three independent experiments. (B) WT (+/+) and PTP-1B KO (−/−) MEFs and PTP-1B KO MEFs stably expressing Myc-tagged PTP-1B (rescued) were stimulated for 5 and 20 min with 100 ng of hGH/ml. Analysis of cell lysates by SDS-PAGE and immunoblotting were performed as described for panel A. Similar results were obtained with other rescued cell lines. (C) WT (+/+) and TC-PTP KO (−/−) MEFs were stimulated for 5 and 20 min with 100 ng of hGH/ml. Analysis of cell lysates by SDS-PAGE and immunoblotting were as described for panel A, with the additional detection of TC-PTP using a specific antibody. The results shown are representative of three independent experiments.
To confirm that GH-mediated JAK2 hyperphosphorylation was caused by the absence of PTP-1B, Myc-tagged PTP-1B was reexpressed in PTP-1B KO MEFs using retroviral infection (“rescued”). Both WT and rescued MEFs were stimulated with hGH, and the cell lysates were analyzed for JAK2 phosphorylation. Rescued cells had lower levels of PTP-1B than WT cells but similar levels of JAK2 and GHR. Nevertheless, this level of PTP-1B expression was sufficient to restore the profile of JAK2 phosphorylation in response to GH to that seen in WT cells (Fig. 2B).
To further assess the specificity of PTP-1B in GH/JAK2 signaling, we asked whether the absence of the closely related phosphatase TC-PTP would also increase GH-mediated JAK2 phosphorylation. WT and KO TC-PTP MEFs (24, 52) were treated with hGH as before. Immunoblot analysis showed that WT and KO TC-PTP MEFs expressed the same amounts of JAK2 and GHR. More importantly, after hGH treatment, the levels of JAK2 phosphorylation in KO TC-PTP MEFs were indistinguishable from those of WT TC-PTP MEFs (Fig. 2C). Overall, these results indicate that PTP-1B, but not TC-PTP, attenuates GH-mediated JAK2 phosphorylation.
GH-mediated interactions between PTP-1B and JAK2 occur in the membrane compartment.
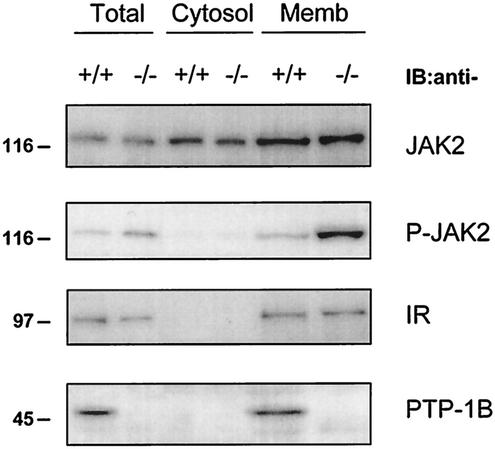
The C terminus of PTP-1B contains a 35-amino-acid hydrophobic stretch that anchors PTP-1B to the endoplasmic reticulum (ER) (15). Unlike PTP-1B, JAK2 is located predominantly in the cytosol and associates with the GHR in a ligand-dependent manner (3). A portion of JAK2 has also been reported to be associated with the membranes of the ER (32). Therefore, we sought to identify the cellular compartment where GH-induced interactions between JAK2 and PTP-1B occurred. WT and KO PTP-1B MEFs were stimulated with 100 ng of hGH/ml for 10 min. The cells were homogenized in an isotonic buffer, the postnuclear supernatant was separated by ultracentrifugation into cytosol and membrane fractions, and aliquots of each fraction (total homogenate, 10%; cytosol, 20%; membrane, 50%) were analyzed by SDS-PAGE and immunoblotting. Proper fractionation was confirmed by exclusive localization of PTP-1B and insulin receptor in the membrane compartment and predominant localization of JAK2 in the cytosol (∼70% of total JAK2) (Fig. 3).
FIG. 3.
PTP-1B dephosphorylates membrane-associated JAK2. WT (+/+) and PTP-1B KO (−/−) MEFs were stimulated with 100 ng of hGH/ml for 10 min. Cell homogenates from a single 10-cm-diameter dish (Total) were separated in cytosolic (Cytosol) and membrane (Memb) fractions. Aliquots of each fraction (total, 10%; cytosol, 20%; membrane, 50%) were analyzed by SDS-PAGE and immunoblotting using specific antibodies against pY1007/1008JAK2 (P-JAK2), JAK2, insulin receptor (IR), and PTP-1B. The IR presence was measured as a control for membrane fractionation. The results shown are representative of three independent experiments.
As shown above, GH induced a higher level of JAK2 phosphorylation in KO than in WT PTP-1B MEFs (Fig. 2). In both cell types, GH-induced phosphorylation of JAK2 was restricted to the membrane, a compartment containing only ∼30% of total JAK2 (Fig. 3). As a consequence, JAK2 hyperphosphorylation in null PTP-1B MEFs was greater for membrane JAK2 than for total JAK2. We conclude that the modulation of GH-induced JAK2 phosphorylation by PTP-1B is restricted to the membrane compartment.
Enhanced GH signaling in PTP-1B-deficient primary fibroblasts.
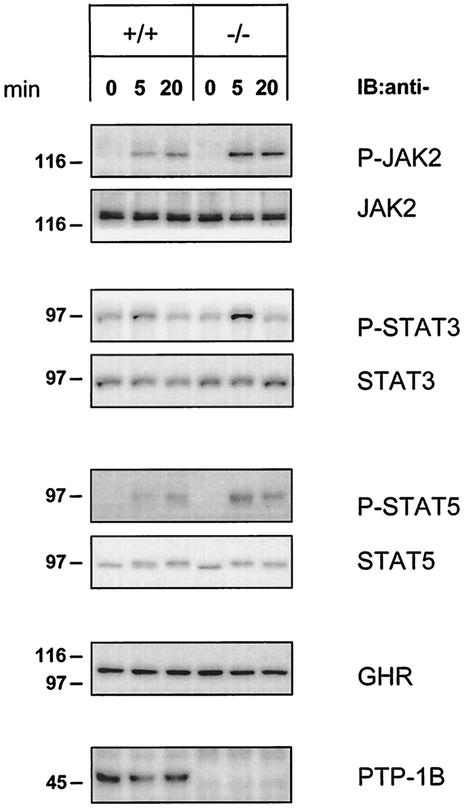
Signaling events following JAK2 phosphorylation include phosphorylation of specific tyrosine residues in the GHR and in STAT1, -3, and -5 (22, 34). After hGH treatment, increased JAK2 phosphorylation in PTP-1B KO MEFs was associated with increased tyrosine phosphorylation of GHR, STAT3, and STAT5 (not shown). However, these cells also had higher expression of STAT3 and STAT5 than WT cells, complicating the interpretation of these results.
To better answer this question, we derived primary fibroblasts from WT and PTB-1B KO mouse embryos (PMEFs). Unlike MEFs, WT and PTP-1B KO PMEFs had identical levels of STAT3 and STAT5 (Fig. 4). Primary cells were stimulated with 100 ng of hGH/ml, and the lysates were analyzed for JAK2, STAT3, and STAT5 phosphorylation. The absence of PTP-1B resulted in a significant increase in JAK2 phosphorylation during the first 20 min of hGH stimulation. JAK2 hyperphosphorylation increased tyrosine-phosphorylated STAT3 after 5 min and increased tyrosine-phosphorylated STAT5 over the entire 20-min treatment period (Fig. 4). These results indicate that, by reducing the GH-dependent phosphorylation of JAK2, PTP-1B limits the activation of downstream components of the GH signaling pathway.
FIG. 4.
JAK2, STAT3, and STAT5 are hyperphosphorylated in PTP-1B-deficient primary fibroblasts upon hGH stimulation. Passage 4 WT (+/+) and PTP-1B KO (−/−) PMEFs were stimulated for 5 and 20 min with hGH (100 ng/ml). Cell lysates (20 μg) were analyzed by SDS-PAGE and immunoblotting (IB) using specific antibodies against total JAK2, STAT3, and STAT5 or their tyrosine-phosphorylated forms (P-JAK2, P-STAT3, and P-STAT5). The levels of the GHR and PTP-1B were also determined using specific antibodies. The results shown are representative of three independent experiments.
Overexpression of PTP-1B reduces GH-mediated transcription in liver cells.
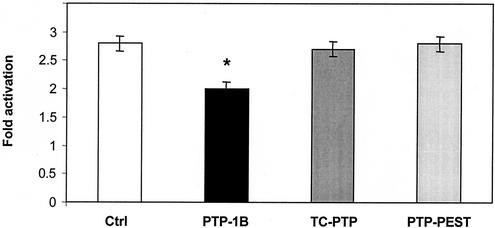
Next, we asked whether PTP-1B could modulate GH-regulated transcription. For these studies, we used the H4-II cells transiently transfected with mALS703WT, a luciferase construct under the control of the GH-dependent mouse ALS promoter. As shown in Fig. 5, GH activates ALS promoter activity 2.8-fold in this system, an effect that we showed to be completely dependent on the binding of STAT5 to a single IFN-γ-activated sequence (39). Overexpression of PTP-1B attenuated this stimulation by 30% (P < 0.05), whereas the related tyrosine phosphatases TC-PTP and PTP-PEST had no effect. These results suggest that the attenuation of GH signaling by PTP-1B is functionally significant.
FIG. 5.
Overexpression of PTP-1B reduces the ability of GH to increase ALS promoter activity in H4-II-E cells. The luciferase plasmid mALS703WT driven by the mouse ALS promoter was transfected in H4-II-E cells with either an empty expression vector (Ctrl) or expression vectors encoding PTP-1B, TC-PTP, or PTP-PEST. The plasmid pRL-TK encoding Renilla luciferase was used to correct for variation in transfection efficiency. Transfected cells were incubated in serum-free medium in the absence or presence of 100 ng of GH/ml. After 24 h, firefly luciferase activity was measured in cell extracts and corrected for Renilla luciferase activity. The n-fold stimulation (mean ± standard error of two experiments) was calculated as the ratio of luciferase activity in the presence and absence of GH. The bar marked with an asterisk differs at P < 0.05, using one-way analysis of variance followed by the Fisher protected least-significant test.
Fasted PTP-1B KO mice have decreased hepatic GH resistance.
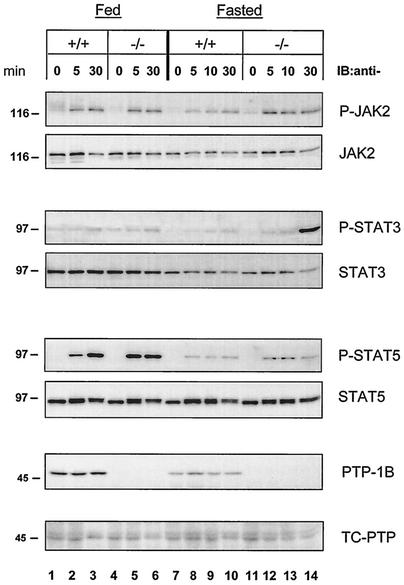
Results in fibroblasts and rat liver cells indicate that PTP-1B plays a negative role in GH signaling. To determine whether PTP-1B played a similar role in vivo, we focused on the liver, a major GH target tissue in postnatal animals (19, 35). Livers were obtained from fed WT and PTP-1B KO mice 5 or 30 min after hGH administration and analyzed by immunoblotting them with phosphospecific antibodies. As shown in Fig. 6, under fed conditions, GH administration increased the amount of phosphorylated JAK2 to similar extents in WT and KO mice at both 5 and 30 min. STAT3 phosphorylation did not change after hGH administration, whereas phosphorylation of STAT5 was slightly higher in KO than in WT livers after 5 min but similar after 30 min. These results suggest that, under conditions associated with normal GH responsiveness, PTP-1B has little impact on the ability of GH to activate signaling in the liver.
FIG. 6.
Fasting-induced GH resistance is impaired in the livers of PTP-1B KO mice. PTP-1B WT (+/+) and PTP-1B KO (−/−) male mice were injected with PBS or hGH (0.5 μg of hGH/g of body weight) when fed or after a 48-h period of fasting. At the indicated time points after hGH injection, the livers were collected and extracts were prepared. The liver extracts were analyzed by SDS-PAGE and immunoblotting (IB) using specific antibodies against total JAK2, STAT3, STAT5, or their tyrosine-phosphorylated forms (P-JAK2, P-STAT3, and P-STAT5), as well as PTP-1B and TC-PTP. The results shown are representative of three independent experiments.
Next, we examined whether the same situation prevailed during fasting when a state of GH resistance develops. In the rat, this state is characterized by a reduced ability of GH to induce tyrosine phosphorylation of JAK2, GHR, and STAT5 despite the unchanged abundance of these proteins (4). Consistent with these findings, JAK2 phosphorylation was reduced throughout the study period in the livers of WT mice fasted for 48 h (Fig. 6). For example, 30 min after GH administration, the ratio of phosphorylated to total JAK2 (pJAK2/total JAK2) in fasted livers was only 50% of that observed in fed livers (1.01 versus 2.01 arbitrary units; [Fig. 6, lane 10 versus lane 3]). Hepatic levels of PTP-1B were reduced by fasting, whereas levels of the closely related tyrosine phosphatase TC-PTP remained unchanged.
In PTP-1B KO mice, however, GH-induced JAK2 phosphorylation was as efficient in fasted as in fed livers. Using the 30-min time point for comparison, the ratios of phosphorylated to total JAK2 were similar in fasted and fed livers (2.12 versus 1.95 arbitrary units [Fig. 6, lane 14 versus lane 6]). Increased JAK2 phosphorylation in fasted PTP-1B KO mice led to improved activation of STAT5 over the first 10 min following GH administration (P-STAT5 [Fig. 6, lanes 12 and 13 versus lanes 8 and 9), and after 30 min, it led to a dramatic activation of STAT3 (P-STAT3 [lane 14 versus lane 10]). Overall, these results suggest that under conditions where GH is decreased, such as fasting, levels of PTP-1B are reduced, reflecting perhaps a lesser need for GH signal attenuation. Complete absence of PTP-1B, however, impairs the development of hepatic GH resistance.
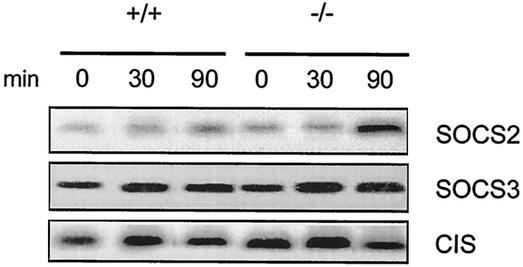
Next, we examined whether expression of SOCS was altered. These proteins have been shown to act as a rapid negative feedback in response to GH (18). Thirty minutes after GH injection, expression of CIS and SOCS3 was very modestly increased, and similarly in both WT and KO mice (Fig. 7). In contrast, SOCS2 expression was increased to a greater extent 90 min after GH administration in KO than in WT mice. This suggests that impaired GH resistance in fasted KO mice has functional consequences, such as a compensatory increase in the expression of other negative modulators of GH signaling, like SOCS2.
FIG. 7.
GH induced a higher expression of SOCS2 in fasted PTP-1B KO mouse livers. WT (+/+) and PTP-1B KO (−/−) mice fasted for 48 h, followed by hGH administration (1 μg/g of body weight). Livers were obtained from individuals before (zero time) and after (30 and 90 min) GH administration. Total RNA was isolated and analyzed by Northern blotting for the abundance of SOCS mRNA using the corresponding mouse cDNA probes. Signals detected with the various probes corresponded to an mRNA of 2.5 kb for CIS, 3.4 kb for SOCS2, and 3.2 kb for SOCS3. Each lane represents RNA from one mouse.
DISCUSSION
Genetic and biochemical evidence that PTP-1B is a critical regulator of metabolism has emerged in recent years. Null PTP-1B mice are hypersensitive to the action of insulin and are resistant to diet-induced obesity (12, 29). Moreover, mice lacking both leptin and PTP-1B are leaner than their leptin-deficient counterparts and are more sensitive to the anorexic and weight-reducing effects of exogenous leptin (10, 53). PTP-1B exerts these actions by dephosphorylating tyrosine residues involved in recruiting and activating signaling molecules (9, 50). In this paper, we demonstrate that PTP-1B also modulates the action of GH, another important metabolic hormone.
Our data indicate that PTP-1B is recruited to JAK2 in response to GH and are consistent with a model in which PTP-1B limits ligand-dependent signaling by dephosphorylating tyrosine residues located in the activation loop of JAK2. PTP-1B is obviously not the only PTP capable of acting on JAK2, as shown by similar dephosphorylation kinetics of pY1007 and -1008 on JAK2 in WT and null PTP-1B fibroblasts treated with GH. Other tyrosine phosphatases shown to be involved in JAK2 dephosphorylation in GH-treated cells include SHP-1, a PTP expressed predominantly in tissues and cells of hematopoietic origin (26), and the widely expressed SHP-2 (28, 46). In the present work, we also considered the possibility that TC-PTP, a phosphatase highly homologous to PTP-1B, could be involved. Our functional data showed that TC-PTP is not involved in JAK2 dephosphorylation in GH-treated fibroblasts, an observation consistent with the ability of TC-PTP to trap JAK1 and JAK3 but not JAK2 (42). A role for TC-PTP in GH signaling remains possible, because we recently demonstrated that its nuclear isoform dephosphorylates STAT1 and STAT3 (48). In contrast to Aoki and Matsuda (2), however, we could not identify STAT5 as a physiological target of TC-PTP (48). This finding is corroborated in the present study by the inability of overexpressed TC-PTP to reduce a GH action dependent on STAT5 activation (i.e., stimulation of the mouse ALS promoter in H4-II-E cells [39]). Finally, the transmembrane tyrosine phosphatase CD45 can dephosphorylate all members of the JAK family, but any role of CD45 in GH signaling would be limited by its near-exclusive expression in hematopoietic tissues (25). Although PTP-1B is not unique in its ability to dephosphorylate JAK2, its widespread expression suggests that it could modulate the action of GH in many target tissues.
Our data do not preclude the possibility that PTP-1B dephosphorylates other components of the GH signaling pathway, such as the GHR, STAT3, and STAT5. In this context, Aoki and Matsuda have suggested that STAT5a and STAT5b are PTP-1B substrates in prolactin-treated cells (1). Whether STAT5 is a genuine PTP-1B substrate at a physiological level remains to be determined, particularly in the context of recent studies of a cocrystal of PTP-1B and the activation segment of the insulin receptor. These studies indicate that PTP-1B has a 70-fold-greater affinity for tandem-pY than for mono-pY containing peptides (41). Moreover, the specificity of this interaction is increased when an acidic and a basic residue flank the tandem pY residues, yielding E/D-pY-pY-R/K as the preferred sequence recognized by PTP-1B (38). This motif is exactly conserved in the activation loop of JAK2 but is absent in STAT5a, STAT5b, STAT3, and GHR. Consistent with its being a physiologically important substrate, JAK2 has also been found to associate with PTP-1B in leptin and IFN-γ signaling (10, 38, 53).
The cellular locations of various tyrosine phosphatases need to be considered to understand their role in GH signaling. For example, cytosolic SHP-1 is recruited directly to JAK2 in response to GH (20), whereas SHP-2 is associated with the GHR itself (28, 46). However, because of its exclusive location on the cytosolic face of the ER, it is not immediately obvious how PTP-1B interacts with JAK2 present in GH receptor complexes. In this context, recent studies using fluorescent energy transfer microscopy have shown that PTP-1B dephosphorylates the EGFR and PDGFR tyrosine kinases on the ER membrane after receptor internalization (21). Consistent with this model, we found that most of the interactions between PTP-1B and phosphorylated JAK2 occurred in the membrane compartment, and others have found that disruption of ER function results in prolongation of JAK2 phosphorylation in GH-treated cells (14). It will be important to determine whether this model holds in the case of the GH and leptin receptors, which do not have intrinsic kinase activity but instead rely on the recruitment of cytosolic JAK2 for signaling.
Decreased GH stimulation of ALS promoter activity in H4-II-E cells overexpressing PTP-1B suggested that PTP-1B could be an important modulator of GH action in the liver. Our in vivo studies of the liver yielded two important findings. First, hepatic expression of PTP-1B was reduced by chronic fasting, whereas expression of the related TC-PTP was unchanged. This reduction in PTP-1B may be the consequence of reduced needs for negative regulation when concentrations of metabolic hormones, such as GH and insulin, in the plasma are reduced. In this context, it has recently been shown that overconsumption of fructose leads to increased hepatic expression of PTP-1B in hamsters (47). Overall, these results suggest that PTP-1B is unique among tyrosine phosphatases in its ability to respond to changes in metabolic stress. Further studies are needed to determine whether this regulation extends to other important metabolic tissues and to identify the basis for altered PTP-1B expression.
Second, our results indicate that, in the context of acute GH actions, the functional importance of PTP-1B varies according to nutritional status. Under fed conditions, absence of PTP-1B does not result in consistent changes in the GH-dependent activation of JAK2, STAT3, and STAT5. During fasting, however, null PTP-1B livers display significantly higher activation of these molecules than WT livers, indicating that the development of GH resistance normally associated with fasting is impaired (4, 49). In the short term, this failure resulted in increased induction of SOCS2 but not of the related SOCS3 or CIS. Greater induction of SOCS2 is interesting, given that it appears to be particularly important in the attenuation of GH action in the liver and in the whole animal (17, 23, 37). Whether the absence of PTP-1B would rescue chronic anabolic actions of GH during states normally associated with hepatic GH resistance, such as inflammation, burn injury, and aging (31, 36, 51), remains to be studied.
Acknowledgments
We thank A. F. Parlow and the National Hormone and Pituitary Program for hGH, Suhad Ali for 293LA cells, W. Baumbach for GHR antibodies, Yosé ChÂteauvert-Lavoie for excellent technical help, and Charity McNamara for performing the transfection assay.
F.G. is the recipient of a Human Frontier Science Program postdoctoral fellowship. N.D. is the recipient of a Canadian Institutes of Health Research doctoral award. This work was supported by a Cancer Research Society Operating Grant to M.L.T. and NIH grant 2R01 DK051624 to Y.R.B.
F. Gu and N. Dubé are joint first authors.
REFERENCES
- 1.Aoki, N., and T. Matsuda. 2000. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J. Biol. Chem. 275:39718-39726. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, N., and T. Matsuda. 2002. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol. Endocrinol. 16:58-69. [DOI] [PubMed] [Google Scholar]
- 3.Argetsinger, L. S., G. S. Campbell, X. Yang, B. A. Witthuhn, O. Silvennoinen, J. N. Ihle, and C. Carter-Su. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244. [DOI] [PubMed] [Google Scholar]
- 4.Beauloye, V., B. Willems, V. de Coninck, S. J. Frank, M. Edery, and J. P. Thissen. 2002. Impairment of liver GH receptor signaling by fasting. Endocrinology 143:792-800. [DOI] [PubMed] [Google Scholar]
- 5.Boisclair, Y. R., J. Wang, J. Shi, K. R. Hurst, and G. T. Ooi. 2000. Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1β on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J. Biol. Chem. 275:3841-3847. [DOI] [PubMed] [Google Scholar]
- 6.Butler, A. A., and D. Le Roith. 2001. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu. Rev. Physiol. 63:141-164. [DOI] [PubMed] [Google Scholar]
- 7.Charest, A., J. Wagner, S. H. Shen, and M. L. Tremblay. 1995. Murine protein tyrosine phosphatase-PEST, a stable cytosolic protein tyrosine phosphatase. Biochem. J. 308:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, A., G. S. Bal, B. P. Kennedy, and M. L. Tremblay. 2001. Attenuation of adhesion-dependent signaling and cell spreading in transformed fibroblasts lacking protein tyrosine phosphatase-1B. J. Biol. Chem. 276:25848-25855. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, A., N. Dube, F. Gu, and M. L. Tremblay. 2002. Coordinated action of protein tyrosine phosphatases in insulin signal transduction Eur. J. Biochem. 269:1050-1059. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, A., N. Uetani, P. D. Simoncic, V. P. Chaubey, A. Lee-Loy, C. J. McGlade, B. P. Kennedy, and M. L. Tremblay. 2002. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2:497-503. [DOI] [PubMed] [Google Scholar]
- 11.Efstratiadis, A. 1998. Genetics of mouse growth. Int. J. Dev. Biol. 42:955-976. [PubMed] [Google Scholar]
- 12.Elchebly, M., P. Payette, E. Michaliszyn, W. Cromlish, S. Collins, A. L. Loy, D. Normandin, A. Cheng, J. Himms-Hagen, C. C. Chan, C. Ramachandran, M. J. Gresser, M. L. Tremblay, and B. P. Kennedy. 1999. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544-1548. [DOI] [PubMed] [Google Scholar]
- 13.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores-Morales, A., L. Fernandez, E. Rico-Bautista, A. Umana, C. Negrin, J. G. Zhang, and G. Norstedt. 2001. Endoplasmic reticulum stress prolongs GH-induced Janus kinase (JAK2)/signal transducer and activator of transcription (STAT5) signaling pathway. Mol. Endocrinol. 15:1471-1483. [DOI] [PubMed] [Google Scholar]
- 15.Frangioni, J. V., P. H. Beahm, V. Shifrin, C. A. Jost, and B. G. Neel. 1992. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell 68:545-560. [DOI] [PubMed] [Google Scholar]
- 16.Frank, S. J. 2001. Growth hormone signalling and its regulation: preventing too much of a good thing. Growth Horm. IGF Res. 11:201-212. [DOI] [PubMed] [Google Scholar]
- 17.Greenhalgh, C. J., P. Bertolino, S. L. Asa, D. Metcalf, J. E. Corbin, T. E. Adams, H. W. Davey, N. A. Nicola, D. J. Hilton, and W. S. Alexander. 2002. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b). Mol. Endocrinol. 16:1394-1406. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh, C. J., and D. J. Hilton. 2001. Negative regulation of cytokine signaling. J. Leukoc. Biol. 70:348-356. [PubMed] [Google Scholar]
- 19.Gronowski, A. M., and P. Rotwein. 1994. Rapid changes in nuclear protein tyrosine phosphorylation after growth hormone treatment in vivo. Identification of phosphorylated mitogen-activated protein kinase and STAT91. J. Biol. Chem. 269:7874-7878. [PubMed] [Google Scholar]
- 20.Hackett, R. H., Y. D. Wang, S. Sweitzer, G. Feldman, W. I. Wood, and A. C. Larner. 1997. Mapping of a cytoplasmic domain of the human growth hormone receptor that regulates rates of inactivation of Jak2 and Stat proteins. J. Biol. Chem. 272:11128-11132. [DOI] [PubMed] [Google Scholar]
- 21.Haj, F. G., P. J. Verveer, A. Squire, B. G. Neel, and P. I. Bastiaens. 2002. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science 295:1708-1711. [DOI] [PubMed] [Google Scholar]
- 22.Herrington, J., and C. Carter-Su. 2001. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol. Metab. 12:252-257. [DOI] [PubMed] [Google Scholar]
- 23.Horvat, S., and J. F. Medrano. 2001. Lack of Socs2 expression causes the high-growth phenotype in mice. Genomics 72:209-212. [DOI] [PubMed] [Google Scholar]
- 24.Ibarra-Sanchez, M. J., J. Wagner, M. T. Ong, C. Lampron, and M. L. Tremblay. 2001. Murine embryonic fibroblasts lacking TC-PTP display delayed G1 phase through defective NF-κB activation. Oncogene 20:4728-4739. [DOI] [PubMed] [Google Scholar]
- 25.Irie-Sasaki, J., T. Sasaki, W. Matsumoto, A. Opavsky, M. Cheng, G. Welstead, E. Griffiths, C. Krawczyk, C. D. Richardson, K. Aitken, N. Iscove, G. Koretzky, P. Johnson, P. Liu, D. M. Rothstein, and J. M. Penninger. 2001. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature 409:349-354. [DOI] [PubMed] [Google Scholar]
- 26.Jiao, H., K. Berrada, W. Yang, M. Tabrizi, L. C. Platanias, and T. Yi. 1996. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol. Cell. Biol. 16:6985-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston, J. A., M. Kawamura, R. A. Kirken, Y. Q. Chen, T. B. Blake, K. Shibuya, J. R. Ortaldo, D. W. McVicar, and J. J. O'Shea. 1994. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature 370:151-153. [DOI] [PubMed] [Google Scholar]
- 28.Kim, S. O., J. Jiang, W. Yi, G. S. Feng, and S. J. Frank. 1998. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J. Biol. Chem. 273:2344-2354. [DOI] [PubMed] [Google Scholar]
- 29.Klaman, L. D., O. Boss, O. D. Peroni, J. K. Kim, J. L. Martino, J. M. Zabolotny, N. Moghal, M. Lubkin, Y. B. Kim, A. H. Sharpe, A. Stricker-Krongrad, G. I. Shulman, B. G. Neel, and B. B. Kahn. 2000. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20:5479-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopchick, J. J., L. L. Bellush, and K. T. Coschigano. 1999. Transgenic models of growth hormone action. Annu. Rev. Nutr. 19:437-461. [DOI] [PubMed] [Google Scholar]
- 31.Lang, C. H., X. Liu, G. J. Nystrom, and R. A. Frost. 2000. Acute response of IGF-I and IGF binding proteins induced by thermal injury. Am. J. Physiol. Endocrinol. Metab. 278:E1087-E1096. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie, C., E. Chevet, L. Roy, N. K. Tonks, A. Fazel, B. I. Posner, J. Paiement, and J. J. Bergeron. 2000. Tyrosine phosphorylation of p97 regulates transitional endoplasmic reticulum assembly in vitro. Proc. Natl. Acad. Sci. USA 97:13637-13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lebrun, J. J., S. Ali, A. Ullrich, and P. A. Kelly. 1995. Proline-rich sequence-mediated Jak2 association to the prolactin receptor is required but not sufficient for signal transduction. J. Biol. Chem. 270:10664-10670. [DOI] [PubMed] [Google Scholar]
- 34.Le Roith, D., C. Bondy, S. Yakar, J. L. Liu, and A. Butler. 2001. The somatomedin hypothesis: 2001. Endocr. Rev. 22:53-74. [DOI] [PubMed] [Google Scholar]
- 35.Lupu, F., J. D. Terwilliger, K. Lee, G. V. Segre, and A. Efstratiadis. 2001. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev. Biol. 229:141-162. [DOI] [PubMed] [Google Scholar]
- 36.Mao, Y., P. R. Ling, T. P. Fitzgibbons, K. C. McCowen, G. P. Frick, B. R. Bistrian, and R. J. Smith. 1999. Endotoxin-induced inhibition of growth hormone receptor signaling in rat liver in vivo. Endocrinology 140:5505-5515. [DOI] [PubMed] [Google Scholar]
- 37.Metcalf, D., C. J. Greenhalgh, E. Viney, T. A. Willson, R. Starr, N. A. Nicola, D. J. Hilton, and W. S. Alexander. 2000. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature 405:1069-1073. [DOI] [PubMed] [Google Scholar]
- 38.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276:47771-47774. [DOI] [PubMed] [Google Scholar]
- 39.Ooi, G. T., K. R. Hurst, M. N. Poy, M. M. Rechler, and Y. R. Boisclair. 1998. Binding of STAT5a and STAT5b to a single element resembling a gamma-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol. Endocrinol. 12:675-687. [DOI] [PubMed] [Google Scholar]
- 40.Pitot, H. C., C. Peraino, P. A. Morse, and V. A. Potter. 1964. Hepatoma in tissue culture compared with adapting liver in vitro. Natl. Cancer Inst. Monogr. 13:229-242. [PubMed] [Google Scholar]
- 41.Salmeen, A., J. N. Andersen, M. P. Myers, N. K. Tonks, and D. Barford. 2000. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol. Cell 6:1401-1412. [DOI] [PubMed] [Google Scholar]
- 42.Simoncic, P. D., A. Lee-Loy, D. L. Barber, M. L. Tremblay, and C. J. McGlade. 2002. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr. Biol. 12:446-453. [DOI] [PubMed] [Google Scholar]
- 43.Smit, L. S., D. J. Meyer, N. Billestrup, G. Norstedt, J. Schwartz, and C. Carter-Su. 1996. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol. Endocrinol. 10:519-533. [DOI] [PubMed] [Google Scholar]
- 44.Sonksen, P. H., F. Salomon, and R. Cuneo. 1991. Metabolic effects of hypopituitarism and acromegaly. Horm. Res. 36:27-31. [DOI] [PubMed] [Google Scholar]
- 45.Starr, R., T. A. Willson, E. M. Viney, L. J. Murray, J. R. Rayner, B. J. Jenkins, T. J. Gonda, W. S. Alexander, D. Metcalf, N. A. Nicola, and D. J. Hilton. 1997. A family of cytokine-inducible inhibitors of signalling. Nature 387:917-921. [DOI] [PubMed] [Google Scholar]
- 46.Stofega, M. R., J. Herrington, N. Billestrup, and C. Carter-Su. 2000. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol. Endocrinol. 14:1338-1350. [DOI] [PubMed] [Google Scholar]
- 47.Taghibiglou, C., F. Rashid-Kolvear, S. C. Van Iderstine, H. Le-Tien, I. G. Fantus, G. F. Lewis, and K. Adeli. 2002. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J. Biol. Chem. 277:793-803. [DOI] [PubMed] [Google Scholar]
- 48.ten Hoeve, J., M. de Jesus Ibarra-Sanchez, Y. Fu, W. Zhu, M. Tremblay, M. David, and K. Shuai. 2002. Identification of a nuclear Sstat1 protein tyrosine phosphatase. Mol. Cell. Biol. 22:5662-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thissen, J. P., L. E. Underwood, and J. M. Ketelslegers. 1999. Regulation of insulin-like growth factor-I in starvation and injury. Nutr. Rev. 57:167-176. [DOI] [PubMed] [Google Scholar]
- 50.Tonks, N. K., and B. G. Neel. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13:182-195. [DOI] [PubMed] [Google Scholar]
- 51.Xu, X., S. A. Bennett, R. L. Ingram, and W. E. Sonntag. 1995. Decreases in growth hormone receptor signal transduction contribute to the decline in insulin-like growth factor I gene expression with age. Endocrinology 136:4551-4557. [DOI] [PubMed] [Google Scholar]
- 52.You-Ten, K. E., E. S. Muise, A. Itie, E. Michaliszyn, J. Wagner, S. Jothy, W. S. Lapp, and M. L. Tremblay. 1997. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 186:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zabolotny, J. M., K. K. Bence-Hanulec, A. Stricker-Krongrad, F. Haj, Y. Wang, Y. Minokoshi, Y. B. Kim, J. K. Elmquist, L. A. Tartaglia, B. B. Kahn, and B. G. Neel. 2002. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2:489-495. [DOI] [PubMed] [Google Scholar]