Abstract
In hereditary hemochromatosis (HH), intestinal absorption of dietary iron is increased, leading to excessive iron accumulation in tissues and resultant organ damage. The HFE protein, which is defective in HH, normally is expressed in crypt enterocytes of the duodenum where it has a unique, predominantly intracellular localization. In placenta, the HFE protein colocalizes with and forms a stable association with the transferrin receptor (TfR), providing a link between the HFE protein and iron transport. In the present study, we examined the relationship of the HFE protein to the TfR in enterocytes of the human duodenum and measured the uptake of transferrin-bound iron and ionic iron by isolated crypt and villus enterocytes. Immunocytochemistry showed that the HFE protein and TfR both are expressed in the crypt enterocytes. Western blots showed that, as was the case in human placenta, the HFE protein in crypt enterocytes is physically associated with the TfR and with β2-microglobulin. The crypt cell fraction exhibited dramatically higher transferrin-bound iron uptake than villus cells. On the other hand, the villus cells showed 2–3 times higher uptake of ionic iron than crypt cells. We propose that the HFE protein modulates the uptake of transferrin-bound iron from plasma by crypt enterocytes and participates in the mechanism by which the crypt enterocytes sense the level of body iron stores. Impairment of this function caused by HFE gene mutations in HH could provide a paradoxical signal in crypt enterocytes that programs the differentiating enterocytes to absorb more dietary iron when they mature into villus enterocytes.
Keywords: β2-microglobulin, hemochromatosis, intestine, iron
Hereditary hemochromatosis (HH) is a common autosomal recessive disorder characterized by increased iron absorption that leads to iron overload of parenchymal cells in many organs (1–4). Clinical consequences of iron accumulation include cirrhosis of the liver, hepatocellular carcinoma, diabetes, heart failure, arthritis, and hypogonadism. Feder et al. (5) cloned a candidate gene (HFE) for HH. In their study, 83% of 178 patients with clinically diagnosed HH were found to be homozygous for the same missense mutation (C282Y) in the HFE gene. The high frequency of the C282Y mutation in patients with HH has been confirmed by other investigators (6–13). Confirmatory evidence that a defective HFE gene causes HH was provided by recent observations in mice with a targeted disruption of the HFE gene (14). By age 10 weeks, the HFE gene knockout mice had elevated transferrin saturations and increased iron storage in hepatocytes, confirming the prediction that a defect in the murine HFE gene would result in excessive absorption of dietary iron.
The human HFE protein predicted from the cDNA sequence is a major histocompatibility complex class I-like integral membrane protein (5). The C282Y mutation was predicted to disrupt a critical disulfide bond in the α3 domain of the HFE protein and abrogate binding of the mutant HFE protein to β2-microglobulin (β2M) (5). Feder et al. (15) and Waheed et al. (16) showed that the C282Y mutant HFE protein does not associate with β2M in human embryonic kidney cells (293 cells) and COS-7 cells transfected with the mutant cDNA. Waheed et al. (16) also demonstrated that much of the C282Y mutant protein remains in high molecular weight aggregates, fails to undergo late Golgi processing, and undergoes accelerated degradation. Although these studies confirmed predictions of the effects of the C282Y mutation on HFE association with β2M and its transport to the cell surface, they did not clarify the link between the HFE protein and iron absorption.
Our previous immunohistochemical studies demonstrated the immunolocalization of HFE protein in gastrointestinal tissues (17) and also showed that HFE protein is expressed in the placenta (18). Here the HFE protein is localized on the apical surface of the syncytiotrophoblast cells and is physically associated with β2M and the transferrin receptor (TfR), which mediates transport of transferrin-bound iron to the fetus via TfR-mediated endocytosis. Independently, Feder et al. (19), Lebrón et al. (20), and Gross et al. (21) reported that association of the expressed recombinant HFE protein with the TfR in cultured cells (19, 21) and in vitro (20) reduces its affinity for its ligand, diferric transferrin, and suggested that the normal HFE protein may play a role in down-regulating TfR-mediated iron uptake. Although this interpretation of the role of the normal HFE could explain how loss of a functional HFE gene could lead to increased uptake of transferrin-bound iron in parenchymal tissues in HH, it fails to explain how such mutations lead to dysregulation of dietary iron absorption by intestinal villus enterocytes in HH patients.
Our previous studies using immunohistochemistry showed that crypt enterocytes of duodenum express the HFE protein (17). In the present study, we demonstrate that the HFE protein in crypt enterocytes is physically associated with the TfR. The cellular colocalization and association of these proteins in the crypt enterocytes leads us to propose that the HFE protein modulates the transport of transferrin-bound iron from the circulation into the crypt enterocytes. This interaction would allow the normal HFE protein to participate in the mechanism by which crypt cells sense the level of body iron stores and influence the iron level in crypt cells, which, in turn, regulates the developmental program that determines the amount of dietary iron they will absorb when they mature into villus enterocytes.
EXPERIMENTAL PROCEDURES
Reagents.
The production and characterization of the HFE-CT16 antibody raised against a polypeptide of 16 C-terminal amino acids based on the cDNA of the HFE protein have been described (17). By using a corresponding peptide-Affigel 10 resin, peptide-specific IgG was affinity-purified and stored in 50% glycerol at −20°C. Rabbit anti-human β2M antibody was purchased from Sigma. Monoclonal anti-human TfR antibody was a product of Zymed. Human apotransferrin and nitrilotriacetic acid (NTA) were purchased from Sigma, and 59FeSO4 was purchased from Amersham Pharmacia. Apotransferrin was labeled with 59Fe in the presence of NTA as described (22).
Preparation of Duodenum Specimens and Isolation of Intestinal Cells.
Duodenum specimens were obtained during surgical operations for pancreatic carcinoma. They were collected after informed consent, and the research was carried out according to the provisions of the Declaration of Helsinki. Some specimens were fixed in Carnoy’s fluid and embedded in paraffin as described (17). One specimen was cleaned with saline, frozen in liquid nitrogen, and stored at −80°C before use for the Western blots, immunoprecipitation, and functional studies. Different cell fractions of duodenal mucosa were obtained by using a differential scraping technique as described (23). Using the short edge of a microscope slide, the mucosa from a thawed duodenum sample was gently scraped to remove cells in fraction 1 containing predominantly villus cells. The scraping process was repeated with increasing degrees of pressure for fractions 2 and 3. The final scraping with the greatest pressure yielded fraction 4, containing a majority of the crypt cells. The scrapings were quickly transferred to ice-cold PBS. The cells were recovered after centrifugation at 15,000 × g for 10 min at 4°C. The cell pellets were resuspended in PBS for further experiments.
Association of the HFE Protein with TfR and β2M.
Fractions containing enriched villus (fraction 1) or crypt cells (fraction 4) containing 500 μg of protein were lysed by sonication in 20 mM Na-phosphate buffer, pH 7.0, containing 1% Nonidet P-40 and protease inhibitors. The immunoprecipitation of the HFE protein complex was carried out by using the HFE-CT16 antibody as described (17, 18). The immunocomplex was recovered and analyzed by SDS/PAGE. The polypeptides associated with the HFE protein were visualized by using Western blot.
SDS/PAGE and Western Blot Analysis.
Samples containing 20 μg of protein were analyzed by SDS/PAGE under reducing conditions according to Laemmli (24). The polypeptides were electrophoretically transferred to Immobilon-P membranes (Millipore). After transblotting, the polypeptides were immunostained by using HFE-CT16 antibody, polyclonal rabbit anti-human β2M antibody, or monoclonal mouse anti-human TfR antibody followed by incubation with peroxidase-conjugated goat anti-rabbit IgG (Sigma) or peroxidase-conjugated sheep anti-mouse IgG (Sigma). The peroxidase activity was visualized by using a chemiluminescent substrate.
Immunocytochemistry.
The HFE protein and TfR were immunostained by the biotin-streptavidin complex method, using the following steps: (i) pretreatment of the sections with undiluted cow colostral whey for 40 min and rinsing in PBS, (ii) incubation for 1 h with the primary antibody (5 μg/microscope slide) in 1% BSA in PBS, (iii) incubation with cow colostral whey for 40 min and rinsing in PBS, (iv) incubation for 1 h with biotinylated swine anti-rabbit IgG (Dakopatts, Glostrup, Denmark) or rabbit anti-mouse IgG (Dakopatts) diluted 1:300 in 1% BSA-PBS, (v) incubation with cow colostral whey for 5 min and rinsing in PBS, (vi) incubation for 30 min with peroxidase-conjugated streptavidin (Dakopatts) diluted 1:500 in PBS, and (vii) incubation for 2 min in a solution containing 9 mg of 3,3′-diaminobenzidine tetrahydrochloride (Fluka) in 15 ml of PBS +10 μl of 30% H2O2. The sections were washed three times for 10 min in PBS after incubation steps ii, iv and vi. All of the incubations and washings were carried out at room temperature, and the sections finally were mounted in Permount (Fisher Scientific).
The double-immunostaining of resuspended cells for the HFE protein and TfR was performed by using an immunofluorescence technique. Enriched crypt cells from fraction 4 were spread onto the microscope slides and fixed in 4% paraformaldehyde in PBS for 20 min. Saponin (0.05%) was used to permeabilize the cells. The steps in the double-immunostaining process for the cells and tissue sections were essentially the same as described by Waheed et al. (16) and Saarnio et al. (25).
Uptake of 59Fe-NTA and 59Fe-Transferrin by Isolated Cells.
The uptake of 59Fe-NTA and 59Fe-transferrin by isolated cells of each fraction was carried out at 37°C for 1 h in a final volume of 0.5 ml of PBS containing 1 mg/ml of BSA and 200 μM of 59Fe-NTA or 59Fe-transferrin. Nonspecific binding was measured in a parallel experiment with 100-fold excess of nonradioactive Fe-NTA or Fe-transferrin. After incubation, the cells were removed by centrifugation and washed twice with 1 ml of cold PBS. The cell-associated radioactivity was measured, and the results were expressed as cpm bound/mg cell protein.
RESULTS
Colocalization of the HFE Protein and TfR in Human Duodenum.
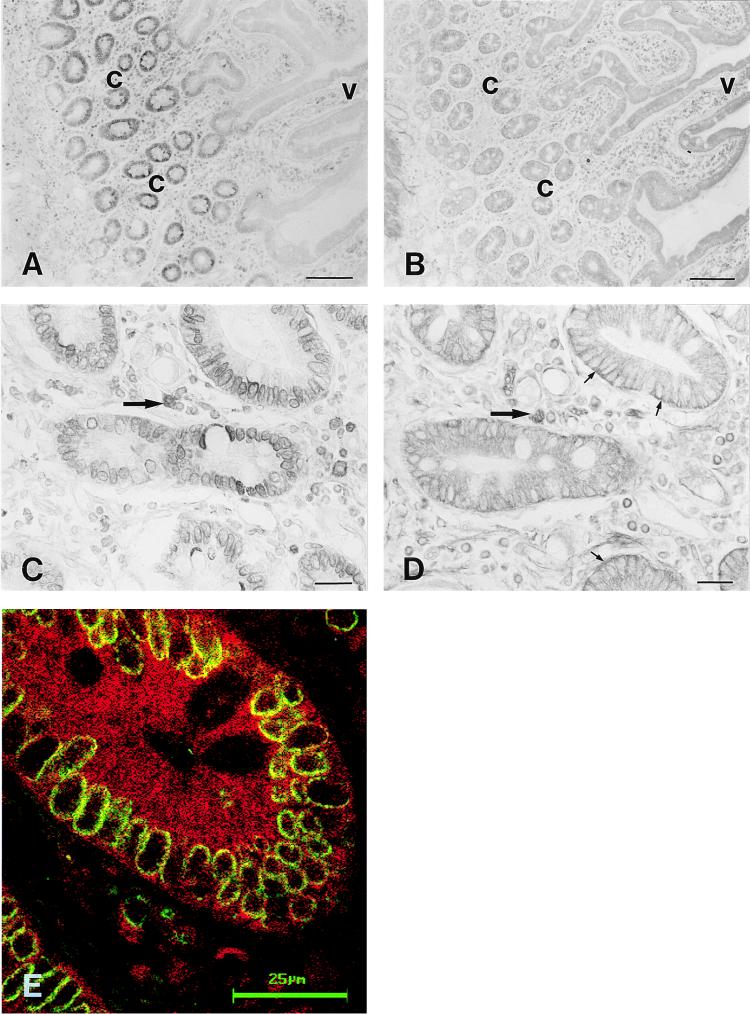
Two different immunostaining techniques were used to study the cellular distribution of the HFE protein and TfR in human duodenum: (i) both proteins were localized in serial tissue sections by using an immunoperoxidase method, and (ii) both proteins were immunostained on the same section by using a double immunofluorescence method. The low-magnification views of the HFE and TfR immunostainings demonstrate that the HFE protein was predominantly confined to the crypt region (Fig. 1A). The TfR showed a more widespread distribution pattern along the crypt-villus axis (Fig. 1B). Both proteins were coexpressed in the same crypt enterocytes, although some differences were observed in the subcellular distribution. In the crypts, the HFE protein showed a prominent, perinuclear immunoreaction (Fig. 1 C and E), whereas the TfR showed both intracellular and basolateral plasma membrane-associated signals (Fig. 1 D and E). In Fig. 1E, the yellow color indicates colocalization of HFE protein (green fluorescence) and TfR (red fluorescence). Colocalization of the signals in the crypt cells appears to be predominantly intracellular. In the villi, the HFE protein was barely seen, whereas the TfR showed more distinct intracellular immunoreactivity. In addition to the staining seen in epithelial cells, numerous leukocytes staining for HFE protein and TfR were seen in the cellular lamina propria (Fig. 1 C and D).
Figure 1.
Immunohistochemistry of HFE protein and TfR in human duodenum. Immunoperoxidase staining of the HFE protein shows that the most intense signal is confined to the crypt enterocytes (A and C). The TfR shows wider distribution along the crypt-villus axis (B), but heavier staining at the basolateral surface of the crypt cells (D). Double immunostaining of the HFE protein and TfR demonstrates that both proteins are expressed in the same crypt enterocytes (E). The HFE protein shows a strong perinuclear staining pattern (C and E), whereas the TfR shows more diffuse intracellular as well as basolateral membrane-associated immunoreactions (small arrows in D). Leukocytes in the cellular lamina propria express both proteins (large arrows in C and D). The green and red colors in E indicate the HFE protein and TfR immunoreactions, respectively. Colocalization of HFE and TfR proteins is indicated by the yellow color. c, crypt region; v, villus. (Bars: A and B = 100 μm; C and D = 20 μm; and E = 25 μm.)
Expression of the HFE Protein and TfR in Isolated Villus and Crypt Cells of Duodenal Mucosa.
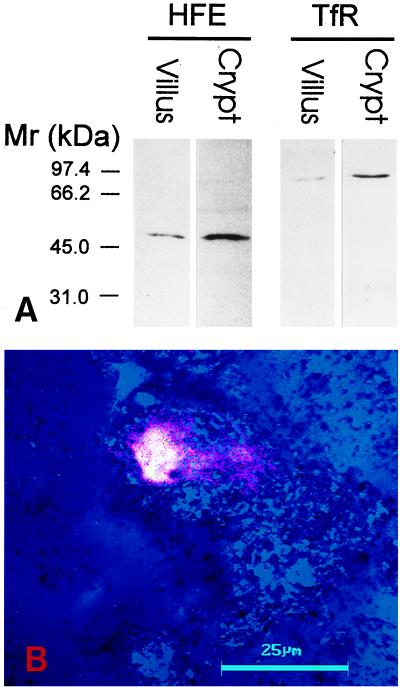
Four different fractions of mucosal cells were obtained from a duodenum sample by using a scraping method. Fractions 1 and 4, comprising primarily villus and crypt cells, respectively, first were analyzed for the expression of the HFE protein and TfR. Western blots of these fractions are shown in Fig. 2A. A single band with the expected Mr of the HFE protein (48 kDa) was detected by the HFE-CT16 antibody in both fractions. However, the signal for HFE protein was much more intense in the crypt cells. The signal for the TfR polypeptide was also greater in the crypt cell fraction. The isolated crypt cell-enriched cell fraction was also double-immunostained for the HFE protein and TfR. Fig. 2B shows a single crypt cell where the overlapping fluorescence for HFE and TfR produces the strong whitish-yellow color indicating coexpression of both proteins in the same subcellular localization.
Figure 2.
Expression of HFE protein and TfR in crypt and villus cells of human duodenum. Aliquots of cell homogenates from fraction 1 (villus cells) and fraction 4 (crypt cells) containing 20 μg of protein were analyzed by SDS/PAGE under reducing conditions followed by immunostaining using the anti-HFE-CT16 or anti-TfR antibodies (A). Both HFE protein and TfR show more prominent signals in the crypt cells. (B) A confocal laser scanning microscopy image of the HFE protein and TfR in a crypt cell. Blue color (reflection image) demonstrates the location of two cells. The green color for HFE and red color for TfR produce a strong whitish yellow color reaction where the immunostainings colocalize. (Bar = 25 μm.)
Association of the HFE Protein with the TfR and β2M.
The association of the HFE protein with β2M and TfR recently was demonstrated in placental membranes (17). As recently pointed out by Andrews and Levy (26), an important question is whether a similar association occurs in duodenum where dietary iron absorption is regulated. To address this question, the proteins from fraction 1 and 4 containing enriched villus and crypt cells, respectively, first were subjected to immunoprecipitation using HFE-CT16 antibody. Then, the immunocomplexes were analyzed by Western blot using antibodies against TfR and β2M. Fig. 3 demonstrates that the HFE immunoprecipitate from the crypt cell fraction contained coimmunoprecipitated TfR and β2M. The HFE protein is less abundant in the villus cells (Fig. 2) and the amounts of coimmunoprecipitated TfR and β2M from the fraction containing predominantly villus cells were correspondingly lower.
Figure 3.
Western blot of TfR and β2M coimmunoprecipitated with HFE from lysates of crypt and villus cell fractions from human duodenum. The TfR and β2M in immunocomplexes isolated by immunoprecipitation with the anti-human HFE-CT16 antibody were identified on Western blot by a mixture of anti-TfR and anti-human β2M antibodies.
Uptake of 59Fe-Transferrin and 59Fe-NTA by Mucosal Cell Fractions.
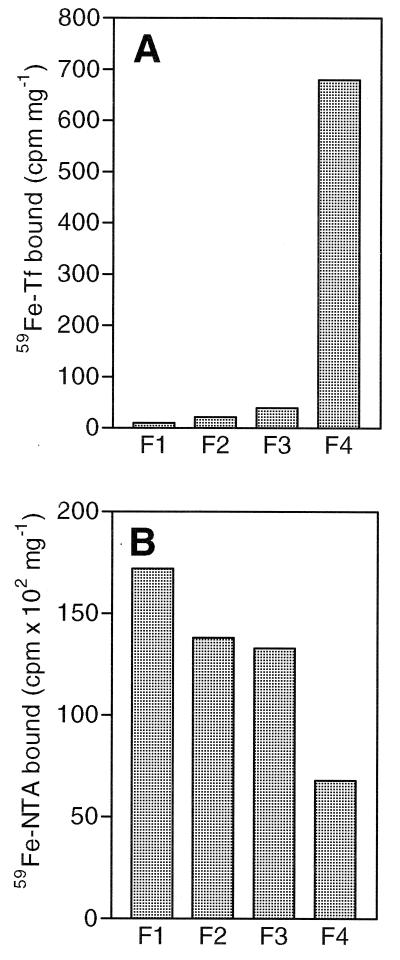
The four fractions of the duodenal cells were analyzed for uptake of 59Fe-transferrin and 59Fe-NTA and the results are shown in Fig. 4. The uptake of transferrin-bound iron was found to be very low in the first three fractions (Fig. 4A) and dramatically higher in fraction 4 containing crypt cells. The uptake by this fraction was 68 times higher than that of the most luminal (villus) fraction (fraction 1). In contrast, the uptake of 59Fe-NTA, which presumably is mediated by the recently identified divalent metal transporter (DMT1)‖, was highest in the first fraction containing mainly villus cells, and slightly lower in the second and third fractions (Fig. 3B). In fraction 4 (crypt cell), the uptake of 59Fe-NTA was 50–70% lower than that seen by the other three fractions.
Figure 4.
Uptake of transferrin-bound iron and ionic iron by fractionated mucosal cells. (A) The uptake of transferrin-bound iron is low in the first three fractions (F1–F3) and is much higher in the fraction most greatly enriched for crypt cells (F4). (B) Uptake of ionic iron (59Fe-NTA) is higher in the fractions having the greatest enrichment for villus cells (F1–F3).
DISCUSSION
The absorption of dietary iron is a tightly regulated process, occurring mainly in the proximal small intestine. Under normal conditions, the amount of absorbed iron is closely linked to the body iron needs (27–29). Iron absorption is increased when body iron stores are low and tissue iron requirements are high. Conversely, iron absorption is depressed if body iron stores are increased. There is considerable evidence that this regulation is defective in HH (27, 30), but the precise mechanism of the mucosal abnormality in this disorder is not known. Physiological studies by McLaren et al. (31) suggested that the increased iron absorption in HH may be mediated primarily by an increase in the rate constant for transfer of mucosal iron to the plasma. Our previous studies showed that the HFE protein, which is defective in HH, is expressed in the epithelial cells of the gastrointestinal tract (17). The unique localization in the crypt enterocytes of the duodenum suggested that the HFE protein may participate in the regulation of intestinal iron absorption. The studies reported here demonstrate that the HFE protein colocalizes with and is physically associated with the TfR in the crypt enterocytes.
Our findings that crypt cells take up much more transferrin-bound iron than villus cells, whereas the villus cells show higher uptake of ionic iron, are in agreement with previous studies indicating differences in the transport of iron along the crypt-villus axis. For example, Conrad and Crosby (32) showed by autoradiography that the epithelial cells of the mid to upper villus are able to absorb iron from the diet, whereas crypt enterocytes take up iron from body iron stores that is delivered to them by the circulation. Oates et al. (33) showed that crypt cells not only showed greater uptake of transferrin-bound iron, but that this uptake was regulated by dietary iron consumption in that greater uptake of transferrin-bound iron was seen when dietary loading raised the serum transferrin saturation. Presumably, this uptake of transferrin-bound iron leads to a regulatory response that down-regulates further absorption of dietary iron.
A key question in understanding HH is how this transferrin-bound iron uptake by crypt cells regulates absorption of dietary iron by the villus cells. There is physiological evidence that the iron concentration within the crypt cells is the most important determinant of regulation of dietary iron absorption (27, 32). When serum transferrin-bound iron levels are low, reflecting low body iron stores, the reduced transferrin-bound iron uptake by crypt cells programs the differentiating enterocytes to absorb more dietary iron in the villus (27). The 2- to 3-day time interval required for newly formed crypt cells to mature into absorptive enterocytes explains the delay between a physiological stimulus to alter iron absorption and the actual change in the iron absorption rate. Presumably, this transition to mature ionic iron-absorptive enterocytes involves regulation of the level of iron regulatory proteins, which stabilize the iron-responsive mRNA for DMT1, leading to increased synthesis of DMT1 in the differentiating enterocytes (34, 35). Increased expression of DMT1 in the villus, the scenario reported in iron deprivation (34), would in turn lead to greater absorption of dietary iron at the apical surface.
What then is the role of the normal HFE protein in this process and how do the mutations in this protein in HH lead to elevated levels of dietary iron absorption, even in the face of iron overload? We propose that the normal HFE protein participates in the regulation and uptake of transferrin-bound iron in the crypt cells, and that the HH mutations disrupt this function, leading to decreased transferrin-bound iron uptake by the crypt cells. The lower than appropriate levels of iron in the crypt cells, relative to the total body iron stores, would lead to an inappropriate signal to absorb more dietary iron in the differentiating villus cells.
The normal HFE protein, which we show here associates with the TfR in crypt cells, could modulate the uptake of transferrin-bound iron by the crypt cells by one or more of several mechanisms (21). First, it might enhance the cycling rate of the TfR cycle, expediting the delivery of transferrin-bound iron into the cells. Second, it might facilitate the endosomal egress of iron released during the TfR cycle. Third, it might change the affinity of the TfR for its ligand, diferric transferrin, and thus indirectly influence the uptake of iron. Actually, the HFE protein has been reported to reduce the affinity of the TfR for its ligand (19–21). However, the nanomolar changes in affinity reported seem unlikely to influence the TfR occupancy significantly at physiological levels of transferrin (≈25 μM). An intracellular effect of HFE protein on TfR trafficking resulting in increased iron uptake by crypt cells seems more consistent with the predominantly intracellular localization of the HFE protein in crypt enterocytes of the duodenum (17). Whatever the mechanism by which the HFE protein regulates the uptake of transferrin-bound iron in crypt enterocytes, we propose that the effect of HH mutations is to impair uptake of transferrin-bound iron. Lower than appropriate intracellular iron levels, in turn, would signal the need for greater iron absorption at the luminal surface of the villus, even in the face of excess transferrin-bound iron in serum and iron storage in parenchymal cells, as are characteristic of HH.
The one clear prediction of this model is that the anticipated low levels of iron in crypt enterocytes in HH should signal up-regulation of enterocyte DMT1 mRNA through its iron response element (36). The availability of the mouse model of HH in which the murine HFE gene has been disrupted should allow a direct test of this prediction.
Acknowledgments
We acknowledge Elizabeth Torno for editorial assistance. The work was supported by Grants DK53405 (W.S.S.), DK40163 (W.S.S.), GM34182 (W.S.S.), and DK41816 (B.R.B.) from the National Institutes of Health, and by a grant from the Sigrid Juselius Foundation (S.P.).
ABBREVIATIONS
- TfR
transferrin receptor
- HH
hereditary hemochromatosis
- NTA
nitrilotriacetic acid
- β2M
β2-microglobulin
- DMT1
divalent metal ion transporter
Footnotes
DMT1 was cloned independently on the basis of homology to Nramp, a macrophage gene product and initially called Nramp2; it also was identified by expression cloning as a divalent cation transporter and called DCT1. In this paper it is referred to as DMT1 to indicate its specificity for metal cations (36).
References
- 1.Cartwright G E, Edwards C Q, Kravitz K, Skolnick M, Amos D B, Johnson A, Buskjaer L. N Engl J Med. 1979;301:175–179. doi: 10.1056/NEJM197907263010402. [DOI] [PubMed] [Google Scholar]
- 2.Cox T M, Lord D K. Eur J Haematol. 1989;42:113–125. doi: 10.1111/j.1600-0609.1989.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 3.Edwards C Q, Griffen L M, Goldgar D, Drummond C, Skolnick M H, Kushner J P. N Engl J Med. 1988;318:1355–1362. doi: 10.1056/NEJM198805263182103. [DOI] [PubMed] [Google Scholar]
- 4.Bacon B R, Tavill A S. In: Hepatology: A Textbook of Liver Disease. 3rd Ed. Zakim D, Boyer T D, editors. Philadelphia: Saunders; 1996. pp. 1439–1472. [Google Scholar]
- 5.Feder J N, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy D A, Basava A, Dormishian F, Domingo R, Jr, Ellis M C, Fullan A, et al. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 6.Beutler E, Gelbart T, West C, Lee P, Adams M, Blackstone R, Pockros P, Kosty M, Venditti C P, Phatak P D, et al. Blood Cells Mol Dis. 1996;22:187–194. doi: 10.1006/bcmd.1996.0027. [DOI] [PubMed] [Google Scholar]
- 7.Jouanolle A M, Gandon G, Jézéquel P, Blayau M, Campion M L, Yaouanq J, Mosser J, Fergelot P, Chauvel B, Bouric P, et al. Nat Genet. 1996;14:251–252. doi: 10.1038/ng1196-251. [DOI] [PubMed] [Google Scholar]
- 8.Jazwinska E C, Cullen L M, Busfield F, Pyper W R, Webb S I, Powell L W, Morris C P, Walsh T P. Nat Genet. 1996;14:249–251. doi: 10.1038/ng1196-249. [DOI] [PubMed] [Google Scholar]
- 9.Carella M, D’Ambrosio L, Totaro A, Grifa A, Valentino M A, Piperno A, Girelli D, Roetto A, Franco B, Gasparini P, Camaschella C. Am J Hum Genet. 1997;60:828–832. [PMC free article] [PubMed] [Google Scholar]
- 10.Borot N, Roth M-P, Malfroy L, Demangel C, Vinel J-P, Pascal J-P, Coppin H. Immunogenetics. 1997;45:320–324. doi: 10.1007/s002510050211. [DOI] [PubMed] [Google Scholar]
- 11.Datz C, Lalloz M R A, Vogel W, Graziadei I, Hackl F, Vautier G, Layton D M, Maier-Dobersberger T, Ferenci P, Penner E, et al. J Hepatol. 1997;27:773–779. doi: 10.1016/s0168-8278(97)80312-1. [DOI] [PubMed] [Google Scholar]
- 12.Sham R L, Ou C-Y, Cappuccio J, Braggins C, Dunnigan K, Phatak P D. Blood Cells Mol Dis. 1997;23:314–320. doi: 10.1006/bcmd.1997.0148. [DOI] [PubMed] [Google Scholar]
- 13.Beckman L E, Saha N, Spitsyn V, Vanlandeghem G, Beckman L. Hum Heredity. 1997;47:263–267. doi: 10.1159/000154422. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X Y, Tomatsu S, Fleming R E, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt E M, Ruddy D A, Prass C E, et al. Proc Natl Acad Sci USA. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feder J N, Tsuchihashi Z, Irrinki A, Lee V K, Mapa F A, Morikang E, Prass C E, Starnes S M, Wolff R K, Parkkila S, et al. J Biol Chem. 1997;272:14025–14028. doi: 10.1074/jbc.272.22.14025. [DOI] [PubMed] [Google Scholar]
- 16.Waheed A, Parkkila S, Zhou X Y, Tomatsu S, Tsuchihashi Z, Feder J N, Schatzman R C, Britton R S, Bacon B R, Sly W S. Proc Natl Acad Sci USA. 1997;94:12384–12389. doi: 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkkila S, Waheed A, Britton R S, Feder J N, Tsuchihashi Z, Schatzman R C, Bacon B R, Sly W S. Proc Natl Acad Sci USA. 1997;94:2534–2539. doi: 10.1073/pnas.94.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkkila S, Waheed A, Britton R S, Bacon B R, Zhou X Y, Tomatsu S, Fleming R E, Sly W S. Proc Natl Acad Sci USA. 1997;94:13198–13202. doi: 10.1073/pnas.94.24.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feder J N, Penny D M, Irrinki A, Lee V K, Lebron J A, Watson N, Tsuchihashi Z, Sigal E, Bjorkman P J, Schatzman R C. Proc Natl Acad Sci USA. 1998;95:1475–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebrón J A, Bennett M J, Vaughn D E, Chirino A J, Snow P M, Mintier G A, Feder J N, Bjorkman P J. Cell. 1998;93:111–123. doi: 10.1016/s0092-8674(00)81151-4. [DOI] [PubMed] [Google Scholar]
- 21.Gross C N, Irrinki A, Feder J N, Enns C A. J Biol Chem. 1998;273:22068–22074. doi: 10.1074/jbc.273.34.22068. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan J, Jordan I, Sturrock A. J Biol Chem. 1991;266:2997–3004. [PubMed] [Google Scholar]
- 23.Anderson G J, Powell L W, Halliday J W. Gastroenterology. 1994;106:414–422. doi: 10.1016/0016-5085(94)90600-9. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Saarnio J, Parkkila S, Parkkila A-K, Waheed A, Casey M C, Zhou X Y, Pastoreková S, Pastorek J, Karttunen T, Haukipuro K, et al. J Histochem Cytochem. 1998;46:497–504. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- 26.Andrews N C, Levy J E. Blood. 1998;92:1845–1851. [PubMed] [Google Scholar]
- 27.Anderson G J. J Gastroenterol Hepatol. 1996;11:1030–1032. doi: 10.1111/j.1440-1746.1996.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 28.Beutler E. Am J Clin Nutr. 1997;66:419–420. doi: 10.1093/ajcn/66.2.419. [DOI] [PubMed] [Google Scholar]
- 29.Hallberg L, Hultén L, Gramatkovski E. Am J Clin Nutr. 1997;66:347–356. doi: 10.1093/ajcn/66.2.347. [DOI] [PubMed] [Google Scholar]
- 30.Cox T M, Peters T J. Lancet. 1978;1:123–124. doi: 10.1016/s0140-6736(78)90420-8. [DOI] [PubMed] [Google Scholar]
- 31.McLaren G D, Nathanson M H, Jacobs A, Trevett D, Thomson W. J Lab Clin Med. 1991;117:390–401. [PubMed] [Google Scholar]
- 32.Conrad M E, Crosby W H. Blood. 1963;22:406–415. [PubMed] [Google Scholar]
- 33.Oates P S, Thomas C, Morgan E H. J Gastroenterol Hepatol. 1997;12:829–838. doi: 10.1111/j.1440-1746.1997.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 34.Gunshin H, Mackenzie B, Berger U V, Gunshin Y, Romero M F, Boron W F, Nussberger S, Gollan J L, Hediger M A. Nature (London) 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 35.Fleming M D, Romano M A, Su M A, Garrick L M, Garrick M D, Andrews N C. Proc Natl Acad Sci USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacon B R, Powell L W, Adams P C, Kresina T F, Hoofnagle J H. Gastroenterology. 1999;116:1–17. doi: 10.1016/s0016-5085(99)70244-1. [DOI] [PubMed] [Google Scholar]