Abstract
The nonreceptor c-Abl tyrosine kinase binds to cytosolic 14-3-3 proteins and is targeted to the nucleus in the apoptotic response to DNA damage. The MUC1 oncoprotein is overexpressed by most human carcinomas and blocks the induction of apoptosis by genotoxic agents. Using human carcinoma cells with gain and loss of MUC1 function, we show that nuclear targeting of c-Abl by DNA damage is abrogated by a MUC1-dependent mechanism. The results demonstrate that c-Abl phosphorylates MUC1 on Tyr-60 and forms a complex with MUC1 by binding of the c-Abl SH2 domain to the pTyr-60 site. Binding of MUC1 to c-Abl attenuates phosphorylation of c-Abl on Thr-735 and the interaction between c-Abl and cytosolic 14-3-3. We also show that expression of MUC1 with a mutation at Tyr-60 (i) disrupts the interaction between MUC1 and c-Abl, (ii) relieves the MUC1-induced block of c-Abl phosphorylation on Thr-735 and binding to 14-3-3, and (iii) attenuates the MUC1 antiapoptotic function. These findings indicate that MUC1 sequesters c-Abl in the cytoplasm and thereby inhibits apoptosis in the response to genotoxic anticancer agents.
Keywords: apoptosis, c-Abl, DNA damage, MUC1, 14-3-3
Introduction
DNA damage activates a nuclear complex that consists in part of the nonreceptor c-Abl tyrosine kinase (Kharbanda et al, 1995b; Yuan et al, 1997). c-Abl binds to the p53 tumor suppressor in the response to genotoxic stress (Yuan et al, 1996a, 1996b) and regulates p53 by preventing its nuclear export (Sionov et al, 2001). Other studies have demonstrated that c-Abl interacts with the p73 homolog of p53 in the apoptotic response of cells to DNA damage (Agami et al, 1999; Gong et al, 1999; Yuan et al, 1999). Nuclear c-Abl also activates MEK kinase-1 and thereby the proapoptotic c-Jun N-terminal kinase (JNK) pathway (Kharbanda et al, 1995a, 1995b, 2000). c-Abl contains three nuclear localization signals and one nuclear export signal in the carboxy-terminal region that are responsible for shuttling of c-Abl between the cytoplasm and nucleus (Wen et al, 1996; Taagepera et al, 1998). Recent work has shown that c-Abl is sequestered in the cytoplasm through binding to 14-3-3 proteins (Yoshida et al, 2005). Phosphorylation of c-Abl on Thr-735 by an as yet unidentified kinase serves as a site for direct binding to 14-3-3. In the response to DNA damage, activation of JNK induces phosphorylation of 14-3-3 and the release of c-Abl for targeting to the nucleus (Yoshida et al, 2005). In this regard, expression of a 14-3-3 mutant at the JNK phosphorylation site attenuates DNA damage-induced nuclear import of c-Abl and apoptosis. These findings have provided support for a model in which 14-3-3 is an important regulator of intracellular c-Abl localization and the apoptotic response to genotoxic stress.
The MUC1 transmembrane glycoprotein is expressed as a stable heterodimer following synthesis as a single polypeptide and cleavage into two subunits in the endoplasmic reticulum (Ligtenberg et al, 1992). MUC1 is normally localized to the apical borders of secretory epithelial cells (Kufe et al, 1984). With transformation and loss of polarity, the MUC1 heterodimer is aberrantly overexpressed on the entire cell membrane (Kufe et al, 1984). The MUC1 N-terminal subunit (MUC1-N) contains variable numbers of 20 amino-acid tandem repeats that are modified by O-linked glycans (Gendler et al, 1988; Siddiqui et al, 1988). MUC1-N is tethered to the cell membrane by the MUC1 C-terminal subunit (MUC1-C), which includes a transmembrane region and a 72 amino-acid cytoplasmic domain (MUC1-CD) (Merlo et al, 1989). MUC1-CD associates with β-catenin (Yamamoto et al, 1997; Li et al, 1998; Huang et al, 2005) and the p53 tumor suppressor (Wei et al, 2005). In addition, MUC1-CD is phosphorylated by the epidermal growth factor receptor (Li et al, 2001b), c-Src (Li et al, 2001a) and glycogen synthase kinase 3β (Li et al, 1998). Other studies have shown that overexpression of MUC1 confers transformation (Li et al, 2003; Huang et al, 2005) and blocks the apoptotic response to genotoxic stress (Raina et al, 2004; Ren et al, 2004). In this regard and in addition to expression at the cell membrane, MUC1-C localizes to the mitochondrial outer membrane and inhibits DNA damage-induced release of apoptogenic factors (Ren et al, 2004, 2006). Mitochondrial targeting of MUC1-C is attenuated by mutation of the cytoplasmic domain at Tyr-46 (Ren et al, 2004, 2006). Moreover, expression of MUC1 with a Y46F mutation attenuates the antiapoptotic function of MUC1 in the response to DNA damage (Ren et al, 2004). These findings indicate that MUC1 blocks in part the apoptotic response to genotoxic anticancer agents by disrupting the intrinsic mitochondrial pathway.
The overexpression of MUC1 in most human carcinomas, the antiapoptotic function of MUC1 in the response of cancer cells to genotoxic anticancer agents and the role of c-Abl in inducing apoptosis in the DNA damage response prompted us to investigate whether MUC1 interacts with c-Abl. Our results demonstrate that MUC1 binds directly to c-Abl and sequesters c-Abl in the cytoplasm. We also show that MUC1 blocks nuclear targeting of c-Abl and thereby the apoptotic response to genotoxic anticancer agents.
Results
MUC1 attenuates DNA damage-induced targeting of c-Abl to the nucleus
To investigate whether MUC1 regulates nuclear targeting of c-Abl, HeLa cells were transfected to stably express an empty vector or MUC1. The MUC1 N-terminal (MUC1-N) and C-terminal (MUC1-C) subunits were detectable in HeLa/MUC1, but not HeLa/vector, cells (Figure 1A). Moreover, c-Abl levels were similar in the absence and presence of MUC1 (Figure 1A). Consistent with previous results (Yoshida et al, 2005), treatment of HeLa/vector cells with the DNA damaging agent adriamycin (ADR) was associated with targeting of c-Abl to the nucleus (Figure 1B). By contrast, there was little if any nuclear targeting of c-Abl in the ADR-treated HeLa/MUC1 cells (Figure 1B). As controls, the nuclear fractions were immunoblotted with antibodies against the nuclear lamin B and cytoplasmic IκBα proteins (Figure 1B). Treatment of HeLa/vector and HeLa/MUC1 cells with cisplatin (CDDP) confirmed that MUC1 attenuates nuclear targeting of c-Abl by genotoxic anticancer agents (Figure 1C). In concert with these results, CDDP treatment was associated with a decrease in cytosolic c-Abl in HeLa/vector, but not HeLa/MUC1, cells (Figure 1D).
Figure 1.
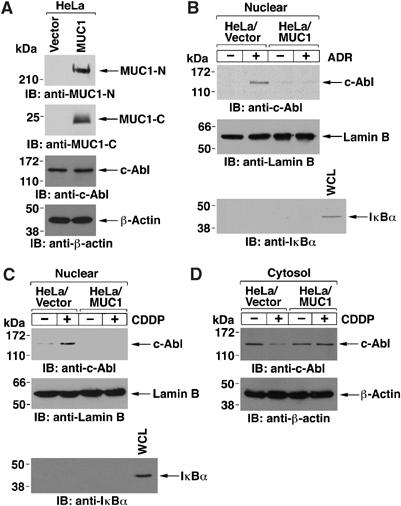
Exogenous MUC1 attenuates nuclear targeting of c-Abl in HeLa cells. (A) Lysates from HeLa cells were subjected to immunoblotting with anti-MUC1-N, anti-MUC1-C, anti-c-Abl or anti-β-actin. (B, C). HeLa/vector and HeLa/MUC1 cells were treated either with adriamycin (ADR) (B) or cisplatin (CDDP) (C) for 2 h. Nuclear fractions were subjected to immunoblot analysis with anti-c-Abl, anti-lamin B or anti-IκBα. (D) HeLa/vector and HeLa/MUC1 cells were treated with CDDP for 2 h. Cytosolic extracts were subjected to immunoblot analysis with anti-c-Abl or anti-β-actin.
As reported previously, endogenous MUC1 expression was knocked-down with a MUC1siRNA in human ZR-75-1 breast cancer cells (Ren et al, 2004). Silencing MUC1 in the ZR-75-1 cells had no effect on c-Abl levels (Figure 2A). When ZR-75-1/vector cells were treated with CDDP, there was no detectable targeting of c-Abl to the nucleus (Figure 2B). However, in ZR-75-1/MUC1siRNA cells silenced for MUC1, nuclear targeting of c-Abl was increased about six-fold in response to CDDP treatment (Figure 2B). Cytosolic c-Abl was also decreased by CDDP treatment of ZR-75-1/MUC1siRNA, but not ZR-75-1/vector, cells (Figure 2C). To further analyze the effects of MUC1 on c-Abl localization, transient overexpression of GFP-tagged c-Abl in 293 cells resulted in targeting of c-Abl to the nucleus that was detectable constitutively and increased by ADR treatment (Supplementary Figure S1A). By contrast, constitutive and ADR-induced nuclear targeting of c-Abl was attenuated by coexpression of MUC1 (Supplementary Figure S1A). Similar results were obtained when the transfected cells were treated with CDDP (data not shown). The c-Abl mutant with deletion of the nuclear export signal (c-AblNES) localizes exclusively in the nucleus (Yoshida et al, 2005). Importantly, MUC1 expression had little effect on nuclear localization of GFP-c-AblΔNES (Supplementary Figure S1B). These findings indicate that MUC1 blocks targeting of cytosolic c-Abl to the nucleus in the response to genotoxic anticancer agents.
Figure 2.
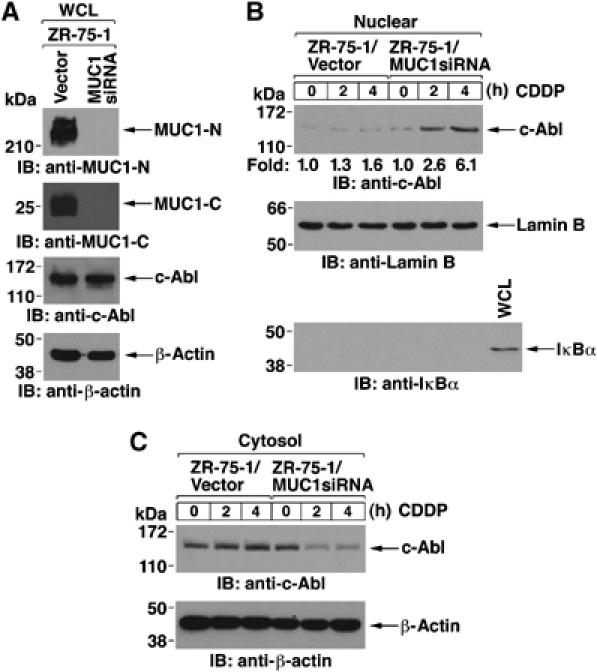
c-Abl is targeted to the nucleus by silencing MUC1 in ZR-75-1 cells. (A) Lysates from ZR-75-1/vector and ZR-75-1/MUC1siRNA cells were subjected to immunoblotting with anti-MUC1-N, anti-MUC1-C, anti-c-Abl or anti-β-actin. (B and C) ZR-75-1/vector and ZR-75-1/MUC1siRNA cells were treated with CDDP for 2 and 4 h. Nuclear (B) and cytosolic (C) fractions were subjected to immunoblot analysis with the indicated antibodies.
MUC1 associates with c-Abl at the cell membrane
To determine if MUC1 associates with c-Abl, lysates from HeLa/MUC1 cells were precipitated with anti-MUC1-N or, as a control, IgG. Immunoblot analysis of the precipitates with anti-c-Abl demonstrated that MUC1 associates with c-Abl (Figure 3A). Coimmunoprecipitation studies performed on ZR-75-1 cells confirmed that MUC1 forms a complex with c-Abl (Figure 3B). MUC1 is expressed at the cell membrane as a heterodimer of MUC1-N and MUC1-C. Consequently, we asked if c-Abl localizes to the cell membrane as a function of MUC1 expression. Immunoblot analysis showed a substantially higher level of c-Abl in HeLa/MUC1 cell membranes as compared to those purified from HeLa/vector cells (Figure 3C). Equal loading and purity of the cell membrane fraction was confirmed by immunoblotting with antibodies against the cell membrane-associated platelet-derived growth factor receptor, endoplasmic reticulum-associated calnexin and cytosolic IκBα proteins (Figure 3C). Consistent with the results in HeLa cells, localization of c-Abl to the cell membrane was significantly decreased, but not completely abrogated, by silencing MUC1 in ZR-75-1 cells (Figure 3D). Consistent with these results, c-Abl is known to bind to other cell membrane-associated proteins, such as scramblase 1 and caveolin 1 (Sun et al, 2001; Sanguinetti and Mastick, 2003). Confocal microscopy further showed colocalization of MUC1 and c-Abl at the cell membrane (Figure 3E). These findings indicate that c-Abl associates with MUC1 at the cell membrane.
Figure 3.
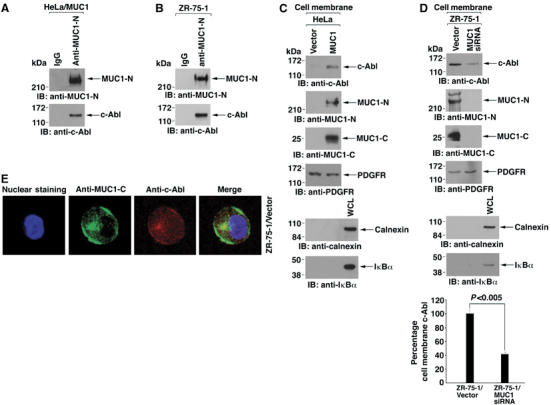
MUC1 forms complexes with c-Abl at the cell membrane. (A, B). Lysates from HeLa/MUC1 (A) and ZR-75-1/vector (B) cells were subjected to immunoprecipitation (IP) with mouse IgG or anti-MUC1-N. The precipitates were immunoblotted with anti-c-Abl or anti-MUC1-N. (C) Cell membrane fractions from HeLa/vector and HeLa/MUC1 cells were subjected to immunoblot analysis with the indicated antibodies. (D) Cell membrane fractions from ZR-75-1/vector or ZR-75-1/MUC1siRNA cells were subjected to immunoblot analysis with the indicated antibodies. Immunoblots from three separate experiments were subjected to densitometric scanning to determine the effects of MUC1 silencing on localization of c-Abl to the cell membrane. The results are expressed as the percentage (mean±s.d.) of c-Abl localization for ZR-75-1/vector cells (assigned a value of 100%) and ZR-75-1/MUC1siRNA cells. (E) Confocal microscopy of ZR-75/vector cells stained with anti-MUC1-C or anti-c-Abl. Nuclei were stained with TO-PRO-3.
c-Abl phosphorylates the MUC1 cytoplasmic domain
To identify the region of MUC1 that associates with c-Abl, studies were performed on HCT116 colon cancer cells. As found in other cell types (Kharbanda et al, 1995b; Yuan et al, 1997), treatment of the HCT116 cells with CDDP was associated with induction of c-Abl activity (Supplementary Figure S2). Consequently, we studied HCT116 cells that stably express the empty vector or a Flag-tagged MUC1 cytoplasmic domain (MUC1-CD) (Huang et al, 2003) (Figure 4A). Expression of Flag-MUC1-CD had no detectable effect on c-Abl levels (Figure 4A). Immunoblot analysis of anti-Flag precipitates with anti-c-Abl further showed that MUC1-CD is sufficient for the association with c-Abl (Figure 4B). To determine if MUC1-CD is a substrate for c-Abl, we incubated purified kinase-active c-Abl and MUC1-CD in the presence of [γ-32P]ATP. Analysis of the reaction products by SDS–PAGE and autoradiography showed phosphorylation of MUC1-CD (Figure 4C). As a control, there was no detectable phosphorylation when MUC1-CD was incubated with a purified kinase-inactive c-Abl(K-R) (Kharbanda et al, 1995b) (Figure 4C). MUC1-CD contains a consensus c-Abl phosphorylation site (YXXP) at Y60TNP (Figure 4D). Consequently, we asked if c-Abl phosphorylation of MUC1-CD is decreased by a Y-60->F mutation. Compared to MUC1-CD, c-Abl phosphorylation was substantially decreased with the MUC1-CD(Y60F) mutant (Figure 4D), indicating that c-Abl phosphorylates MUC1, at least in large part, on Tyr-60 in the cytoplasmic domain.
Figure 4.
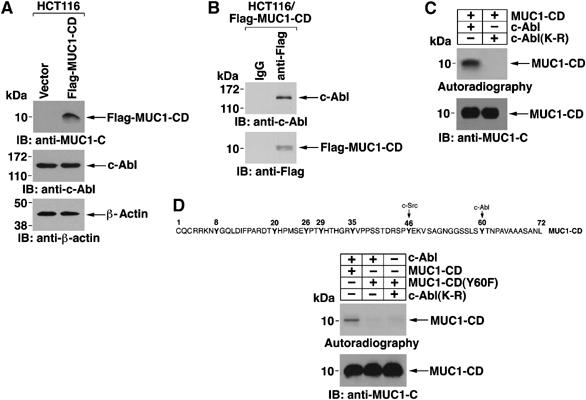
c-Abl phosphorylates the MUC1 cytoplasmic domain. (A) Lysates from HCT116/vector and HCT116/Flag-MUC1-CD cells were immunoblotted with anti-MUC1-C, anti-c-Abl or anti-β-actin. (B) Lysates from HCT116/Flag-MUC1-CD cells were immunoprecipitated with mouse IgG or anti-Flag. The precipitates were immunoblotted with anti-c-Abl or anti-Flag. (C) Purified GST-MUC1-CD was cleaved with thrombin to remove the GST tag and then incubated with GST-c-Abl or GST-c-Abl(K-R) and [γ-32P]ATP for 15 min at 30°C. The reaction products were analyzed by SDS–PAGE and autoradiography (top panel) or by immunoblotting with anti-MUC1-C (lower panel). (D) Amino-acid sequence of MUC1-CD (upper panel). Purified MUC1-CD or MUC1-CD(Y60F) was incubated with GST-c-Abl or GST-c-Abl(K-R) and [γ-32P]ATP for 15 min at 30°C. Reaction products were analyzed by SDS–PAGE and autoradiography or by immunoblotting with anti-MUC1-C (lower panels).
c-Abl SH2 domain binds to the MUC1 pY60TNP site
MUC1 with the Y60F mutation was stably expressed in HCT116 cells. Immunoblot analysis of two separately isolated clones showed somewhat higher levels of MUC1(Y60F) as compared to the wild-type protein in stably transfected HCT116/MUC1 cells (Figure 5A). As found for MUC1, expression of the MUC1(Y60F) mutant had no effect on c-Abl levels (Figure 5A). Notably, c-Abl was detectable in anti-MUC1-N immunoprecipitates from HCT116/MUC1, but not HCT116/MUC1(Y60F), cells (Figure 5B). As a control, the Y60F mutation had no effect on the association between MUC1-N and MUC1-C (Figure 5B). Similar results were obtained with the two HCT116/MUC1(Y60F) clones (data not shown). Localization of c-Abl to the cell membrane was also decreased in HCT116/MUC1(Y60F), as compared to that in HCT116/MUC1, cells (Figure 5C). In 293 cells, binding of MUC1 to c-Abl was also attenuated by the Y60F mutation (Figure 5D). To determine if the pY60TNP motif functions as a binding site for the c-Abl SH2 domain, we incubated MUC1-CD or MUC1-CD(Y60F) with kinase-active truncated Abl (no SH2 domain) and ATP. Incubation of the reaction products with GST-c-Abl SH2 demonstrated that binding of the c-Abl SH2 domain to phosphorylated MUC1-CD is abrogated by the Y60F mutation (Figure 5E). To extend these results, we generated a c-Abl SH2 point mutant in which Arg-152 in the conserved FLVRES sequence was modified to Leu. In vitro studies demonstrated that the R152L mutation abrogates binding of GST-c-Abl SH2 to c-Abl phosphorylated MUC1-CD (Supplementary Figure S3A). We also found that MUC1 is effective in blocking nuclear targeting of GFP-c-Abl, but not GFP-c-Abl(R152L), in the response to DNA damage (Supplementary Figure 3B–D). These findings indicate that MUC1 and c-Abl interact directly through binding of the c-Abl SH2 domain to the MUC1 pYTNP motif.
Figure 5.
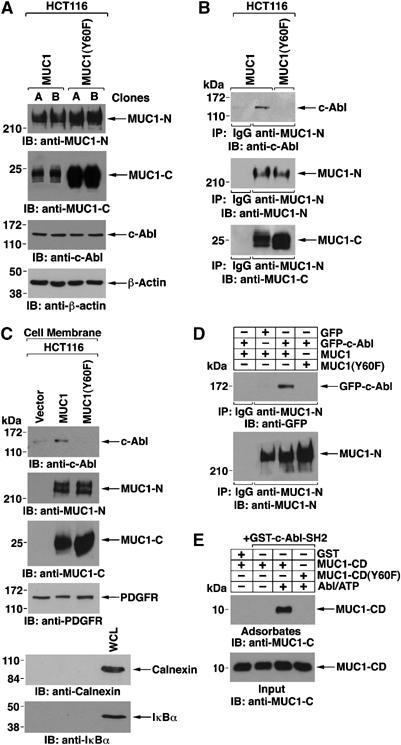
c-Abl-SH2 domain binds to the MUC1 pY60TNP site. (A) Lysates from HCT116/MUC1 (clones A and B) and HCT116/MUC1(Y60F) (clones A and B) were subjected to immunoblotting with anti-MUC1-N, anti-MUC1-C, anti-c-Abl or anti-β-actin. (B) Lysates from HCT116/MUC-1 and HCT116/MUC1(Y60F) cells were immunoprecipitated with mouse IgG and anti-MUC1-N. The precipitates were immunoblotted with anti-c-Abl, anti-MUC1-N and anti-MUC1-C. (C) Cell membrane fractions from HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) cells were immunoblotted with the indicated antibodies. (D) 293T cells were transfected with GFP and MUC1, GFP-c-Abl and MUC1, and GFP-c-Abl and MUC1(Y60F). Lysates were subjected to immunoprecipitation with anti-MUC1-N or control IgG and immunoblotting of the precipitates with anti-GFP and anti-MUC1-N. (E) Purified MUC1-CD and MUC1-CD (Y60F) were incubated in the presence or absence of recombinant truncated Abl (no SH2 domain) and 200 μM ATP for 30 min at 30°C. GST or GST-c-Abl-SH2 bound to GST-beads was then added and incubated for 1 h at 4°C. Adsorbed (upper panel) and input (lower panel) proteins were subjected to immunoblot analysis with anti-MUC1-C.
MUC1 blocks binding of c-Abl to 14-3-3 proteins
c-Abl contains a RSVpT735LP sequence that confers binding to 14-3-3 proteins as a mechanism for regulating nuclear targeting of c-Abl in the response to DNA damage (Yoshida et al, 2005). To determine if MUC1 affects phosphorylation of Thr-735, anti-c-Abl precipitates from HeLa/vector and HeLa/MUC1 cells were immunoblotted with an anti-phospho-c-Abl-Thr-735 antibody. The results show that MUC1 blocks c-Abl Thr-735 phosphorylation (Figure 6A). In concert with these results, c-Abl Thr-735 phosphorylation was increased in ZR-75-1 cells silenced for MUC1 (Figure 6B). In addition, c-Abl Thr-735 phosphorylation was attenuated by expression of MUC1, but not MUC1(Y60F), in HCT116 cells, indicating that the interaction between MUC1 and c-Abl blocks phosphorylation of the Thr-735 site (Figure 6C). Consistent with a block in Thr-735 phosphorylation, we found that MUC1 inhibits the association between c-Abl and 14-3-3 in HeLa cells (Figure 6D) and ZR-75-1 cells (Figure 6E). MUC1 also blocked binding of c-Abl to 14-3-3 in HCT116 cells (Figure 6F). By contrast, MUC1(Y60F) had little effect on binding of c-Abl to 14-3-3 (Figure 6F). These results indicate that binding of MUC1 and c-Abl blocks phosphorylation of c-Abl on Thr-735 and thereby the association between c-Abl and 14-3-3.
Figure 6.
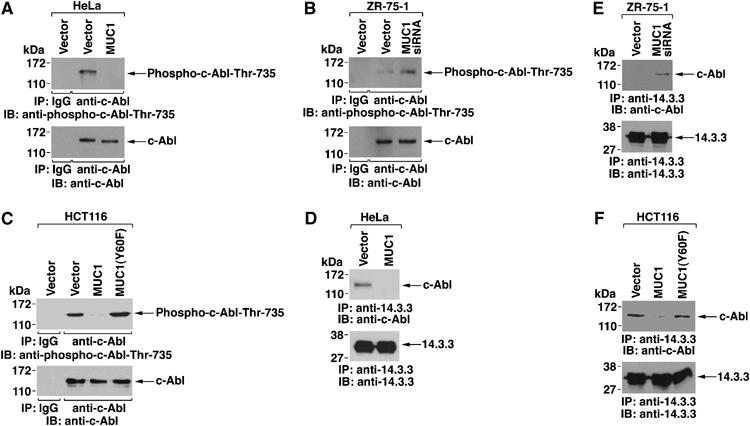
MUC1 blocks c-Abl Thr-735 phosphorylation and binding of c-Abl to 14-3-3. (A–C) Lysates from HeLa/vector, HeLa/MUC1 (A), ZR-75-1/vector, ZR-75-1/MUC1siRNA (B), HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) (C) cells were immunoprecipitated with anti-c-Abl. The precipitates were immunoblotted with anti-phospho-c-Abl-Thr-735 and anti-c-Abl. (D–F) Cytoplasmic fractions of HeLa/vector, HeLa/MUC1 (D), ZR-75-1/vector, ZR-75-1/MUC1siRNA (E), HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) (F) cells were immunoprecipitated with anti-14-3-3. The precipitates were immunoblotted with anti-c-Abl and anti-14-3-3.
Binding of MUC1 and c-Abl attenuates DNA damage-induced apoptosis
HCT116/vector cells express a low level of nuclear c-Abl and, in the response to CDDP treatment, c-Abl was targeted to the nucleus (Figure 7A). As found in the other cell types, expression of MUC1 in HCT116 cells blocked nuclear targeting of c-Abl (Figure 7A). Moreover, there was less than a two-fold increase in nuclear MUC1-C levels in response to CDDP, consistent with the effects on c-Abl (Supplementary Figure S4). Importantly, however, MUC1(Y60F) had little if any effect on CDDP-induced targeting of c-Abl to the nucleus (Figure 7A). In concert with the block in nuclear targeting of c-Abl by wild-type MUC1, CDDP treatment increased binding of MUC1, but not MUC1(Y60F), with c-Abl (Figure 7B and data not shown). DNA damage activates the intrinsic apoptotic pathway with caspase-3-mediated cleavage of protein kinase Cδ (Emoto et al, 1995; Ghayur et al, 1996). Cleavage of PKCδ was attenuated by MUC1, but not the MUC1(Y60F) mutant (Figure 7C). Analysis of the cells for sub-G1 DNA further demonstrated that MUC1 blocks DNA damage-induced apoptosis and that this inhibition is relieved by the Y60F mutation (Supplementary Figure S5). Similar results were obtained in repetitive experiments (Figure 7D) and when the HCT116 cells were treated with ADR (data not shown). To assess the role of c-Abl in the induction of apoptosis, we treated the cells with CDDP in the absence and presence of STI571, an inhibitor of the c-Abl kinase function (Druker et al, 1996). STI751 significantly attenuated CDDP-induced apoptosis in HCT116/vector and HCT116/MUC1(Y60F) cells, but had no significant effect on HCT116/MUC1 cells (Figure 7E). To substantiate these results, c-Abl expression was downregulated in HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) cells by transient transfection with a pool of c-Abl siRNAs (Supplementary Figure S6A). Consistent with the effects of STI571, c-Abl downregulation was associated with attenuation of CDDP-induced apoptosis in HCT116/vector and HCT116/MUC1(Y60F) cells (Figure 7F, Supplementary Figure S6B and S6D). By contrast, silencing c-Abl in HCT116/MUC1 cells had no significant effect on the induction of apoptosis (Figure 7F, Supplementary Figure S6C). These findings demonstrate that binding of MUC1 to c-Abl and sequestration of c-Abl in the cytosol blocks the proapoptotic function of c-Abl in the response to DNA damage.
Figure 7.
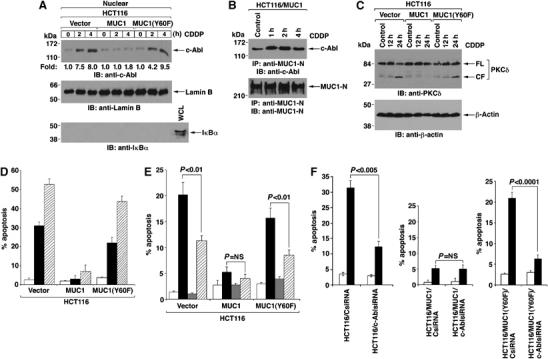
Binding of MUC1 and c-Abl attenuates DNA damage-induced apoptosis. (A) HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) cells were treated with CDDP for 2 and 4 h. Nuclear lysates were immunoblotted with anti-c-Abl, anti-lamin B and anti-IκBα. Intensity of the signals was determined by densitometric scanning and compared to that for untreated cells. (B) Anti-MUC1-N precipitates from HCT116/MUC1 cells treated with CDDP for the indicated times were immunoblotted with anti-c-Abl and anti-MUC1-N. (C) HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) cells were treated with CDDP for indicated times. Lysates were immunoblotted with anti-PKCδ and anti-β-actin. FL: full-length. CF: cleaved fragments. (D) The results from sub-G1 DNA analysis of HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) cells treated with CDDP for 0 h (open bars), 12 h (solid bars) or 24 h (hatched bars) are expressed as the percentage apoptosis (mean±s.d. of three separate experiments). (E) The results from sub-G1 DNA analysis of HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) cells left untreated (open bars) or treated with CDDP alone for 12 h (solid bars), STI571 alone for 12 h (shaded bars) or both CDDP and STI571 for 12 h (hatched bars) are expressed as the percentage apoptosis (mean±s.d. of three separate experiments). (F) The results from sub-G1 DNA analysis of HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) cells transfected with the control CsiRNA or c-AblsiRNA and left untreated (open bars) or treated with CDDP for 12 h (solid bars) are expressed as the percentage of apoptosis (mean+s.d. of three separate experiments). NS, not significant.
Selectivity of the MUC1 Tyr-60 site for the regulation of c-Abl
Previous work showed that the antiapoptotic effects of MUC1 are attenuated by mutation of Tyr-46 (Ren et al, 2004). To determine whether the loss of MUC1 function by the MUC1(Y60F) and MUC1(Y46F) mutants is due to similar or distinct mechanisms, we compared the effects of stably expressing these proteins in HCT116 cells (Supplementary Figure S7A). As found for MUC1(Y60F), the MUC1(Y46F) mutant had no apparent effect on c-Abl levels (Supplementary Figure S7A). Coimmunoprecipitation studies further demonstrated that, like MUC1, the MUC1(Y46F) mutant associates with c-Abl (Supplementary Figure S7B). Moreover, in contrast to MUC1(Y60F), MUC1(Y46F) blocked CDDP-induced targeting of cytosolic c-Abl to the nucleus (Supplementary Figures S7C and S7D). MUC1 localizes to the outer mitochondrial membrane and blocks the release of apoptogenic factors (Ren et al, 2004, 2006). In addition, mitochondrial localization of MUC1 is substantially attenuated by mutation of Tyr-46 (Ren et al, 2004, 2006), but not the Tyr-60 site (Supplementary Figure S7E). These findings indicate that MUC1 Tyr-60, and not Tyr-46, regulates c-Abl function. Conversely, MUC1 Tyr-46, and not Tyr-60, regulates mitochondrial localization.
Discussion
MUC1 binds directly to c-Abl
Nuclear c-Abl is activated by diverse genotoxic agents and induces apoptosis (Kharbanda et al, 1995b; Yuan et al, 1997). Conversely, overexpression of MUC1, as found in most human carcinomas, blocks the induction of apoptosis by genotoxic anticancer agents (Raina et al, 2004; Ren et al, 2004). The present studies demonstrate that MUC1 associates with c-Abl. MUC1 is expressed at the cell membrane as a heterodimer of the MUC1-N and MUC1-C subunits. MUC1-N extends beyond the cell glycocalyx and is tethered to the cell membrane by the MUC1-C transmembrane subunit. The observation that c-Abl is detectable in anti-MUC1-N precipitates suggested that c-Abl interacts with the cell membrane-associated MUC1 heterodimer. Colocalization of MUC1 and c-Abl at the cell membrane was confirmed by confocal microscopy. Moreover, cell membrane-associated c-Abl was increased by an MUC1-dependent mechanism. The results further demonstrate that c-Abl interacts directly with the MUC1 cytoplasmic domain (MUC1-CD). The results show that c-Abl phosphorylates MUC1-CD on Tyr-60. In turn the pY60TNP motif functions as a binding site for the c-Abl SH2 domain. Consistent with this mechanism of interaction, mutation of Tyr-60 abrogated binding of c-Abl to MUC1 in vitro. Moreover, stable expression of MUC1 with a Y60F mutation in cells abrogated the formation of MUC1–c-Abl complexes. These findings provide the first evidence for an interaction between the antiapoptotic MUC1 and proapoptotic c-Abl proteins.
MUC1 blocks binding of c-Abl to 14-3-3 proteins
Cytosolic 14-3-3 proteins bind to the consensus RSXpS/TXP sequence and disrupt nuclear import of binding proteins by blocking their nuclear localization signals (Muslin and Xing, 2000; Tzivion and Avruch, 2002). The c-Abl RSVT735LP motif conforms to the consensus 14-3-3 binding site and is phosphorylated by a presently unidentified kinase (Yoshida et al, 2005). In this context, c-Abl binds to 14-3-3 and mutation of Thr-735 abrogates this interaction (Yoshida et al, 2005). Using cells with gain and loss of MUC1 function, the present results demonstrate that MUC1 blocks phosphorylation of c-Abl on Thr-735. Importantly, phosphorylation of the c-Abl Thr-735 site was not blocked by MUC1 with the Y60F mutation, indicating that direct binding of MUC1 and c-Abl is responsible for maintaining Thr-735 in the unphosphorylated state. Consistent with these results, MUC1 blocked binding of c-Abl to 14-3-3 and the c-Abl-14-3-3 interaction was unaffected by MUC1(Y60F). These results indicate that MUC1 blocks phosphorylation of c-Abl on Thr-735 by direct binding to c-Abl. However, whereas the c-Abl Thr-735 kinase is not known, our results do not exclude the possibility that MUC1 may inhibit that kinase. JNK is activated in the response to DNA damage (Kharbanda et al, 1995b) and phosphorylates 14-3-3 as a mechanism for the release of Bax to mitochondria (Tsuruta et al, 2004). JNK phosphorylation of 14-3-3 similarly induces the release of c-Abl from 14-3-3 for targeting of c-Abl to the nucleus (Yoshida et al, 2005). However, our results support a model in which MUC1 subverts this mechanism for DNA damage-induced shuttling of c-Abl between the cytoplasm and nucleus by blocking binding of c-Abl to 14-3-3.
MUC1 blocks DNA damage-induced targeting of c-Abl to the nucleus and thereby the apoptotic response
Nuclear c-Abl functions in activating pro-apoptotic signals in the DNA damage response (Kharbanda et al, 1995b; Yuan et al, 1997). The Bcr-Abl fusion protein, which also contains the three NLSs, localizes to the cytoplasm (Wetzler et al, 1993) and confers survival (Raitano et al, 1997). Conversely, forced entrapment of Bcr-Abl in the nucleus induces apoptosis (Vigneri and Wang, 2001). The present studies demonstrate that MUC1 sequesters c-Abl, at least in large part, at the cell membrane. Moreover and importantly, MUC1 blocks DNA damage-induced targeting of c-Abl to the nucleus. The results indicate that the direct binding between MUC1 and c-Abl is responsible for the block in localization of c-Abl to the nucleus. In this regard, expression of MUC1 with the Y60F mutation was ineffective in preventing DNA damage-induced nuclear targeting of c-Abl. Our results also provide support for a model in which binding of MUC1 to c-Abl blocks the apoptotic response to DNA damage. c-Abl-deficient cells are resistant to DNA damage-induced apoptosis (Yuan et al, 1997) and activation of nuclear c-Abl is essential for its proapoptotic function (Agami et al, 1999; Gong et al, 1999; Yuan et al, 1999; Yoshida et al, 2002). Thus, we found that the antiapoptotic effects of MUC1 are inhibited by blocking the c-Abl interaction with MUC1(Y60F). Moreover, DNA damage-induced apoptosis in the presence of MUC1(Y60F) was attenuated by inhibiting c-Abl activation with STI571 or by downregulating c-Abl expression. These findings indicate that the antiapoptotic effects of MUC1 are in large part dependent on sequestering c-Abl in the cytoplasm.
Why would MUC1 inhibit c-Abl-induced apoptosis?
MUC1 contributes to a physical barrier that protects epithelia from damage induced by diverse forms of stress that occurs at the interface with the external environment. In the epithelial stress response, damage induces the disruption of tight junctions, loss of polarity and the activation of a repair and survival program (Vermeer et al, 2003). The available evidence indicates that MUC1 contributes to this survival program through targeting of the MUC1-C subunit to the outer mitochondrial membrane where it blocks release of apoptogenic factors (Ren et al, 2004, 2006). In this context, targeting of MUC1 to mitochondria is attenuated by mutation of the Tyr-46 site (Ren et al, 2004, 2006). Moreover, the antiapoptotic function of MUC1 is attenuated by expression of MUC1(Y46F) (Ren et al, 2004, 2006). The present results indicate that MUC1 also contributes to the epithelial survival program by blocking nuclear targeting of c-Abl. Notably, MUC1(Y46F) and MUC1(Y60F) appear to inhibit the antiapoptotic function of MUC1 by different mechanisms. In contrast to MUC1(Y60F), the MUC1(Y46F) mutant binds to c-Abl and attenuates DNA damage-induced targeting of c-Abl to the nucleus. Conversely, unlike MUC1(Y46F), we found that MUC1(Y60F) localizes to mitochondria. These findings suggest that MUC1 blocks DNA damage-induced apoptosis by two mechanisms involving (i) localization to the mitochondrial outer membrane through phosphorylation of the Tyr-46 site and (ii) sequestration of c-Abl at the cell membrane through phosphorylation of the Tyr-60 site. The results suggest that both pathways in which MUC1 is phosphorylated on Tyr-46 by c-Src and on Tyr-60 by c-Abl are integral in conferring the MUC1 antiapoptotic function. Notably, c-Abl functions as a substrate and effector of c-Src in the response to growth factor stimulation (Plattner et al, 1999; Furstoss et al, 2002). As such, activation of the c-Src → c-Abl pathway may be integrated with MUC1 as a mechanism for attenuating signals that induce an apoptotic response. Thus, overexpression of MUC1 as found in diverse human tumors may confer resistance to genotoxic anticancer agents by exploiting physiologic mechanisms that evolved to protect normal epithelia from stress-induced death.
Materials and methods
Cell culture
Human HeLa cervical cancer, HCT116/vector, HCT116/MUC1, HCT116/MUC1(Y46F) colon cancer (Ren et al, 2002) and 293 cells were cultured in Dulbecco's modified medium with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine. Human ZR-75-1/vector and ZR-75-1/MUC1siRNA breast cancer cells (Ren et al, 2004) were grown in RPMI1640 medium containing 10% heat-inactivated fetal bovine serum and antibiotics. Cells were treated with 1 μM adriamycin (ADR; Calbiochem), 100 μM cisplatin (CDDP; Sigma) or 10 μM STI571 (Gleevec; Novartis, Basel, Switzerland).
Cell transfections
HeLa cells were stably transfected with pCMV or pCMV-MUC1 using LipofactAMINE and selected in the presence of G418. The pIRESpuro2-MUC1(Y60F) and GFP-c-Abl(R152L) vectors were constructed by site-directed mutagenesis as described (Li et al, 2001a). HCT116 cells were transfected with pIRESpuro2-MUC1(Y60F) using LipofectAMINE and selected in the presence of puromycin (Calbiochem-Novabiochem Co.). 293 cells were transiently transfected with pIRESpuro2, pIRESpuro2-MUC1, pIRESpuro2-MUC1(Y60F), GFP, GFP-c-Abl, GFP-c-AblΔNES and GFP-c-Abl(R152L) using LipofectAMINE. For downregulation of c-Abl, HCT116/vector, HCT116/MUC1 and HCT116/MUC1(Y60F) cells were seeded (5 × 105/well) on six-well plates. After 24 h, the cells were transfected with 1 nM SMART pool c-Abl or nonspecific control pool siRNAs (Upstate Biotechnology Inc.) using LipofectAMINE. The cells were incubated for an additional 72 h and then harvested for analysis.
Subcellular fractionation
Nuclear fractions were prepared as described (Kharbanda et al, 1996). Cytosolic fractions were prepared as described (Hill et al, 2004). Cell membrane fractions were prepared with the Plasma Membrane Extraction Kit (BioVision Research Products). Mitochondria were purified as described (Ren et al, 2004, 2006).
Immunoprecipitations and immunoblot analysis
Cell lysates were prepared as described (Kharbanda et al, 1995b; Yoshida et al, 2005). Soluble proteins were incubated with anti-MUC1-N (Kufe et al, 1984), anti-MUC1-C (Ab5; Neomarker), anti-Flag (Sigma), anti-c-Abl (Ab3; Oncogene) or anti-14-3-3 (Abcam Inc.) for 2 h at 4°C, followed by precipitation with protein G-Sepharose beads for 1 h. Immune complexes and cell lysates (50 μg) were subjected to immunoblot analysis with anti-MUC1-N, anti-MUC1-C, anti-c-Abl (Calbiochem), anti-lamin B (Oncogene), anti-IκBα (Santa Cruz Biotechnology), anti-GFP (BD Biosciences), anti-Flag (Sigma), anti-β-actin (Sigma), anti-phospho-c-Abl-Thr-735 (Cell Signaling Technology), anti-14-3-3 (Santa Cruz Biotechnology), anti-PKCδ (Santa Cruz Biotechnology), anti-HSP60 (BD Biosciences), anti-PCNA (Oncogene) and anti-calnexin (Stressgen). The antigen–antibody complexes were visualized by chemiluminescence (NEN Life Science Products). Intensity of the signals was determined by densitometric scanning. Statistical significance was determined by the Student's t-test.
In vitro kinase assays
GST-c-Abl or GST-c-Abl(K-R) purified from baculovirus infected Sf9 cells (Yuan et al, 1999) were incubated in kinase buffer (50 mM HEPES, pH 7.4, 10 mM MgCl2, 10 mM MnCl2, 2 mM dithiothreitol, 0.1 mM sodium vanadate) with thrombin-cleaved and purified MUC1-CD or MUC1-CD(Y60F) and [γ-32P]ATP (NEN Life Science Products) for 15 min at 30°C. Reaction products were analyzed by SDS–PAGE and autoradiography.
Immunofluorescence microscopy
Cells cultured on coverslips were fixed in 3.7% formaldehyde, washed with PBS, permeabilized in PBS containing 0.2% Triton X-100 and then postfixed in 3.7% formaldehyde. The cells were blocked with 10% goat serum and stained with anti-MUC1-C, followed by FITC-conjugated secondary antibody. The cells were then incubated with anti-c-Abl (24-11; Santa Cruz Biotechnology), followed by Texas Red-goat anti-mouse Ig conjugate (Jackson ImmunoReasearch Laboratory). Nuclei were stained with 2 μM TO-PRO3 (Molecular Probes). Images were captured with a Zeiss LSM510 confocal microscope at 1024 × 1024 resolution.
Analysis of c-Abl kinase activity
Nuclear lysates were immunoprecipitated with anti-c-Abl (K-12) as described (Kharbanda et al, 1997). The precipitates were resuspended in kinase buffer (20 mM HEPES, pH 7.4, 10 mM MgCl2) containing [γ-32P]ATP, GST-Crk(120-225) for 20 min at 30°C. The reaction products were analyzed by SDS–PAGE and autoradiography.
Binding studies
Purified MUC1-CD and MUC1-CD(Y60F) were incubated in the absence and presence of recombinant truncated Abl (kinase domain; New England Biolabs) and 200 μM ATP for 30 min at 30°C. GST-c-Abl SH2 (Yoshida et al, 2002) or GST-c-Abl SH2(R152L) bound to glutathione beads was then added for 1 h at 4°C. After washing, the precipitated proteins were subjected to immunoblot analysis.
Assessment of apoptosis
DNA content was assessed by staining ethanol-fixed cells with propidium iodide and monitoring by FACScan (Becton Dickinson). The percentage of cells with sub-G1 DNA was determined by the MODFIT LT Program (Verity Software). Statistical, significance was determined using the unpaired Student's t-test and GraftPad software.
Supplementary Material
Supplementary Figure S1A
Supplementary Figure S2
Supplementary Figure S3A
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6A
Supplementary Figure S7A
Acknowledgments
This work was supported by Grants CA097098 and CA29431 awarded by the National Cancer Institute and Grant BC022158 awarded by the US Army. We acknowledge Michelle Ocana, Harvard Center for Neurodegeneration and Repair, for help with the confocal studies and Kamal Chauhan for technical support.
References
- Agami R, Blandino G, Oren M, Shaul Y (1999) Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature 399: 809–813 [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB (1996) Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 2: 561–566 [DOI] [PubMed] [Google Scholar]
- Emoto Y, Manome G, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong WW, Kamen R, Weichselbaum R, Kufe D (1995) Proteolytic activation of protein kinase Cδ by an ICE-like protease in apoptotic cells. EMBO J 14: 6148–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furstoss O, Dorey K, Simon V, Barila D, Superti-Furga G, Roche S (2002) c-Abl is an effector of Src for growth factor-induced c-myc expression and DNA synthesis. EMBO J 21: 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell JA (1988) A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem 263: 12820–12823 [PubMed] [Google Scholar]
- Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D (1996) Proteolytic activation of protein kinase Cδ by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med 184: 2399–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Costanzo A, Yang H, Melino G, Kaelin JR W, Levrero M, Wang JYJ (1999) The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399: 806–809 [DOI] [PubMed] [Google Scholar]
- Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ (2004) Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J 23: 2134–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D (2005) MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of β-catenin. Cancer Res 65: 10413–10422 [DOI] [PubMed] [Google Scholar]
- Huang L, Ren J, Chen D, Li Y, Kharbanda S, Kufe D (2003) MUC1 cytoplasmic domain coactivates Wnt target gene transcription and confers transformation. Cancer Biol Ther 2: 702–706 [PubMed] [Google Scholar]
- Kharbanda S, Pandey P, Jin S, Inoue S, Bharti A, Yuan Z-M, Weichselbaum R, Weaver D, Kufe D (1997) Functional interaction of DNA-PK and c-Abl in response to DNA damage. Nature 386: 732–735 [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Pandey P, Ren R, Feller S, Mayer B, Zon L, Kufe D (1995a) c-Abl activation regulates induction of the SEK1/stress activated protein kinase pathway in the cellular response to 1-β-D-arabinofuranosylcytosine. J Biol Chem 270: 30278–30281 [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Pandey P, Yamauchi T, Kumar S, Kaneki M, Kumar V, Bharti A, Yuan Z, Ghanem L, Rana A, Weichselbaum R, Johnson G, Kufe D (2000) Activation of MEK kinase-1 by the c-Abl protein tyrosine kinase in response to DNA-damaging agents. Mol Cell Biol 20: 4979–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda S, Ren R, Pandey P, Shafman TD, Feller SM, Weichselbaum RR, Kufe DW (1995b) Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376: 785–788 [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Saleem A, Yuan Z-M, Kraeft S, Weichselbaum R, Chen LB, Kufe D (1996) Nuclear signaling induced by ionizing radiation involves colocalization of the activated p56/p53lyn tyrosine kinase with p34cdc2. Cancer Res 56: 3617–3621 [PubMed] [Google Scholar]
- Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J (1984) Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma 3: 223–232 [DOI] [PubMed] [Google Scholar]
- Li Y, Bharti A, Chen D, Gong J, Kufe D (1998) Interaction of glycogen synthase kinase 3β with the DF3/MUC1 carcinoma-associated antigen and β-catenin. Mol Cell Biol 18: 7216–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kuwahara H, Ren J, Wen G, Kufe D (2001a) The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3β and β-catenin. J Biol Chem 276: 6061–6064 [DOI] [PubMed] [Google Scholar]
- Li Y, Liu D, Chen D, Kharbanda S, Kufe D (2003) Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene 22: 6107–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ren J, Yu W-H, Li G, Kuwahara H, Yin L, Carraway KL, Kufe D (2001b) The EGF receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and β-catenin. J Biol Chem 276: 35239–35242 [DOI] [PubMed] [Google Scholar]
- Ligtenberg MJ, Kruijshaar L, Buijs F, van Meijer M, Litvinov SV, Hilkens J (1992) Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem 267: 6171–6177 [PubMed] [Google Scholar]
- Merlo G, Siddiqui J, Cropp C, Liscia DS, Lidereau R, Callahan R, Kufe D (1989) DF3 tumor-associated antigen gene is located in a region on chromosome 1q frequently altered in primary human breast cancer. Cancer Res 49: 6966–6971 [PubMed] [Google Scholar]
- Muslin AJ, Xing H (2000) 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal 12: 703–709 [DOI] [PubMed] [Google Scholar]
- Plattner R, Kadlec L, DeMali K, Kazlauskas A, Pendergast A (1999) c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev 13: 2400–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina D, Kharbanda S, Kufe D (2004) The MUC1 oncoprotein activates the anti-apoptotic PI3K/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem 279: 20607–20612 [DOI] [PubMed] [Google Scholar]
- Raitano AB, Whang YE, Sawyers CL (1997) Signal transduction by wild-type and leukemogenic Abl proteins. Biochem Biophys Acta 1333: F201–F216 [DOI] [PubMed] [Google Scholar]
- Ren J, Agata N, Chen D, Li Y, Yu W-H, Huang L, Raina D, Chen W, Kharbanda S, Kufe D (2004) Human MUC1 carcinoma-associated protein confers resistance to genotoxic anti-cancer agents. Cancer Cell 5: 163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Bharti A, Raina D, Chen W, Ahmad R, Kufe D (2006) MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene 25: 20–31 [DOI] [PubMed] [Google Scholar]
- Ren J, Li Y, Kufe D (2002) Protein kinase C δ regulates function of the DF3/MUC1 carcinoma antigen in β-catenin signaling. J Biol Chem 277: 17616–17622 [DOI] [PubMed] [Google Scholar]
- Sanguinetti AR, Mastick CC (2003) c-Abl is required for oxidative stress-induced phosphorylation of caveolin-1 on tyrosine 14. Cell Signal 15: 289–298 [DOI] [PubMed] [Google Scholar]
- Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D (1988) Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci USA 85: 2320–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionov R, Coen S, Goldberg Z, Berger M, Bercovich B, Ben-Neriah Y, Ciechanover A (2001) c-Abl regulates p53 levels under normal and stress conditions by preventing its nuclear export and ubiquitination. Mol Cell Biol 21: 5869–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhao J, Schwartz MA, Wang JY, Wiedmer T, Sims PJ (2001) c-Abl tyrosine kinase binds and phosphorylates phospholipid scramblase 1. J Biol Chem 276: 28984–28990 [DOI] [PubMed] [Google Scholar]
- Taagepera S, McDonald D, Loeb J, Whitaker L, McElroy A, Wang J, Hope T (1998) Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci USA 95: 7457–7462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y (2004) JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J 23: 1889–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzivion G, Avruch J (2002) 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem 277: 3061–3064 [DOI] [PubMed] [Google Scholar]
- Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ (2003) Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422: 322–326 [DOI] [PubMed] [Google Scholar]
- Vigneri P, Wang JY (2001) Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR-ABL tyrosine kinase. Nat Med 7: 228–234 [DOI] [PubMed] [Google Scholar]
- Wei X, Xu H, Kufe D (2005) Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell 7: 167–178 [DOI] [PubMed] [Google Scholar]
- Wen S-T, Jackson PK, Van Etten RA (1996) The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J 15: 1583–1595 [PMC free article] [PubMed] [Google Scholar]
- Wetzler M, Talpaz M, Van Etten RA, Hirsh-Ginsberg C, Beran M, Kurzrock R (1993) Subcellular localization of Bcr, Abl, and Bcr-Abl proteins in normal and leukemic cells and correlation of expression with myeloid differentiation. J Clin Invest 92: 1925–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Bharti A, Li Y, Kufe D (1997) Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem 272: 12492–12494 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Komatsu K, Wang H-G, Kufe D (2002) c-Abl tyrosine kinase regulates the human Rad9 checkpoint protein in response to DNA damage. Mol Cell Biol 22: 3292–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Yamaguchi T, Natsume T, Kufe D, Miki Y (2005) JNK phosphorylation of 14-3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat Cell Biol 7: 278–285 [DOI] [PubMed] [Google Scholar]
- Yuan Z, Huang Y, Ishiko T, Kharbanda S, Weichselbaum R, Kufe D (1997) Regulation of DNA damage-induced apoptosis by the c-Abl tyrosine kinase. Proc Natl Acad Sci USA 94: 1437–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZM, Huang Y, Fan M, Sawers C, Kharbanda S, Kufe D (1996a) Genotoxic drugs induce interaction of the c-Abl tyrosine kinase and the tumor suppressor protein p53. J Biol Chem 271: 26457–26460 [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Huang Y, Whang Y, Sawyers C, Weichselbaum R, Kharbanda S, Kufe D (1996b) Role for the c-Abl tyrosine kinase in the growth arrest response to DNA damage. Nature 382: 272–274 [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Shioya H, Ishiko T, Sun X, Huang Y, Lu H, Kharbanda S, Weichselbaum R, Kufe D (1999) p73 is regulated by the c-Abl tyrosine kinase in the apoptotic response to DNA damage. Nature 399: 814–817 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1A
Supplementary Figure S2
Supplementary Figure S3A
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6A
Supplementary Figure S7A


