Abstract
Earlier studies from this laboratory provided evidence for restricted cytokine expression in the T cell population in RA tissues. Specifically, IL-2, IL-4, IL-6 and interferon-gamma (IFN-gamma) gene expression levels were low. The selective chemoattractant and activation effects of chemokines on leucocytes identify them as potentially ideal candidates in mediating selective inflammatory processes in RA. Accordingly, we undertook studies to examine constitutive chemokine gene expression in RA tissues. RANTES, monocyte chemotactic protein-1 (MCP-1) and MIP-1 beta gene expression was examined in both the T and non-T cell populations in RA peripheral blood (PB), synovial fluid (SF) and synovial tissues (ST). Our results identified elevated levels of both RANTES and MIP-1 beta gene expression in circulating RA PB and SF T cells. By contrast, MCP-1 expression was virtually absent in RA PB, yet elevated MCP-1 mRNA levels were detected primarily in the non-T cell populations of the SF and ST samples. Histological examination of affected rheumatoid joints revealed extensive RANTES and MIP-1 beta expression in sites of lymphocyte infiltration and cell proliferation, namely the synovial lining and sublining layers. Fractionation or RA ST patient samples revealed that RANTES expression was restricted to the T cells, whereas MIP-1 beta expression was detected in both T and non-T fractions. These data suggest that MCP-1, MIP-1 beta and RANTES may have a central role in the trafficking of reactive molecules involved in immunoregulation and in the inflammatory processes in RA.
Full text
PDF

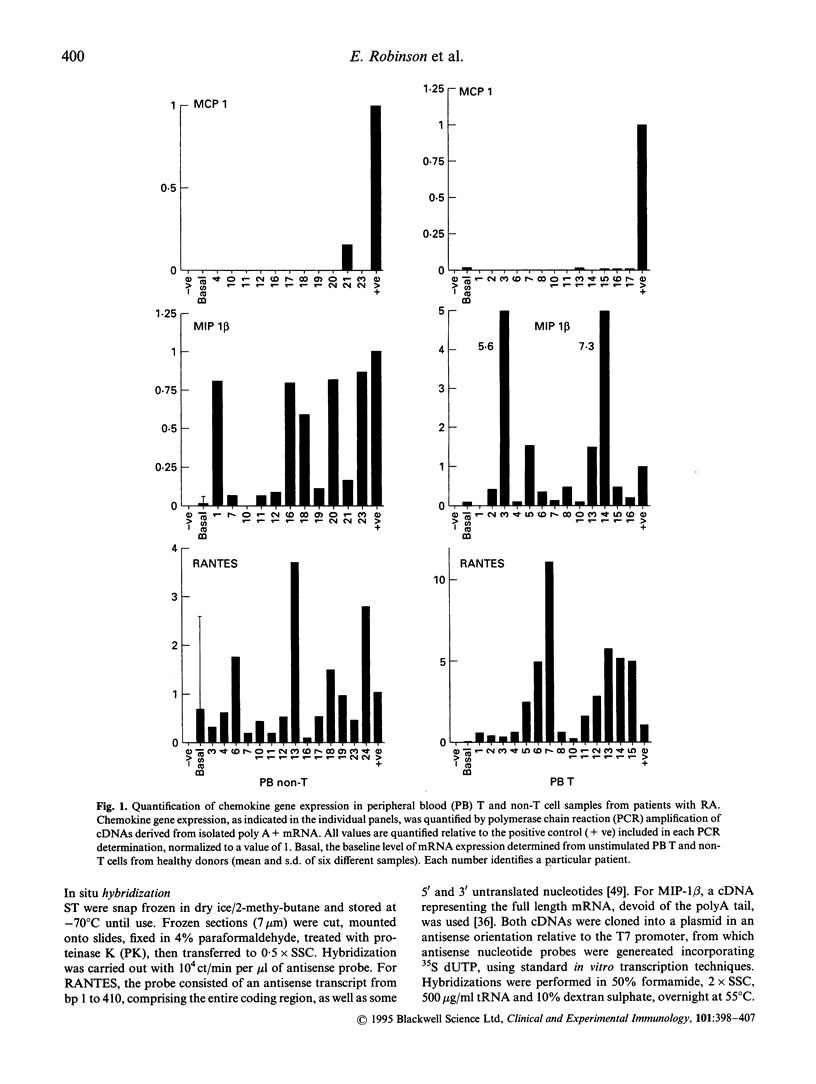
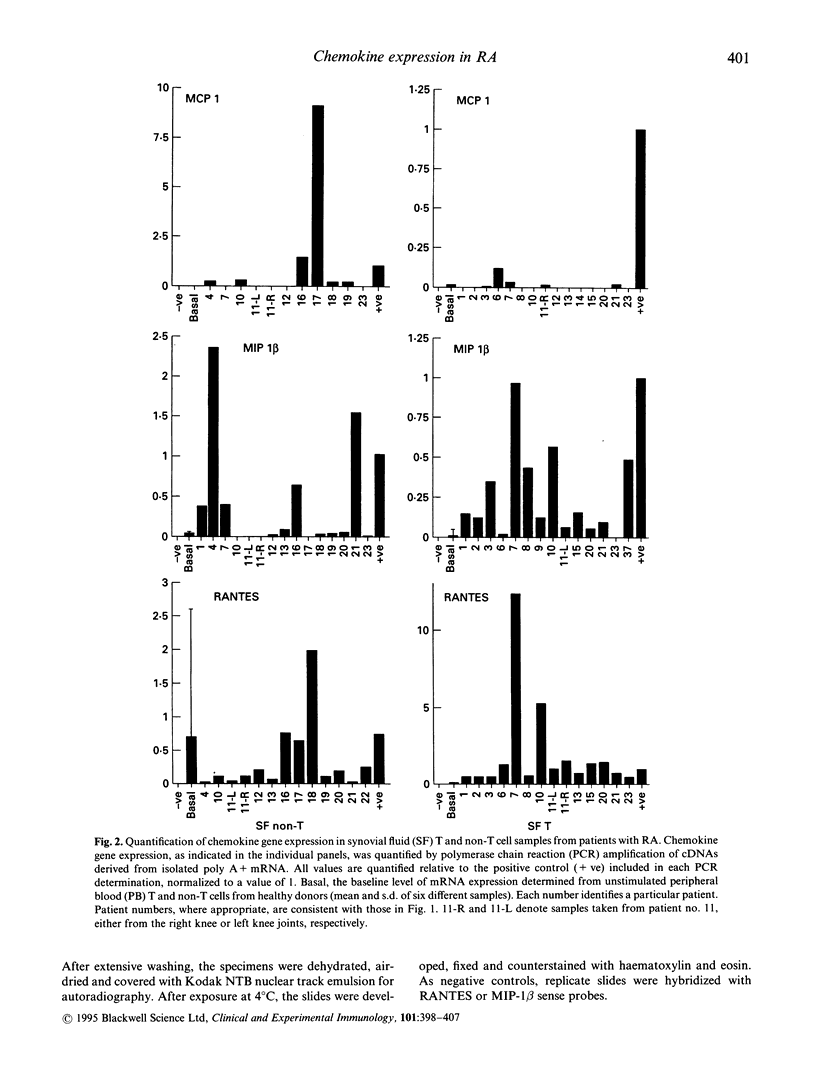
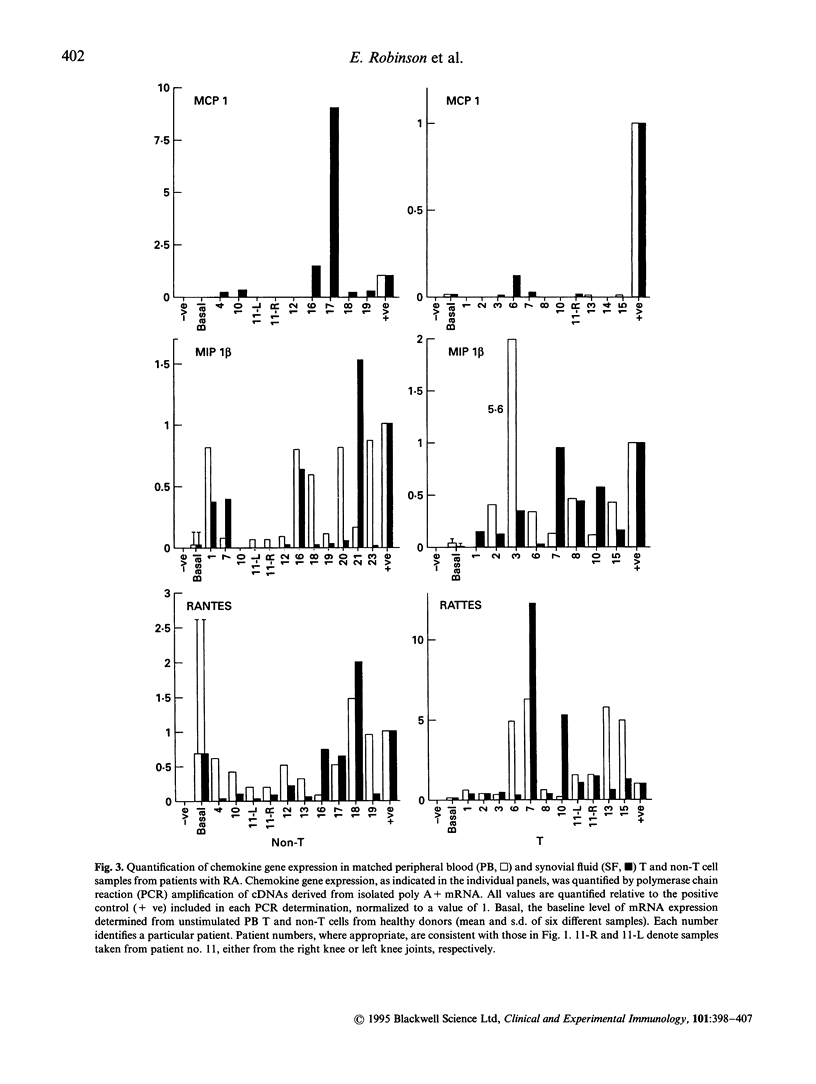
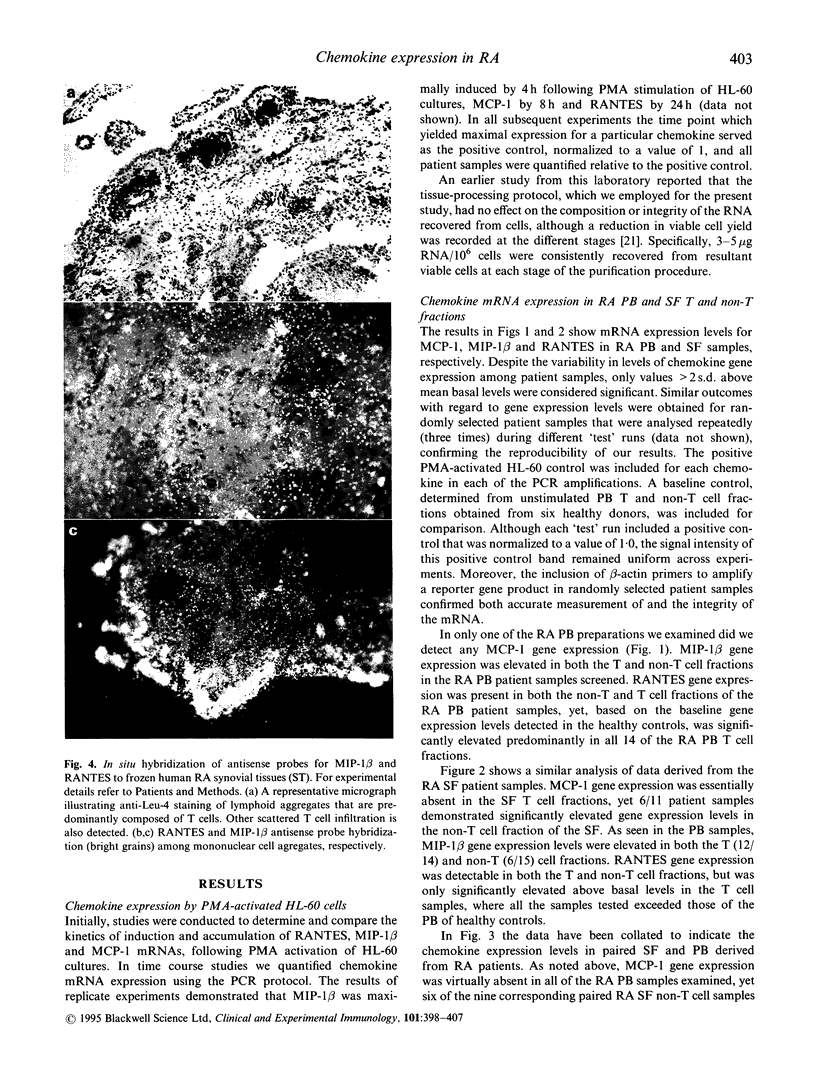
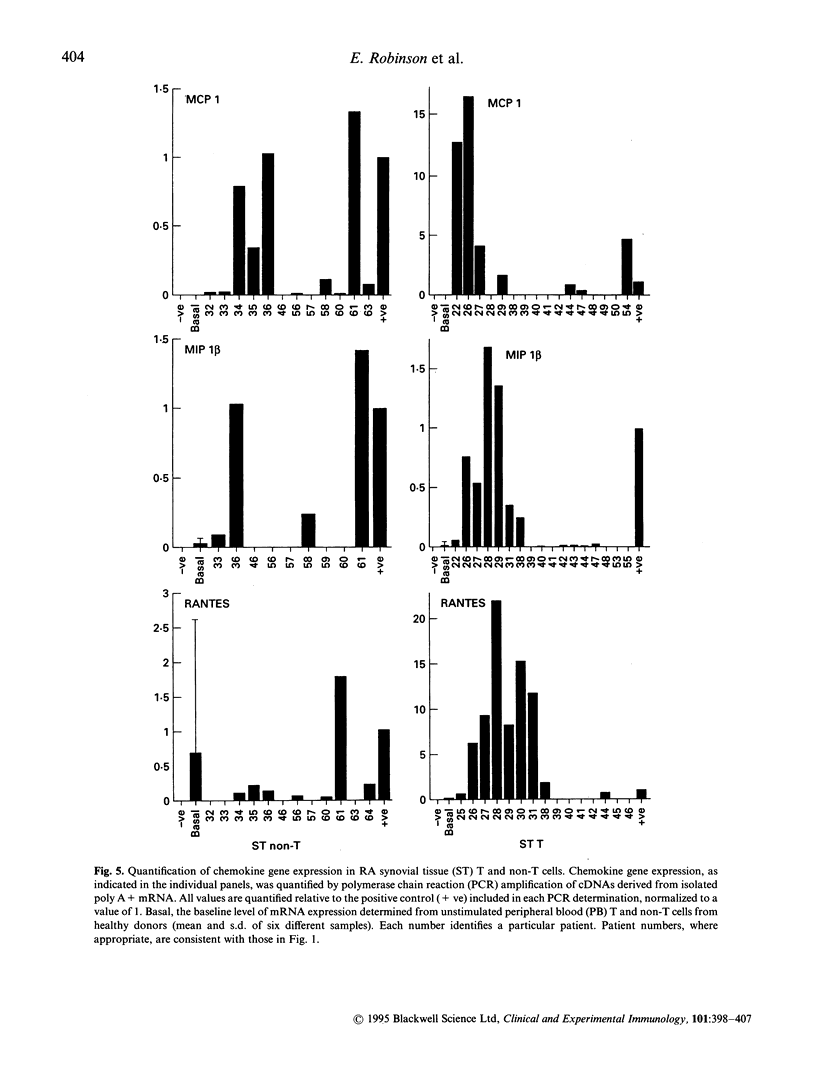


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahoshi T., Wada C., Endo H., Hirota K., Hosaka S., Takagishi K., Kondo H., Kashiwazaki S., Matsushima K. Expression of monocyte chemotactic and activating factor in rheumatoid arthritis. Regulation of its production in synovial cells by interleukin-1 and tumor necrosis factor. Arthritis Rheum. 1993 Jun;36(6):762–771. doi: 10.1002/art.1780360605. [DOI] [PubMed] [Google Scholar]
- Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990 Dec;86(6):1790–1798. doi: 10.1172/JCI114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Chen E., Keystone E. C., Fish E. N. Restricted cytokine expression in rheumatoid arthritis. Arthritis Rheum. 1993 Jul;36(7):901–910. doi: 10.1002/art.1780360706. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Allard S., Abney E., Feldmann M., Maini R. N. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol. 1992 Oct;31(10):653–661. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- Combe B., Pope R. M., Fischbach M., Darnell B., Baron S., Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985 Mar;59(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., Gentry K. C., Mackay I. R., Muirden K. D., Rowley M. Deficiency of the suppressor inducer subset of T lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1987 Aug;30(8):849–856. doi: 10.1002/art.1780300802. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Xu W. D., Townsend K., Broide D., Alvaro-Gracia J., Glasebrook A., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988 Nov 1;168(5):1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987 Aug;30(8):864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- Goto M., Miyamoto T., Nishioka K., Uchida S. T cytotoxic and helper cells are markedly increased, and T suppressor and inducer cells are markedly decreased, in rheumatoid synovial fluids. Arthritis Rheum. 1987 Jul;30(7):737–743. doi: 10.1002/art.1780300703. [DOI] [PubMed] [Google Scholar]
- Hachicha M., Rathanaswami P., Schall T. J., McColl S. R. Production of monocyte chemotactic protein-1 in human type B synoviocytes. Synergistic effect of tumor necrosis factor alpha and interferon-gamma. Arthritis Rheum. 1993 Jan;36(1):26–34. doi: 10.1002/art.1780360106. [DOI] [PubMed] [Google Scholar]
- Harigai M., Hara M., Yoshimura T., Leonard E. J., Inoue K., Kashiwazaki S. Monocyte chemoattractant protein-1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium. Clin Immunol Immunopathol. 1993 Oct;69(1):83–91. doi: 10.1006/clin.1993.1153. [DOI] [PubMed] [Google Scholar]
- Hertzog P. J., Emery P., Cheetham B. F., Mackay I. R., Linnane A. W. Interferons in rheumatoid arthritis: alterations in production and response related to disease activity. Clin Immunol Immunopathol. 1988 Aug;48(2):192–201. doi: 10.1016/0090-1229(88)90083-9. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988 Jul;73(1):88–92. [PMC free article] [PubMed] [Google Scholar]
- Husby G., Williams R. C., Jr Immunohistochemical studies of interleukin-2 and gamma-interferon in rheumatoid arthritis. Arthritis Rheum. 1985 Feb;28(2):174–181. doi: 10.1002/art.1780280212. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Beller D. I., Frendl G., Graves D. T. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992 Apr 15;148(8):2423–2428. [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Harlow L. A., Johnson B., Evanoff H. L., Haines G. K., Burdick M. D., Pope R. M., Strieter R. M. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992 Sep;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky H. P., Bauer K., Pope R. M. Increased helper inducer and decreased suppressor inducer phenotypes in the rheumatoid joint. Arthritis Rheum. 1988 Jan;31(1):52–59. doi: 10.1002/art.1780310108. [DOI] [PubMed] [Google Scholar]
- Lemmel E. M., Franke M., Gaus W., Hartl P. W., Hofschneider P. H., Miehlke K., Machalke K., Obert H. J. Results of a phase-II clinical trial on treatment of rheumatoid arthritis with recombinant interferon-gamma. Rheumatol Int. 1987;7(3):127–132. doi: 10.1007/BF00270465. [DOI] [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Naviliat M., Dupuy d'Angeac A., Sany J., Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor beta in rheumatoid synovitis. Arthritis Rheum. 1990 Aug;33(8):1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Hirano T., Nakagawa N., Yoshizaki K., Kishimoto T. Effect of recombinant IL 2 and gamma-IFN on proliferation and differentiation of human B cells. J Immunol. 1985 Feb;134(2):959–966. [PubMed] [Google Scholar]
- Nouri A. M., Panayi G. S. Cytokines and the chronic inflammation of rheumatic disease. III. Deficient interleukin-2 production in rheumatoid arthritis is not due to suppressor mechanisms. J Rheumatol. 1987 Oct;14(5):902–906. [PubMed] [Google Scholar]
- Postlethwaite A. E., Raghow R., Stricklin G. P., Poppleton H., Seyer J. M., Kang A. H. Modulation of fibroblast functions by interleukin 1: increased steady-state accumulation of type I procollagen messenger RNAs and stimulation of other functions but not chemotaxis by human recombinant interleukin 1 alpha and beta. J Cell Biol. 1988 Feb;106(2):311–318. doi: 10.1083/jcb.106.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathanaswami P., Hachicha M., Sadick M., Schall T. J., McColl S. R. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993 Mar 15;268(8):5834–5839. [PubMed] [Google Scholar]
- Rollins B. J., Walz A., Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991 Aug 15;78(4):1112–1116. [PubMed] [Google Scholar]
- Roth M. J., Tanese N., Goff S. P. Purification and characterization of murine retroviral reverse transcriptase expressed in Escherichia coli. J Biol Chem. 1985 Aug 5;260(16):9326–9335. [PubMed] [Google Scholar]
- Rubin L. A., Snow K. M., Kurman C. C., Nelson D. L., Keystone E. C. Serial levels of soluble interleukin 2 receptor in the peripheral blood of patients with rheumatoid arthritis: correlations with disease activity. J Rheumatol. 1990 May;17(5):597–602. [PubMed] [Google Scholar]
- Rème T., Portier M., Frayssinoux F., Combe B., Miossec P., Favier F., Sany J. T cell receptor expression and activation of synovial lymphocyte subsets in patients with rheumatoid arthritis. Phenotyping of multiple synovial sites. Arthritis Rheum. 1990 Apr;33(4):485–492. doi: 10.1002/art.1780330404. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Camp R. D., Kaspari J. W., Goeddel D. V. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993 Jun 1;177(6):1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J. Biology of the RANTES/SIS cytokine family. Cytokine. 1991 May;3(3):165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Jongstra J., Dyer B. J., Jorgensen J., Clayberger C., Davis M. M., Krensky A. M. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988 Aug 1;141(3):1018–1025. [PubMed] [Google Scholar]
- Schober I., Braun R., Reiser H., Munk K., Leroux M., Kirchner H. la-positive T lymphocytes are the producer cells of interferon gamma. Exp Cell Res. 1984 Jun;152(2):348–356. doi: 10.1016/0014-4827(84)90636-0. [DOI] [PubMed] [Google Scholar]
- Tabata H., Hara M., Kitani A., Hirose T., Norioka K., Harigai M., Suzuki K., Kawakami M., Kawagoe M., Nakamura H. Expression of TLiSA1 on T cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Immunol Immunopathol. 1989 Sep;52(3):366–375. doi: 10.1016/0090-1229(89)90151-7. [DOI] [PubMed] [Google Scholar]
- Talal N., Flescher E. Rheumatoid arthritis: an editorial perspective based on cytokine imbalance. J Autoimmun. 1988 Aug;1(4):309–317. doi: 10.1016/0896-8411(88)90001-7. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Conlon K., Lloyd A. R., Oppenheim J. J., Kelvin D. J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993 Apr 16;260(5106):355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Varga J., Jimenez S. A. Stimulation of normal human fibroblast collagen production and processing by transforming growth factor-beta. Biochem Biophys Res Commun. 1986 Jul 31;138(2):974–980. doi: 10.1016/s0006-291x(86)80591-5. [DOI] [PubMed] [Google Scholar]
- Villiger P. M., Terkeltaub R., Lotz M. Monocyte chemoattractant protein-1 (MCP-1) expression in human articular cartilage. Induction by peptide regulatory factors and differential effects of dexamethasone and retinoic acid. J Clin Invest. 1992 Aug;90(2):488–496. doi: 10.1172/JCI115885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger P. M., Terkeltaub R., Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992 Jul 15;149(2):722–727. [PubMed] [Google Scholar]
- Yamagata N., Kobayashi K., Kasama T., Fukushima T., Tabata M., Yoneya I., Shikama Y., Kaga S., Hashimoto M., Yoshida K. Multiple cytokine activities and loss of interleukin 2 inhibitor in synovial fluids of patients with rheumatoid arthritis. J Rheumatol. 1988 Nov;15(11):1623–1627. [PubMed] [Google Scholar]
- Yocum D. E., Esparza L., Dubry S., Benjamin J. B., Volz R., Scuderi P. Characteristics of tumor necrosis factor production in rheumatoid arthritis. Cell Immunol. 1989 Aug;122(1):131–145. doi: 10.1016/0008-8749(89)90154-8. [DOI] [PubMed] [Google Scholar]
- Zachariae C. O., Anderson A. O., Thompson H. L., Appella E., Mantovani A., Oppenheim J. J., Matsushima K. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990 Jun 1;171(6):2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]