Abstract
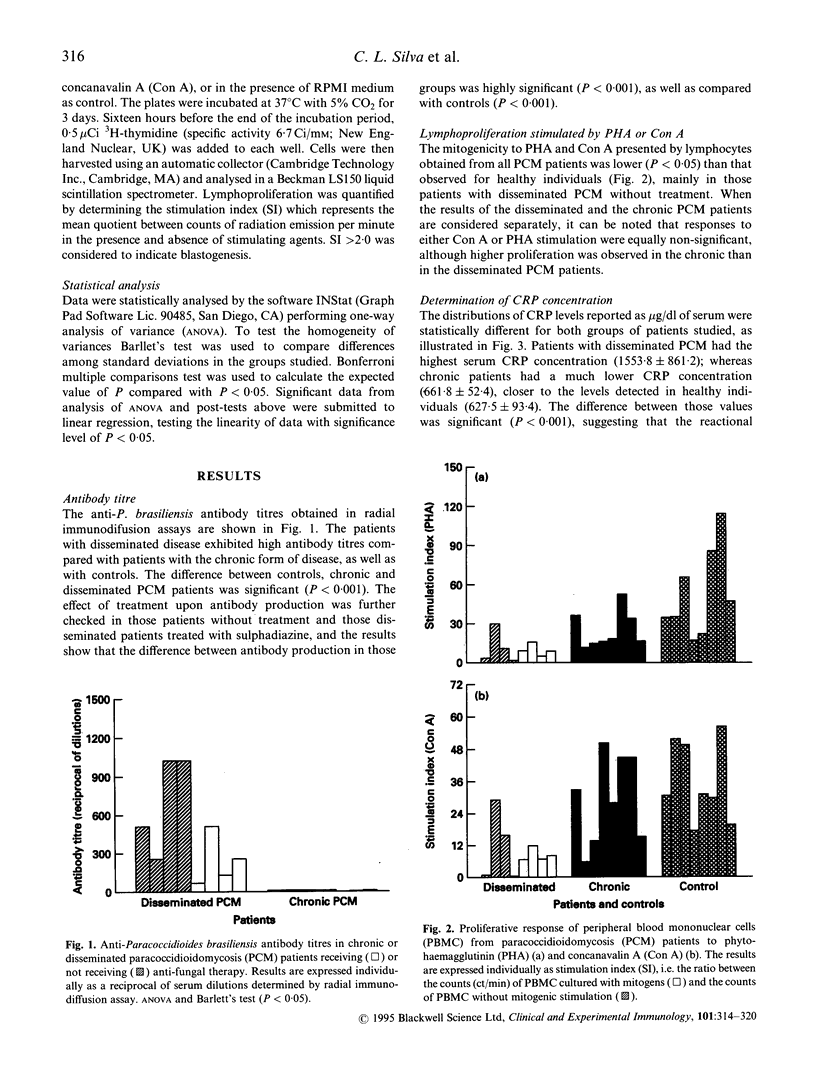
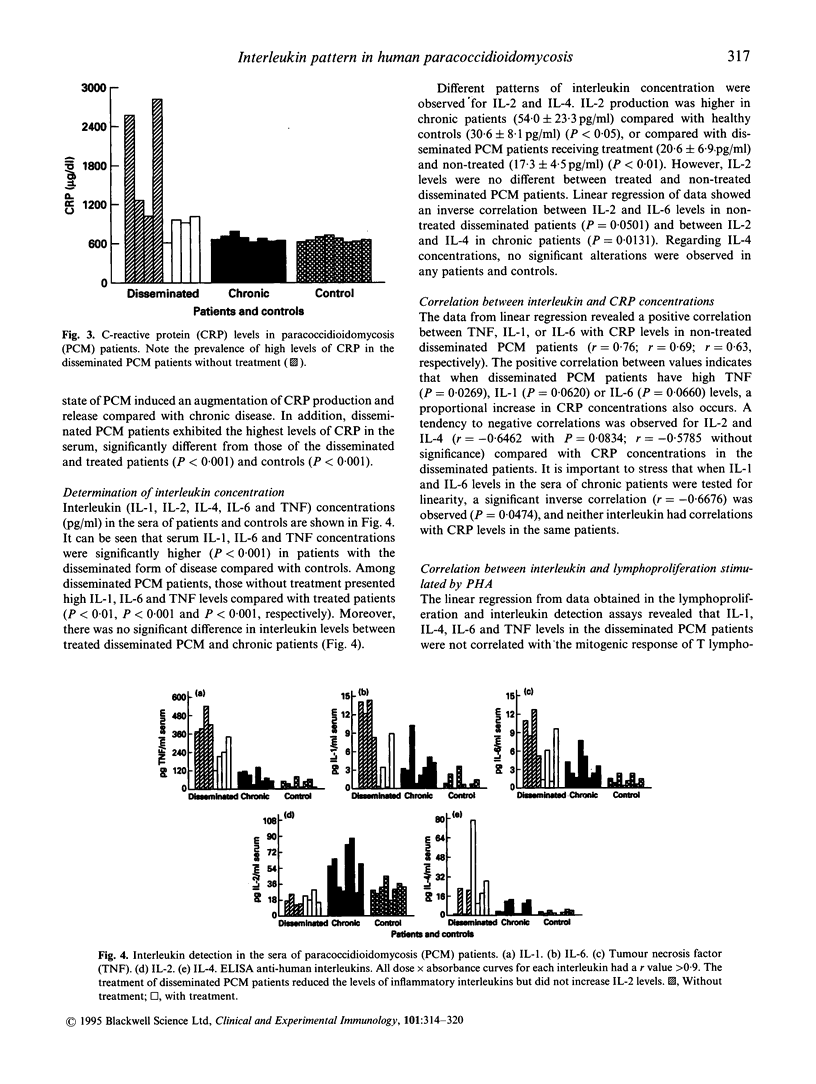
In an attempt to understand better the immunoregulatory disorders in paracoccidioidomycosis (PCM), the possible correlation between interleukin pattern, lymphoproliferation, C-reactive protein (CRP) and specific antibody levels was investigated in the polarized clinical forms of this disease. We studied 16 PCM patients, eight with the disseminated disease (four under treatment and four non-treated) and eight with the chronic disease. The patients with disseminated disease exhibited high antibody titres specific to Paracoccidioides brasiliensis antigen compared with patients with the chronic form of disease. Tumour necrosis factor (TNF), IL-1, IL-6 and CRP in the serum of non-treated disseminated PCM patients were increased, which correlated positively with the low mitogenic response of peripheral blood mononuclear cells (PBMC) to phytohaemagglutinin (PHA) (P < 0.01) and with the high antibody titres (P < 0.001) of these patients. Moreover, we found in the disseminated PCM patients positive correlations between IL-1 and IL-6 (P = 0.0007); IL-1 and TNF (P = 0.0045); IL-1 and IL-6 with the high antibody titres (P = 0.0834 and P = 0.0631, respectively); IL-1, IL-6 and TNF with CRP levels. By contrast, no correlations were found with those interleukins in the treated disseminated and chronic patients or in controls. It was interesting to find an inverse correlation between IL-4 and antibody production in non-treated disseminated PCM (r = -0.4770); moreover, a significant correlation (P = 0.0820) was found in chronic PCM patients with respect to the low level of either IL-4 and antibody titres against fungus antigen. Chronic PCM patients also had IL-2 levels inversely correlated with antibody production (r = -0.6313; P = 0.0628). Inverse correlations were also observed between IL-2 and IL-6 levels in non-treated disseminated patients (P = 0.0501) and between IL-2 and IL-4 in chronic patients (P = 0.0131). The inflammatory cytokines might have a pivotal role in the genesis and in control of some aspects of the disease, such as granulomatous reaction, hypergammaglobulinaemia and depression of T cell-mediated immunity in PCM.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Geiger T., Hirano T., Kishimoto T., Heinrich P. C. Action of recombinant human interleukin 6, interleukin 1 beta and tumor necrosis factor alpha on the mRNA induction of acute-phase proteins. Eur J Immunol. 1988 May;18(5):739–746. doi: 10.1002/eji.1830180513. [DOI] [PubMed] [Google Scholar]
- Angela Restrepo M. Immune response to Paracoccidioides brasiliensis in human and animal hosts. Curr Top Med Mycol. 1988;2:239–277. doi: 10.1007/978-1-4612-3730-3_7. [DOI] [PubMed] [Google Scholar]
- Chequer-Bou-Habib D., Daniel-Ribeiro C., Banic D. M., do Valle A. C., Galvao-Castro B. Polyclonal B cell activation in paracoccidioidomycosis. Polyclonal activation in paracoccidioidomycosis. Mycopathologia. 1989 Nov;108(2):89–93. doi: 10.1007/BF00436058. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Crott L. S., Valim Y. M., Silva C. L., Barbosa J. E. The role of the complement system in the neutrophil functions stimulated in vitro by an alkali-insoluble cell wall fraction of Paracoccidioides brasiliensis. J Med Vet Mycol. 1993;31(1):17–27. [PubMed] [Google Scholar]
- Cáceres-Dittmar G., Tapia F. J., Sánchez M. A., Yamamura M., Uyemura K., Modlin R. L., Bloom B. R., Convit J. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin Exp Immunol. 1993 Mar;91(3):500–505. doi: 10.1111/j.1365-2249.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccioli L. H., Souza G. E., Cunha F. Q., Poole S., Ferreira S. H. Recombinant interleukin-1 and tumor necrosis factor induce neutrophil migration "in vivo" by indirect mechanisms. Agents Actions. 1990 Jun;30(3-4):344–349. doi: 10.1007/BF01966298. [DOI] [PubMed] [Google Scholar]
- Figueiredo F., Alves L. M., Silva C. L. Tumour necrosis factor production in vivo and in vitro in response to Paracoccidioides brasiliensis and the cell wall fractions thereof. Clin Exp Immunol. 1993 Aug;93(2):189–194. doi: 10.1111/j.1365-2249.1993.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss N. T., de Oliveira E. B., Silva C. L. Correlation between TNF production, increase of plasma C-reactive protein level and suppression of T lymphocyte response to concanavalin A during erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1993 Jun;61(2):218–226. [PubMed] [Google Scholar]
- Franco M., Montenegro M. R., Mendes R. P., Marques S. A., Dillon N. L., Mota N. G. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev Soc Bras Med Trop. 1987 Apr-Jun;20(2):129–132. doi: 10.1590/s0037-86821987000200012. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C. C-reactive protein. A historical overview. Ann N Y Acad Sci. 1989;557:9–18. [PubMed] [Google Scholar]
- Hostetler J. S., Brummer E., Coffman R. L., Stevens D. A. Effect of anti-IL-4, interferon-gamma and an antifungal triazole (SCH 42427) in paracoccidioidomycosis: correlation of IgE levels with outcome. Clin Exp Immunol. 1993 Oct;94(1):11–16. doi: 10.1111/j.1365-2249.1993.tb05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. H., Volanakis J. E. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974 Jun;112(6):2135–2147. [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Kushner I., Ganapathi M., Schultz D. The acute phase response is mediated by heterogeneous mechanisms. Ann N Y Acad Sci. 1989;557:19–30. doi: 10.1111/j.1749-6632.1989.tb23996.x. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Musatti C. C., Rezkallah M. T., Mendes E., Mendes N. F. In vivo and in vitro evaluation of cell-mediated immunity in patients with paracoccidiodomycosis. Cell Immunol. 1976 Jun 15;24(2):365–378. doi: 10.1016/0008-8749(76)90220-3. [DOI] [PubMed] [Google Scholar]
- Parronchi P., Macchia D., Piccinni M. P., Biswas P., Simonelli C., Maggi E., Ricci M., Ansari A. A., Romagnani S. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4538–4542. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Blas G. Paracoccidioidomycosis and its etiologic agent Paracoccidioides brasiliensis. J Med Vet Mycol. 1993;31(2):99–113. [PubMed] [Google Scholar]
- Severo L. C., Geyer G. R., Londero A. T., Porto N. S., Rizzon C. F. The primary pulmonary lymph node complex in paracoccidioidomycosis. Mycopathologia. 1979 May 31;67(2):115–118. doi: 10.1007/BF00440683. [DOI] [PubMed] [Google Scholar]
- Silva C. L., Alves L. M., Figueiredo F. Involvement of cell wall glucans in the genesis and persistence of the inflammatory reaction caused by the fungus Paracoccidioides brasiliensis. Microbiology. 1994 May;140(Pt 5):1189–1194. doi: 10.1099/13500872-140-5-1189. [DOI] [PubMed] [Google Scholar]
- Silva C. L., Fazioli R. A. A Paracoccidioides brasiliensis polysaccharide having granuloma-inducing, toxic and macrophage-stimulating activity. J Gen Microbiol. 1985 Jun;131(6):1497–1501. doi: 10.1099/00221287-131-6-1497. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Yokota S., Vilcek J., Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1094–1100. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Kimball K., Okusawa S., Vachino G., Margolis N., Sohn J. W., Li J. J., Wakabayashi G., McAdam K., Burke J. F. Cytokines, acute phase proteins, and tissue injury. C-reactive protein opsonizes dead cells for debridement and stimulates cytokine production. Ann N Y Acad Sci. 1990;587:351–361. doi: 10.1111/j.1749-6632.1990.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Zahedi K., Tebo J. M., Siripont J., Klimo G. F., Mortensen R. F. Binding of human C-reactive protein to mouse macrophages is mediated by distinct receptors. J Immunol. 1989 Apr 1;142(7):2384–2392. [PubMed] [Google Scholar]
- de Brito T., Franco M. F. Granulomatous inflammation. Rev Inst Med Trop Sao Paulo. 1994 Mar-Apr;36(2):185–192. doi: 10.1590/s0036-46651994000200016. [DOI] [PubMed] [Google Scholar]
- de Oliveira S. L., da Silva M. F., Soares A. M., Silva C. L. Cell wall fractions from Paracoccidioides brasiliensis induce hypergammaglobulinemia. Mycopathologia. 1993 Jan;121(1):1–5. doi: 10.1007/BF01103346. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]


