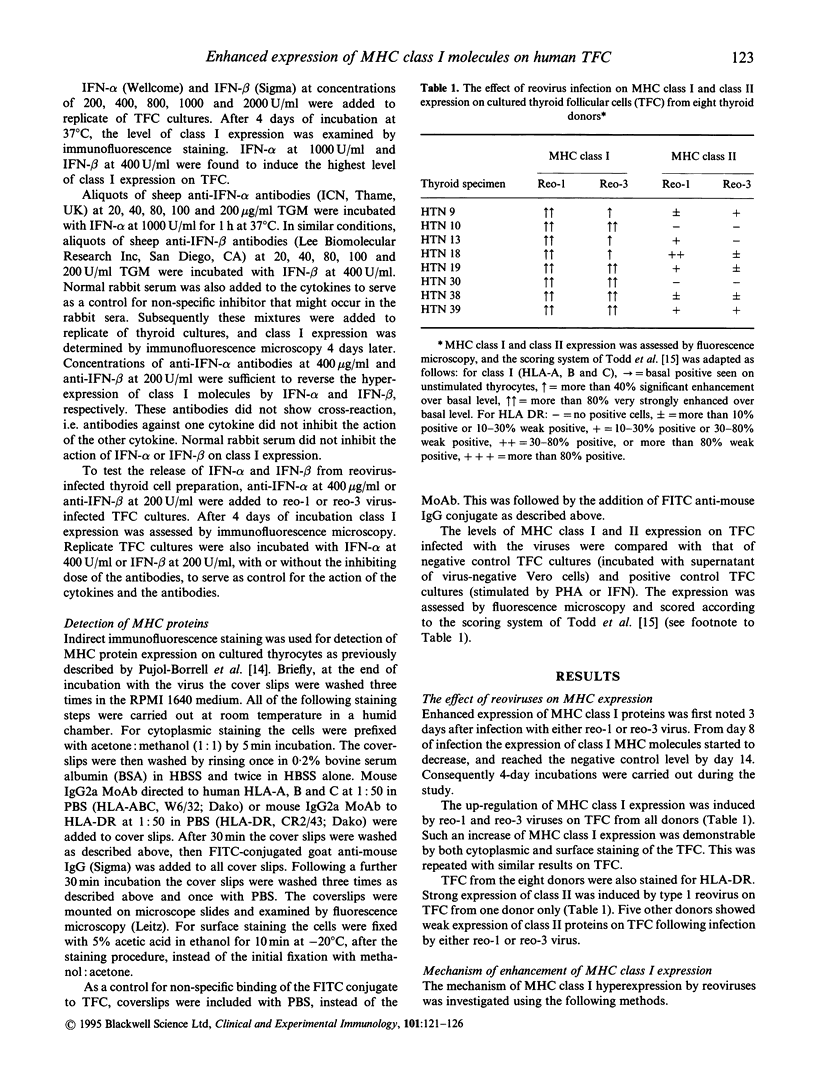
Abstract
Certain viruses are known to modulate the cellular expression of MHC molecules. We have investigated whether reovirus types 1 or 3 can alter the normal MHC molecule expression on cultured human thyroid follicular cells (TFC). Primary TFC cultures were established from eight human thyroid donors and MHC class I and II expression was assessed by indirect immunofluorescence microscopy. Both types of reovirus enhanced MHC class I expression on TFC from all thyroid donors. Class II MHC protein was strongly induced by type 1 reovirus on TFC from one donor, while weak induction of expression, by either reo-1 or reo-3 virus, was noted on the TFC of five other donors. Studies on the mechanism(s) of MHC class I hyperexpression showed that mouse MoAb against the type 3 reovirus haemagglutinin (anti-HA3) reduced the ability of the virus to induce hyperexpression of class I MHC molecules on TFC. However, supernatant harvested from type 3 reovirus-infected TFC cultures maintained its ability to enhance class I expression after incubation with anti-HA3. Moreover, adding rabbit anti-sera to interferon-alpha (IFN-alpha) or IFN-beta inhibited the increased class I MHC expression on TFC by both types of reovirus. These data suggest that reoviruses (types 1 and 3) can enhance MHC class I on cultured TFC. The mechanism of MHC class I enhancement is most probably through the release of IFN-alpha and IFN-beta.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco M., Caretto A., Olive D., Pedini B., Canonica G. W., Betterle C. Expression of intercellular adhesion molecule-1 (ICAM-1) on thyroid epithelial cells in Hashimoto's thyroiditis but not in Graves' disease or papillary thyroid cancer. Clin Exp Immunol. 1991 Feb;83(2):309–313. doi: 10.1111/j.1365-2249.1991.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. L., Harrison L. C., Ashcroft R. G., Jack I. Reovirus infection enhances expression of class I MHC proteins on human beta-cell and rat RINm5F cell. Diabetes. 1988 Mar;37(3):362–365. doi: 10.2337/diab.37.3.362. [DOI] [PubMed] [Google Scholar]
- Ciampolillo A., Napolitano G., Mirakian R., Miyasaki A., Giorgino R., Bottazzo G. F. Intercellular adhesion molecule-1 (ICAM-1) in Graves' disease: contrast between in vivo and in vitro results. Clin Exp Immunol. 1993 Dec;94(3):478–485. doi: 10.1111/j.1365-2249.1993.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. J., Barker R. N., Thompson S. J., Williams N. A. Immunologically ignorant autoreactive T cells, epitope spreading and repertoire limitation. Immunol Today. 1995 Feb;16(2):71–76. doi: 10.1016/0167-5699(95)80091-3. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. The immune response to Moloney murine leukemia virus-induced tumors: induction of cytolytic T lymphocytes specific for both viral and tumor-associated antigens. J Immunol. 1986 Dec 15;137(12):3968–3972. [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A., Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987 May;30(5):333–343. doi: 10.1007/BF00299027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A., Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987 Dec 19;2(8573):1423–1427. doi: 10.1016/s0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Stein M. E., Safko B., Stadecker M. J. Direct induction of Ia antigen on murine thyroid-derived epithelial cells by reovirus. J Immunol. 1989 Jun 1;142(11):3821–3825. [PubMed] [Google Scholar]
- Guerin V., Todd I., Hammond L. J., Bottazzo G. F. Suppression of HLA class II expression on thyrocytes by interferon-alpha 1. Clin Exp Immunol. 1990 Mar;79(3):341–345. doi: 10.1111/j.1365-2249.1990.tb08093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Hardcastle J. D., Farrands P. A., Balfour T. W., Chamberlain J., Amar S. S., Sheldon M. G. Controlled trial of faecal occult blood testing in the detection of colorectal cancer. Lancet. 1983 Jul 2;2(8340):1–4. doi: 10.1016/s0140-6736(83)90001-6. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Campbell I. L., Allison J., Miller J. F. MHC molecules and beta-cell destruction. Immune and nonimmune mechanisms. Diabetes. 1989 Jul;38(7):815–818. doi: 10.2337/diab.38.7.815. [DOI] [PubMed] [Google Scholar]
- Huang X., Hultgren B., Dybdal N., Stewart T. A. Islet expression of interferon-alpha precedes diabetes in both the BB rat and streptozotocin-treated mice. Immunity. 1994 Sep;1(6):469–478. doi: 10.1016/1074-7613(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Lai M. H., Joklik W. K. The induction of interferon by temperature-sensitive mutants of reovirus, UV-irradiated reovirus, and subviral reovirus particles. Virology. 1973 Jan;51(1):191–204. doi: 10.1016/0042-6822(73)90379-6. [DOI] [PubMed] [Google Scholar]
- Ling P. D., Warren M. K., Vogel S. N. Antagonistic effect of interferon-beta on the interferon-gamma-induced expression of Ia antigen in murine macrophages. J Immunol. 1985 Sep;135(3):1857–1863. [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Long W. F., Burke D. C. Interferon production by double-stranded RNA: a comparison of induction by reovirus to that by a synthetic double-stranded polynucleotide. J Gen Virol. 1971 Jul;12(1):1–11. doi: 10.1099/0022-1317-12-1-1. [DOI] [PubMed] [Google Scholar]
- Madaio M. P., Adler S., Groggel G. C., Couser W. G., Salant D. J. Charge selective properties of the glomerular capillary wall influence antibody binding in rat membranous nephropathy. Clin Immunol Immunopathol. 1986 Apr;39(1):131–138. doi: 10.1016/0090-1229(86)90212-6. [DOI] [PubMed] [Google Scholar]
- Matsunaga M., Eguchi K., Fukuda T., Kurata A., Tezuka H., Shimomura C., Otsubo T., Ishikawa N., Ito K., Nagataki S. Class II major histocompatibility complex antigen expression and cellular interactions in thyroid glands of Graves' disease. J Clin Endocrinol Metab. 1986 Apr;62(4):723–728. doi: 10.1210/jcem-62-4-723. [DOI] [PubMed] [Google Scholar]
- Neufeld D. S., Platzer M., Davies T. F. Reovirus induction of MHC class II antigen in rat thyroid cells. Endocrinology. 1989 Jan;124(1):543–545. doi: 10.1210/endo-124-1-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T., Awaya A. Anti-thyroglobulin antibodies induced with recombinant reovirus infection in BALB/c mice. Immunology. 1990 Dec;71(4):581–585. [PMC free article] [PubMed] [Google Scholar]
- Onodera T., Jenson A. B., Yoon J. W., Notkins A. L. Virus-induced diabetes mellitus: reovirus infection of pancreatic beta cells in mice. Science. 1978 Aug 11;201(4355):529–531. doi: 10.1126/science.208156. [DOI] [PubMed] [Google Scholar]
- Onodera T., Toniolo A., Ray U. R., Jenson A. B., Knazek R. A., Notkins A. L. Virus-induced diabetes mellitus. XX. Polyendocrinopathy and autoimmunity. J Exp Med. 1981 Jun 1;153(6):1457–1473. doi: 10.1084/jem.153.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasappa J., Garzelli C., Onodera T., Ray U., Notkins A. L. Virus-induced thyroiditis. Endocrinology. 1988 Feb;122(2):563–566. doi: 10.1210/endo-122-2-563. [DOI] [PubMed] [Google Scholar]
- Tandon N., Metcalfe R. A., Barnett D., Weetman A. P. Expression of the costimulatory molecule B7/BB1 in autoimmune thyroid disease. Q J Med. 1994 Apr;87(4):231–236. [PubMed] [Google Scholar]
- Tandon N., Weetman A. P. Thyroid cells in Graves' disease and Hashimoto's thyroiditis stimulate allogeneic T cells when pretreated with phorbol ester. Clin Endocrinol (Oxf) 1992 Sep;37(3):274–281. doi: 10.1111/j.1365-2265.1992.tb02322.x. [DOI] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Makgoba M. W., Borysiewicz L. K. Expression of an intercellular adhesion molecule, ICAM-1, by human thyroid cells. J Endocrinol. 1989 Jul;122(1):185–191. doi: 10.1677/joe.0.1220185. [DOI] [PubMed] [Google Scholar]


