Abstract
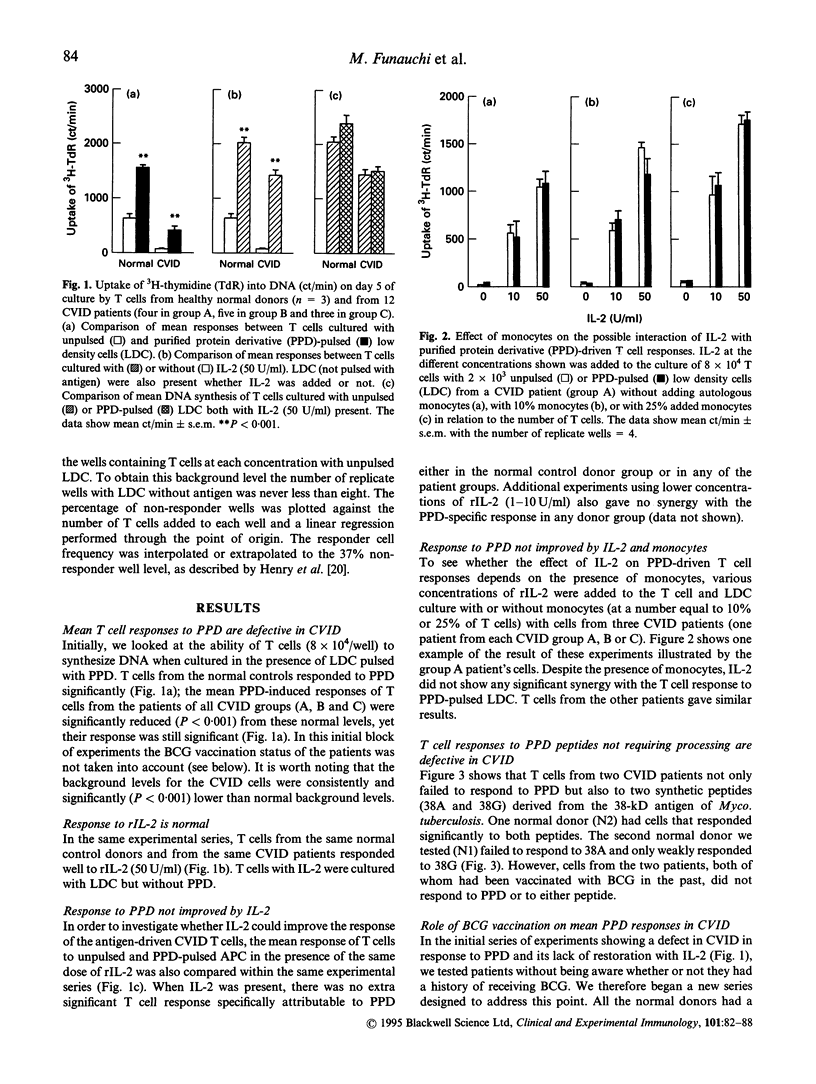
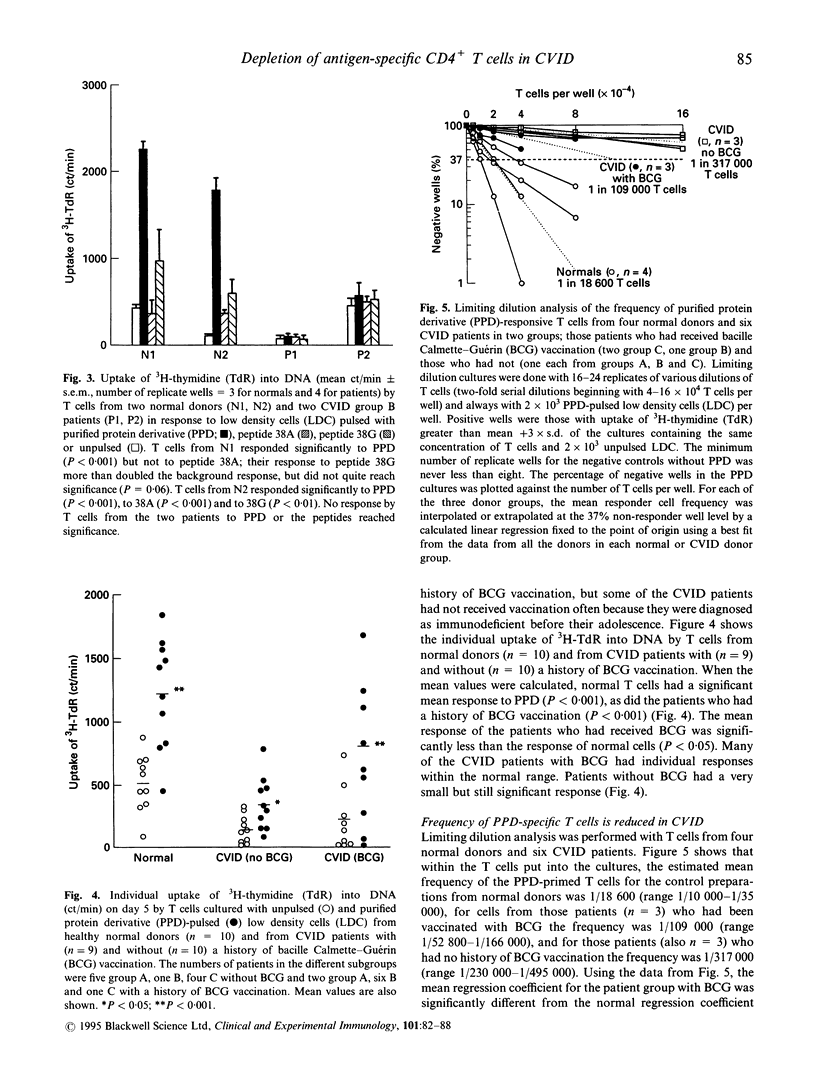
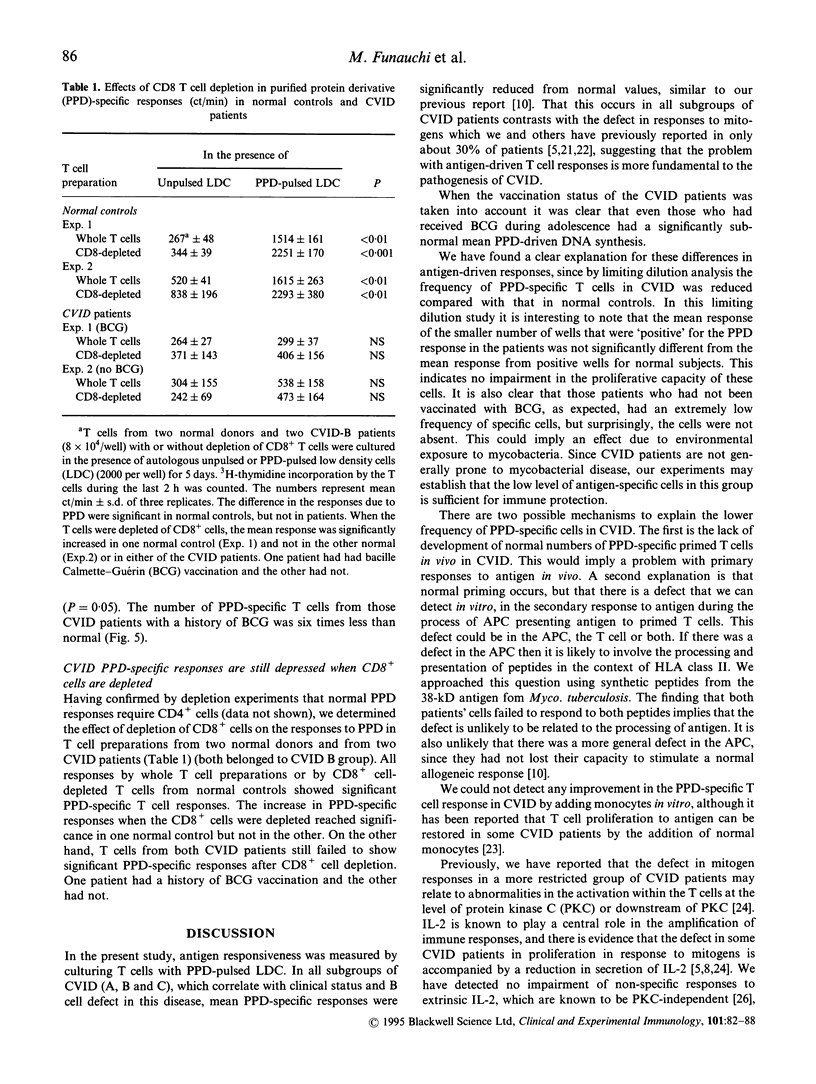
T cells from patients with CVID have defects that may relate to the failure in vivo of B cell production of antibodies. Antigen-driven responses of T cells from CVID patients and normal subjects have been assessed by measuring DNA synthesis in vitro. Low density cells enriched for antigen-presenting dendritic cells were pulsed with purified protein derivative (PPD) and cultured with autologous T cells. Overall, T cells from CVID patients showed a significantly low mean response to PPD, although non-specific DNA synthesis induced in CVID T cells by IL-2 was within the normal range. However, mean PPD-specific T cell responses in CVID were not restored by IL-2 irrespective of the presence of monocytes. Depletion of CD8+ cells also failed to restore the mean PPD response of CVID CD4+ T cells. Limiting dilution analysis showed that in CVID there was a reduced frequency of antigen-specific cells within the T cell preparations. The mean frequency of the PPD-specific T cells in cultures from patients vaccinated with bacille Calmette-Guérin (BCG) was reduced to 1 in 109,000 T cells compared with 1 in 18,600 T cells in BCG-vaccinated normal donors. These data show that the reduced PPD-specific response in CVID is due to a partial peripheral loss of antigen-specific cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverly B., Kang S. M., Lenardo M. J., Schwartz R. H. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992 Jun;4(6):661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- Bryant A., Calver N. C., Toubi E., Webster A. D., Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990 Aug;56(2):239–248. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol. 1989 Jan;9(1):22–33. doi: 10.1007/BF00917124. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C., Mayer L., Sapira E., Mendelsohn L. Restoration of immunoglobulin secretion in vitro in common variable immunodeficiency by in vivo treatment with polyethylene glycol-conjugated human recombinant interleukin-2. Clin Immunol Immunopathol. 1992 Jul;64(1):46–56. doi: 10.1016/0090-1229(92)90058-v. [DOI] [PubMed] [Google Scholar]
- Eibl M. M., Mannhalter J. W., Zlabinger G., Mayr W. R., Tilz G. P., Ahmad R., Zielinski C. C. Defective macrophage function in a patient with common variable immunodeficiency. N Engl J Med. 1982 Sep 23;307(13):803–806. doi: 10.1056/NEJM198209233071307. [DOI] [PubMed] [Google Scholar]
- Farrant J., Clark J. C., Lee H., Knight S. C., O'Brien J. Conditions for measuring DNA synthesis in PHA stimulated human lymphocytes in 20 microliters hanging drops with various cell concentrations and periods of culture. J Immunol Methods. 1980;33(4):301–312. doi: 10.1016/0022-1759(80)90001-0. [DOI] [PubMed] [Google Scholar]
- Farrant J., Spickett G., Matamoros N., Copas D., Hernandez M., North M., Chapel H., Webster A. D. Study of B and T cell phenotypes in blood from patients with common variable immunodeficiency (CVID). Immunodeficiency. 1994;5(2):159–169. [PubMed] [Google Scholar]
- Fischer M. B., Wolf H. M., Eggenbauer H., Thon V., Vogel E., Lokaj J., Litzman J., Mannhalter J. W., Eibl M. M. The costimulatory signal CD28 is fully functional but cannot correct the impaired antigen response in T cells of patients with common variable immunodeficiency. Clin Exp Immunol. 1994 Feb;95(2):209–214. doi: 10.1111/j.1365-2249.1994.tb06512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe J. S., Eisenstein E., Sneller M. C., Strober W. T-cell abnormalities in common variable immunodeficiency. Pediatr Res. 1993 Jan;33(1 Suppl):S24–S28. doi: 10.1203/00006450-199305001-00128. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Kruger G., Welte K., Ciobanu N., Cunningham-Rundles C., Ralph P., Venuta S., Feldman S., Koziner B., Wang C. Y., Moore M. A. Interleukin-2 correction of defective in vitro T-cell mitogenesis in patients with common varied immunodeficiency. J Clin Immunol. 1984 Jul;4(4):295–303. doi: 10.1007/BF00915297. [DOI] [PubMed] [Google Scholar]
- López-Botet M., Fontán G., Garcia Rodriguez M. C., de Landázuri M. O. Relationship between IL 2 synthesis and the proliferative response to PHA in different primary immunodeficiencies. J Immunol. 1982 Feb;128(2):679–683. [PubMed] [Google Scholar]
- North M. E., Akbar A. N., Borthwick N., Sagawa K., Funauchi M., Webster A. D., Farrant J. Co-stimulation with anti-CD28 (Kolt-2) enhances DNA synthesis by defective T cells in common variable immunodeficiency. Clin Exp Immunol. 1994 Feb;95(2):204–208. doi: 10.1111/j.1365-2249.1994.tb06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North M. E., Webster A. D., Farrant J. Defects in proliferative responses of T cells from patients with common variable immunodeficiency on direct activation of protein kinase C. Clin Exp Immunol. 1991 Aug;85(2):198–201. doi: 10.1111/j.1365-2249.1991.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Primary immunodeficiency diseases. Report of a WHO scientific group. Immunodefic Rev. 1992;3(3):195–236. [PubMed] [Google Scholar]
- Rump J. A., Jahreis A., Schlesier M., Dräger R., Melchers I., Peter H. H. Possible role of IL-2 deficiency for hypogammaglobulinaemia in patients with common variable immunodeficiency. Clin Exp Immunol. 1992 Aug;89(2):204–210. doi: 10.1111/j.1365-2249.1992.tb06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawami H., Terada N., Franklin R. A., Okawa H., Uchiyama T., Lucas J. J., Gelfand E. W. Signal transduction by interleukin 2 in human T cells: activation of tyrosine and ribosomal S6 kinases and cell-cycle regulatory genes. J Cell Physiol. 1992 May;151(2):367–377. doi: 10.1002/jcp.1041510218. [DOI] [PubMed] [Google Scholar]
- Schaffer F. M., Palermos J., Zhu Z. B., Barger B. O., Cooper M. D., Volanakis J. E. Individuals with IgA deficiency and common variable immunodeficiency share polymorphisms of major histocompatibility complex class III genes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8015–8019. doi: 10.1073/pnas.86.20.8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmandt R., Fung M., Arima N., Zhang N., Leung B., May C., Gibson S., Hill M., Green W., Mills G. B. T-lymphocyte proliferation: tyrosine kinases in interleukin 2 signal transduction. Baillieres Clin Haematol. 1992 Jul;5(3):551–573. doi: 10.1016/s0950-3536(11)80007-7. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Matamoros N., Farrant J. Lymphocyte surface phenotype in common variable immunodeficiency. Dis Markers. 1992 Mar-Apr;10(2):67–80. [PubMed] [Google Scholar]
- Spickett G. P., Webster A. D., Farrant J. Cellular abnormalities in common variable immunodeficiency. Immunodefic Rev. 1990;2(3):199–219. [PubMed] [Google Scholar]
- Stagg A. J., Funauchi M., Knight S. C., Webster A. D., Farrant J. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID). Clin Exp Immunol. 1994 Apr;96(1):48–53. doi: 10.1111/j.1365-2249.1994.tb06228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg A. J., Harding B., Hughes R. A., Keat A., Knight S. C. The distribution and functional properties of dendritic cells in patients with seronegative arthritis. Clin Exp Immunol. 1991 Apr;84(1):66–71. doi: 10.1111/j.1365-2249.1991.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermeier H. M., Harris D. P., Friscia G., Román E., Surcel H. M., Moreno C., Pasvol G., Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992 Oct;22(10):2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- Vordermeier H. M., Harris D. P., Mehrotra P. K., Roman E., Elsaghier A., Moreno C., Ivanyi J. M. tuberculosis-complex specific T-cell stimulation and DTH reactions induced with a peptide from the 38-kDa protein. Scand J Immunol. 1992 Jun;35(6):711–718. doi: 10.1111/j.1365-3083.1992.tb02979.x. [DOI] [PubMed] [Google Scholar]
- Webster A. D., Asherson G. L. Identification and function of T cells in the peripheral blood of patients with hypogammaglobulinaemia. Clin Exp Immunol. 1974 Dec;18(4):499–504. [PMC free article] [PubMed] [Google Scholar]
- Webster A. D., Lever A., Spickett G., Beattie R., North M., Thorpe R. Recovery of antibody production after HIV infection in 'common' variable hypogammaglobulinaemia. Clin Exp Immunol. 1989 Sep;77(3):309–313. [PMC free article] [PubMed] [Google Scholar]


