Abstract
Felty's syndrome (FS), the association of rheumatoid arthritis (RA) and idiopathic neutropenia, remains an unexplained phenomenon. HLA-DR4 is found in over 90% of cases. Patients with FS may have a T cell lymphocytosis of CD3+CD8+CD57+ large granular lymphocytes (LGL syndrome). In this study of 47 patients with FS, 19% had clear evidence for LGL expansions, while in total 42% had variable evidence for the LGL syndrome using currently available techniques. Of these T cell expansions, 76% were clonal, as demonstrated by Southern blotting and analysis with T cell receptor (TCR) beta chain constant region probes. This technique may fail to detect clonal populations in some patients. Cytofluorographic analysis using antibodies specific for TCR V beta chains identified patients with clonal LGL expansions with results comparable to those obtained with Southern blotting. No evidence for shared V beta usage among expansions from different patients was seen. The role of LGL in RA and FS is currently unclear, but this technique offers a practical and accessible means of identifying patients with LGL expansions, as a starting point for further investigation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I. Heterogeneity of bone marrow-directed immune mechanisms in the pathogenesis of neutropenia of Felty's syndrome. Arthritis Rheum. 1983 Aug;26(8):947–953. doi: 10.1002/art.1780260802. [DOI] [PubMed] [Google Scholar]
- Abe J., Kotzin B. L., Jujo K., Melish M. E., Glode M. P., Kohsaka T., Leung D. Y. Selective expansion of T cells expressing T-cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4066–4070. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akolkar P. N., Gulwani-Akolkar B., Pergolizzi R., Bigler R. D., Silver J. Influence of HLA genes on T cell receptor V segment frequencies and expression levels in peripheral blood lymphocytes. J Immunol. 1993 Apr 1;150(7):2761–2773. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bowman S. J., Sivakumaran M., Snowden N., Bhavnani M., Hall M. A., Panayi G. S., Lanchbury J. S. The large granular lymphocyte syndrome with rheumatoid arthritis. Immunogenetic evidence for a broader definition of Felty's syndrome. Arthritis Rheum. 1994 Sep;37(9):1326–1330. doi: 10.1002/art.1780370909. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Allard S., Londei M., Savill C., Boylston A., Carrel S., Maini R. N., Feldmann M. Heterogeneity of T cell receptor idiotypes in rheumatoid arthritis. Clin Exp Immunol. 1988 Sep;73(3):417–423. [PMC free article] [PubMed] [Google Scholar]
- Bröker B. M., Korthäuer U., Heppt P., Weseloh G., de la Camp R., Kroczek R. A., Emmrich F. Biased T cell receptor V gene usage in rheumatoid arthritis. Oligoclonal expansion of T cells expressing V alpha 2 genes in synovial fluid but not in peripheral blood. Arthritis Rheum. 1993 Sep;36(9):1234–1243. doi: 10.1002/art.1780360908. [DOI] [PubMed] [Google Scholar]
- Callahan J. E., Kappler J. W., Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol. 1993 Dec 15;151(12):6657–6669. [PubMed] [Google Scholar]
- Campion G., Maddison P. J., Goulding N., James I., Ahern M. J., Watt I., Sansom D. The Felty syndrome: a case-matched study of clinical manifestations and outcome, serologic features, and immunogenetic associations. Medicine (Baltimore) 1990 Mar;69(2):69–80. [PubMed] [Google Scholar]
- Clarke G. R., Reyburn H., Lancaster F. C., Boylston A. W. Bimodal distribution of V beta 2+CD4+ T cells in human peripheral blood. Eur J Immunol. 1994 Apr;24(4):837–842. doi: 10.1002/eji.1830240410. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Kissonerghis A. M., Dunne M. J., Watson C. J., Rigby P. W., Owen M. J. Transcripts from an aberrantly re-arranged human T-cell receptor beta-chain gene. EMBO J. 1985 May;4(5):1211–1215. doi: 10.1002/j.1460-2075.1985.tb03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. P., Meyer M. M., Munkirs D. D., Babcock D., Braun M. P., Hayden J. B., Bakke A. C. T-cell receptor variable beta genes show differential expression in CD4 and CD8 T cells. Hum Immunol. 1991 Nov;32(3):194–202. doi: 10.1016/0198-8859(91)90056-f. [DOI] [PubMed] [Google Scholar]
- Dinant H. J., Hissink Muller W., van den Berg-Loonen E. M., Nijenhuis L. E., Engelfriet C. P. HLA-DRw4 in Felty's syndrome. Arthritis Rheum. 1980 Nov;23(11):1336–1336. doi: 10.1002/art.1780231126. [DOI] [PubMed] [Google Scholar]
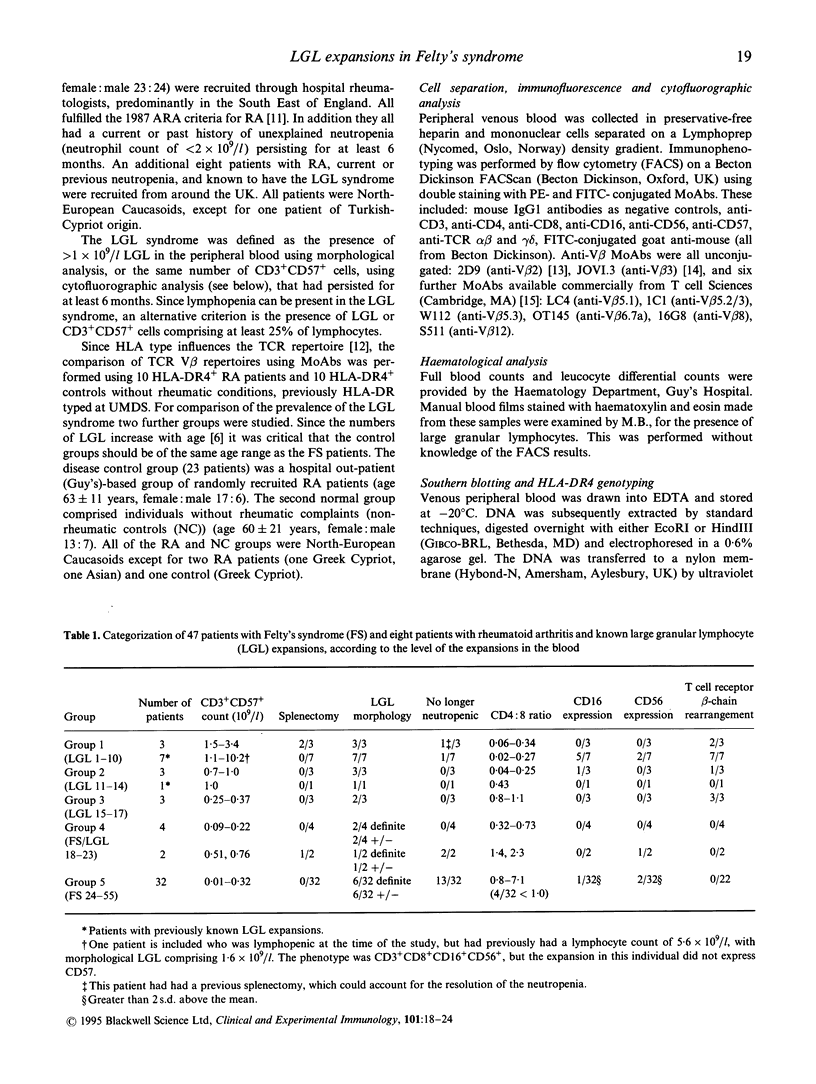
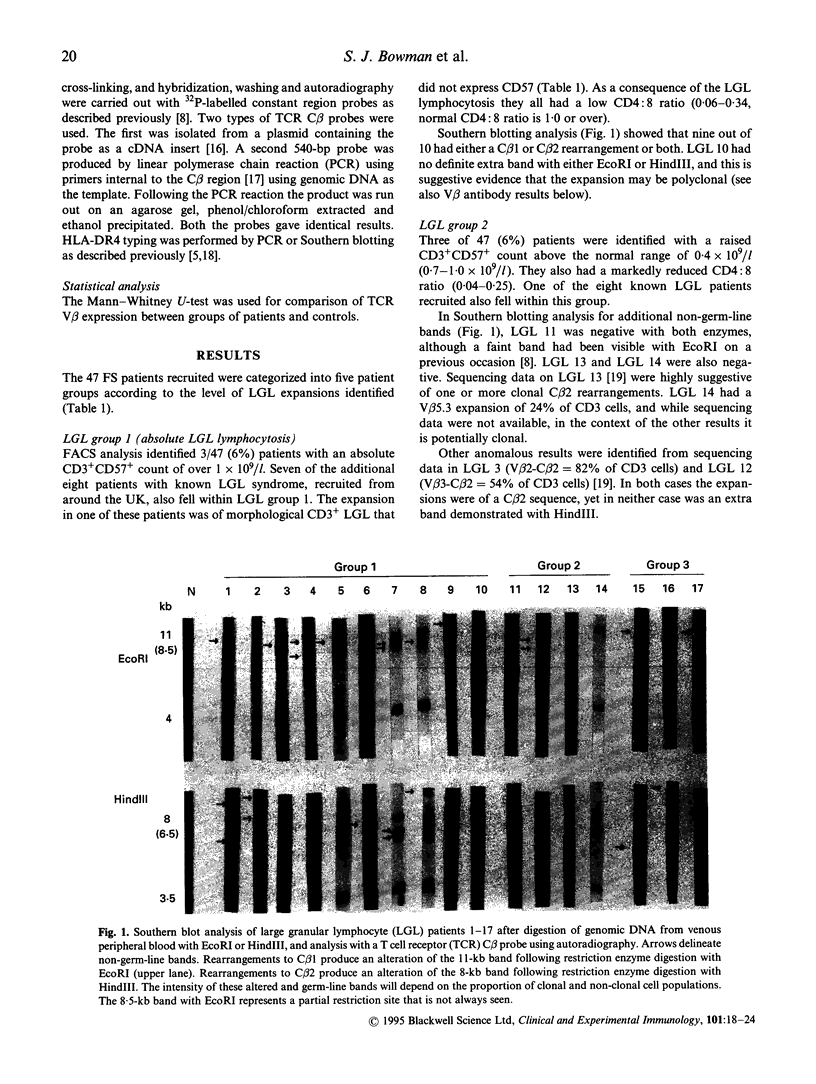
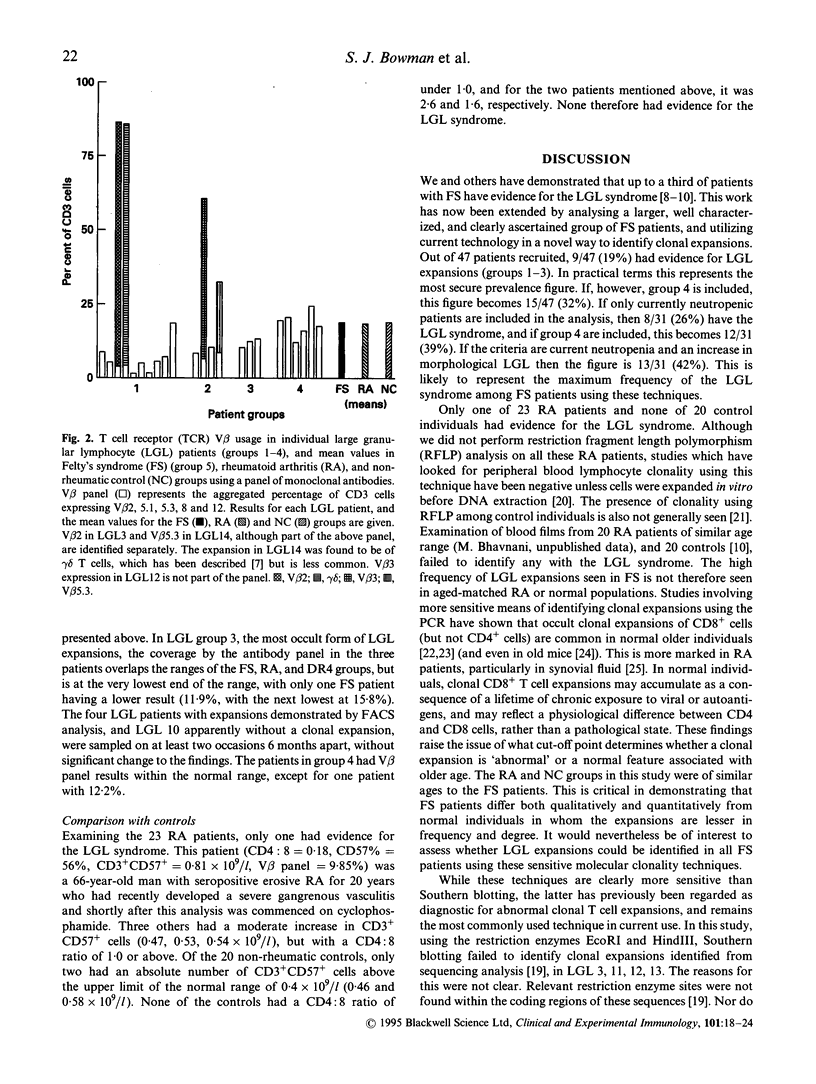
- Drake C. G., Kotzin B. L. Superantigens: biology, immunology, and potential role in disease. J Clin Immunol. 1992 May;12(3):149–162. doi: 10.1007/BF00918083. [DOI] [PubMed] [Google Scholar]
- Favrot M., Janossy G., Tidman N., Blacklock H., Lopez E., Bofill M., Lampert I., Morgenstein G., Powles R., Prentice H. G. T cell regeneration after allogeneic bone marrow transplantation. Clin Exp Immunol. 1983 Oct;54(1):59–72. [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Kardol M., Naipal A. M., Slats J., Den Ouden A., Stijnen T., D'Amaro J., The T. H., Bruning J. W. The influence of cytomegalovirus carrier status on lymphocyte subsets and natural immunity. Clin Exp Immunol. 1987 Jul;69(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Grunewald J., Janson C. H., Wigzell H. Biased expression of individual T cell receptor V gene segments in CD4+ and CD8+ human peripheral blood T lymphocytes. Eur J Immunol. 1991 Mar;21(3):819–822. doi: 10.1002/eji.1830210342. [DOI] [PubMed] [Google Scholar]
- Hingorani R., Choi I. H., Akolkar P., Gulwani-Akolkar B., Pergolizzi R., Silver J., Gregersen P. K. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993 Nov 15;151(10):5762–5769. [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger E. E., Bontrop R. E., Lanchbury J. S. Nucleotide sequences, polymorphism and gene deletion of T cell receptor beta-chain constant regions of Pan troglodytes and Macaca mulatta. J Immunol. 1993 Nov 15;151(10):5301–5309. [PubMed] [Google Scholar]
- Jenkins R. N., Nikaein A., Zimmermann A., Meek K., Lipsky P. E. T cell receptor V beta gene bias in rheumatoid arthritis. J Clin Invest. 1993 Dec;92(6):2688–2701. doi: 10.1172/JCI116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanchbury J. S., Jaeger E. E., Sansom D. M., Hall M. A., Wordsworth P., Stedeford J., Bell J. I., Panayi G. S. Strong primary selection for the Dw4 subtype of DR4 accounts for the HLA-DQw7 association with Felty's syndrome. Hum Immunol. 1991 Sep;32(1):56–64. doi: 10.1016/0198-8859(91)90117-r. [DOI] [PubMed] [Google Scholar]
- Ligthart G. J., van Vlokhoven P. C., Schuit H. R., Hijmans W. The expanded null cell compartment in ageing: increase in the number of natural killer cells and changes in T-cell and NK-cell subsets in human blood. Immunology. 1986 Nov;59(3):353–357. [PMC free article] [PubMed] [Google Scholar]
- Loughran T. P., Jr Clonal diseases of large granular lymphocytes. Blood. 1993 Jul 1;82(1):1–14. [PubMed] [Google Scholar]
- Loughran T. P., Jr, Kadin M. E., Starkebaum G., Abkowitz J. L., Clark E. A., Disteche C., Lum L. G., Slichter S. J. Leukemia of large granular lymphocytes: association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia, and hemolytic anemia. Ann Intern Med. 1985 Feb;102(2):169–175. doi: 10.7326/0003-4819-102-2-169. [DOI] [PubMed] [Google Scholar]
- Lunardi C., Marguerie C., So A. K. An altered repertoire of T cell receptor V gene expression by rheumatoid synovial fluid T lymphocytes. Clin Exp Immunol. 1992 Dec;90(3):440–446. doi: 10.1111/j.1365-2249.1992.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliconi R., Kingsley G. H., Pitzalis C., Sakkas L., Panayi G. S. Analysis of lymphocyte phenotype and T cell receptor genotype in Felty's syndrome. J Rheumatol. 1992 Jul;19(7):1058–1064. [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Lectin-dependent and anti-CD3 induced cytotoxicity are preferentially mediated by peripheral blood cytotoxic T lymphocytes expressing Leu-7 antigen. J Immunol. 1986 Mar 1;136(5):1579–1585. [PubMed] [Google Scholar]
- Pietra B. A., De Inocencio J., Giannini E. H., Hirsch R. TCR V beta family repertoire and T cell activation markers in Kawasaki disease. J Immunol. 1994 Aug 15;153(4):1881–1888. [PubMed] [Google Scholar]
- Posnett D. N., Sinha R., Kabak S., Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to "benign monoclonal gammapathy". J Exp Med. 1994 Feb 1;179(2):609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan N. S., Grunewald J., Janson C. H., Wigzell H. Nearly identical T-cell receptor V-gene usage at birth in two cohorts of distinctly different ethnic origin: influence of environment in the final maturation in the adult. Scand J Immunol. 1992 Jul;36(1):71–78. doi: 10.1111/j.1365-3083.1992.tb02942.x. [DOI] [PubMed] [Google Scholar]
- Sangster R. N., Minowada J., Suciu-Foca N., Minden M., Mak T. W. Rearrangement and expression of the alpha, beta, and gamma chain T cell receptor genes in human thymic leukemia cells and functional T cells. J Exp Med. 1986 Jun 1;163(6):1491–1508. doi: 10.1084/jem.163.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden N., Bhavnani M., Swinson D. R., Kendra J. R., Dennett C., Carrington P., Walsh S., Pumphrey R. S. Large granular T lymphocytes, neutropenia and polyarthropathy: an underdiagnosed syndrome? Q J Med. 1991 Jan;78(285):65–76. [PubMed] [Google Scholar]
- Van Laar J. M., Miltenburg A. M., Verdonk M. J., Daha M. R., De Vries R. R., Van den Elsen P. J., Breedveld F. C. Lack of T cell oligoclonality in enzyme-digested synovial tissue and in synovial fluid in most patients with rheumatoid arthritis. Clin Exp Immunol. 1991 Mar;83(3):352–358. doi: 10.1111/j.1365-2249.1991.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanham G., Kestens L., Gigase P., Colebunders R., Vandenbruaene M., Brijs L., Ceuppens J. L. Evidence for circulating activated cytotoxic T cells in HIV-infected subjects before the onset of opportunistic infections. Clin Exp Immunol. 1990 Oct;82(1):3–9. doi: 10.1111/j.1365-2249.1990.tb05395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney J. L., Prosser H. M., Hewitt C. R., Lamb J. R., Owen M. J. Generation of monoclonal antibodies against a human T cell receptor beta chain expressed in transgenic mice. Hybridoma. 1992 Dec;11(6):701–713. doi: 10.1089/hyb.1992.11.701. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Okumura M., Hirayama K., Sasazuki T. HLA-Bw54-DR4-DRw53-DQw4 haplotype controls nonresponsiveness to hepatitis-B surface antigen via CD8-positive suppressor T cells. Tissue Antigens. 1990 Aug;36(2):69–74. doi: 10.1111/j.1399-0039.1990.tb01802.x. [DOI] [PubMed] [Google Scholar]
- van Laar J. M., Miltenburg A. M., Verdonk M. J., Daha M. R., de Vries R. R., van den Elsen P. J., Breedveld F. C. T-cell receptor beta-chain gene rearrangements of T-cell populations expanded from multiple sites of synovial tissue obtained from a patient with rheumatoid arthritis. Scand J Immunol. 1992 Feb;35(2):187–194. doi: 10.1111/j.1365-3083.1992.tb02849.x. [DOI] [PubMed] [Google Scholar]