Abstract
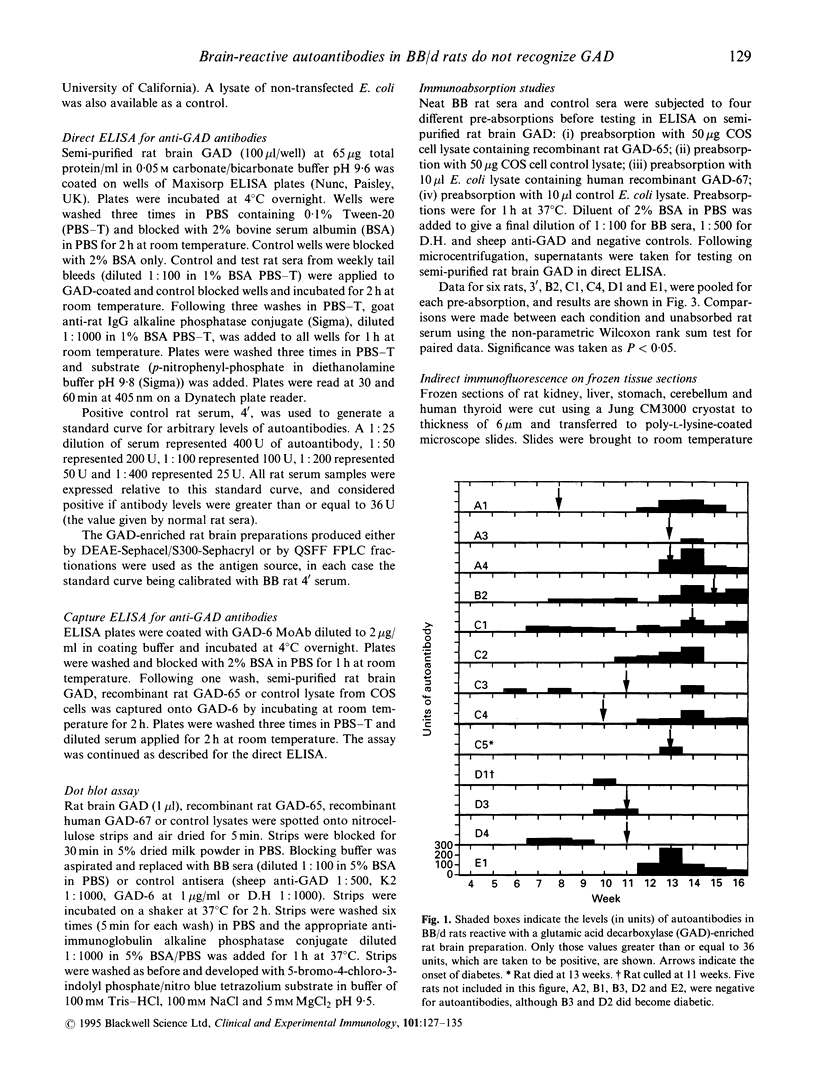
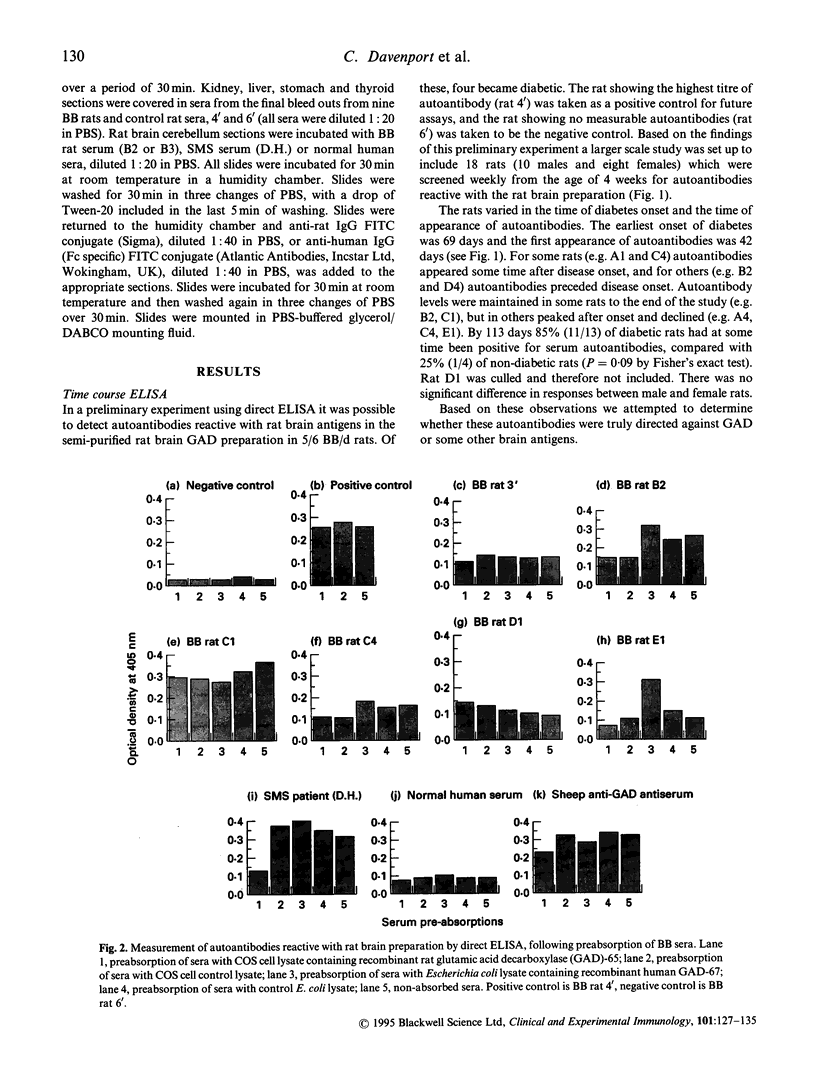
The BB rat spontaneously develops insulin-dependent diabetes mellitus (IDDM) similar to that in humans. The most practical markers of beta cell autoimmunity are circulating antibodies to islet cell components. In particular autoantibodies to the enzyme glutamic acid decarboxylase (GAD) are a common feature of IDDM development in humans. This study aims at investigating the prevalence and levels of autoantibodies in BB rats to antigens in a semipurified, GAD-enriched preparation from rat brain. Eighteen diabetes-prone BB/d rats (10 male and eight female) were tail bled weekly from age 28 days to 113 days and antibodies detected on the rat brain preparation by ELISA. Antibody levels were expressed as arbitrary units relative to a standard positive serum. Individual rats varied in the time and order of antibody appearance and IDDM onset, with the earliest occurrence being 42 days and 69 days, respectively. In some rats antibody production was maintained but declined in others. By 113 days 85% of diabetic rats had at some time been positive for autoantibodies to brain components, compared with 25% of non-diabetics (P = 0.09 by Fisher's exact test). Immunoabsorption studies using recombinant rat GAD-65 or recombinant human GAD-67 failed to inhibit the binding of BB rat sera to the original rat brain preparation. A capture ELISA using GAD-6 MoAb to capture GAD-65 from rat brain preparation or from a preparation of recombinant rat GAD-65, failed to detect anti-GAD antibodies in BB rats. Immunofluorescent staining of tissue sections showed the autoantibodies to be brain-specific, but having distinct staining patterns to the anti-GAD antibodies of Stiff Man Syndrome serum. In conclusion, BB rats possess autoantibodies reactive with rat brain antigens which may be associated with IDDM. However, these are not directed against GAD.
Full text
PDF

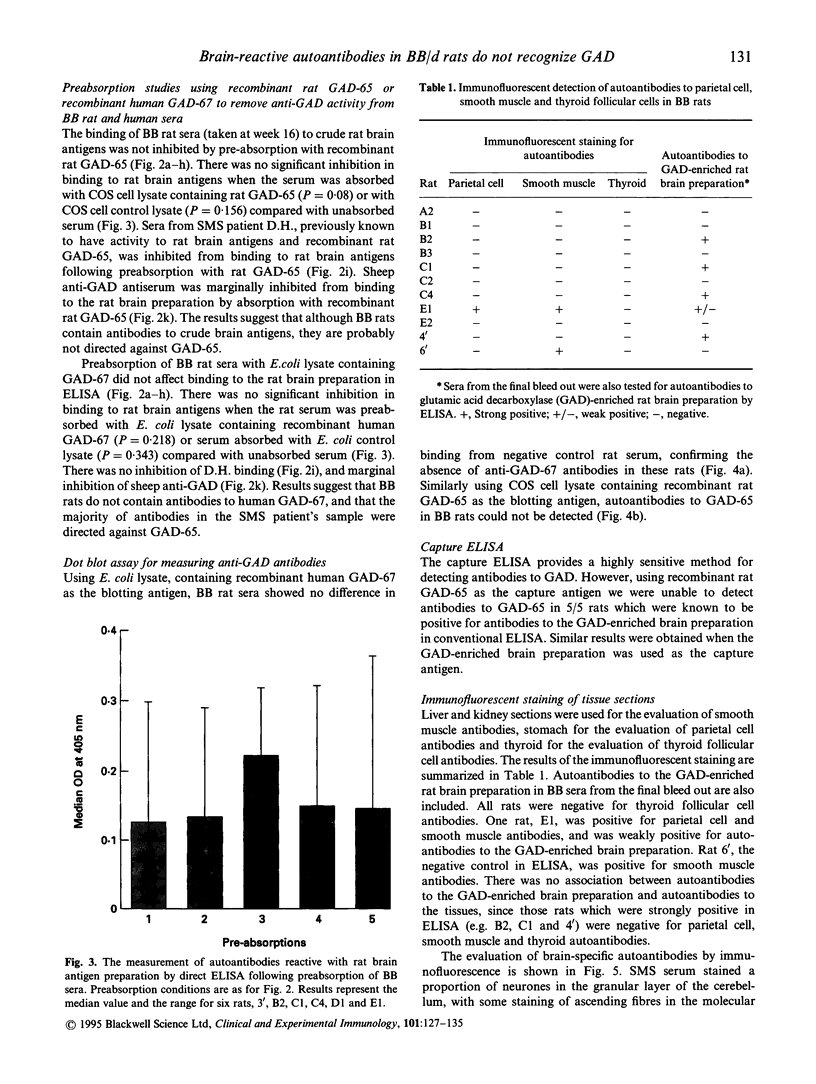
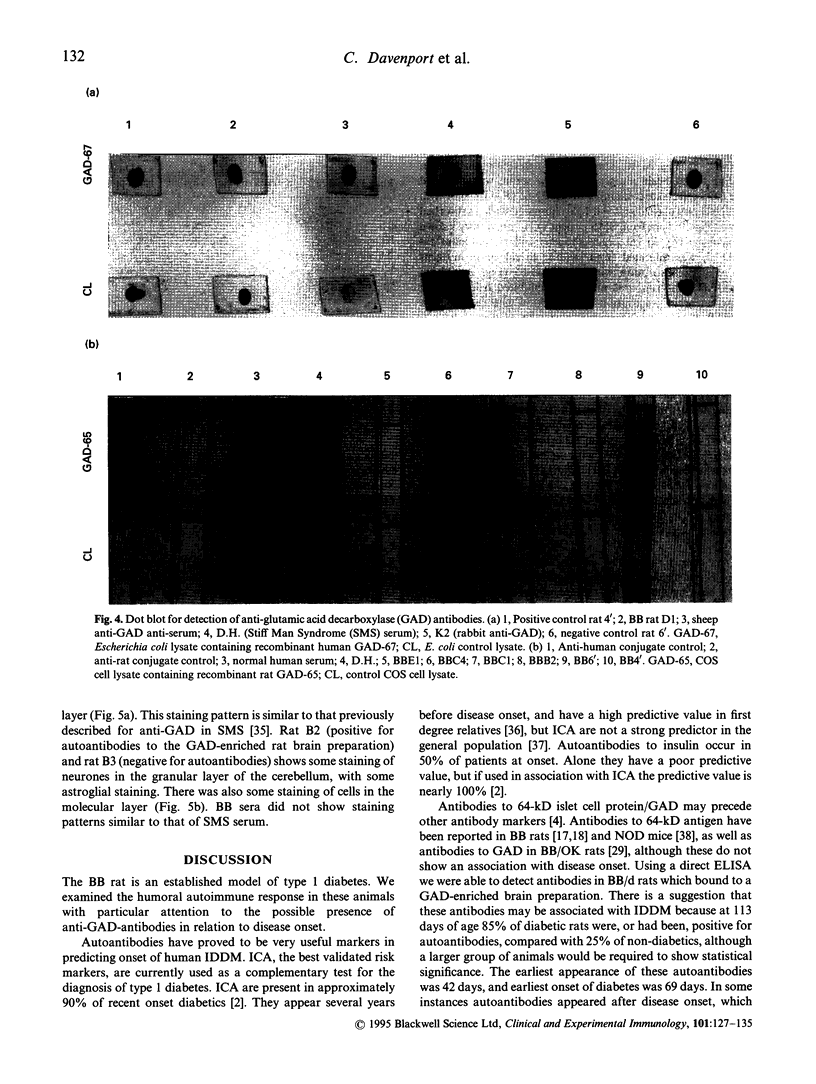
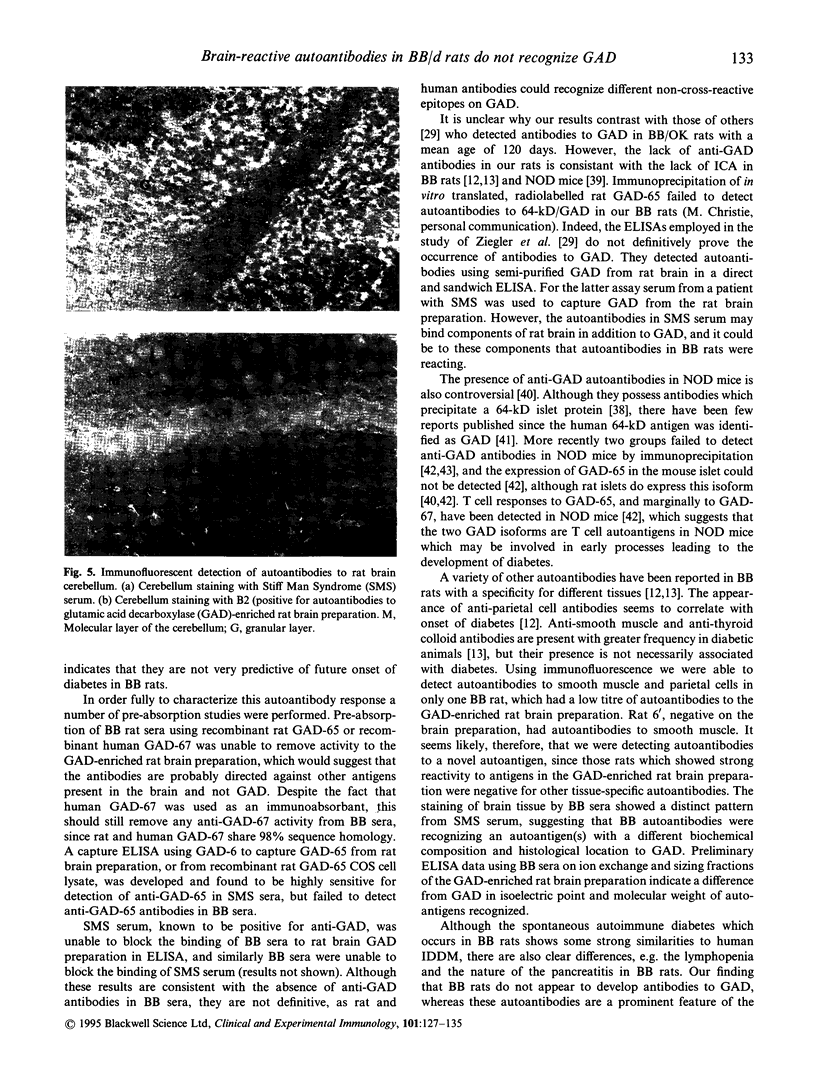


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. A., Maclaren N. K. Autoantibodies in nonobese diabetic mice immunoprecipitate 64,000-Mr islet antigen. Diabetes. 1988 Nov;37(11):1587–1590. doi: 10.2337/diab.37.11.1587. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K., Scharp D. W., Lacy P. E., Riley W. J. 64,000 Mr autoantibodies as predictors of insulin-dependent diabetes. Lancet. 1990 Jun 9;335(8702):1357–1360. doi: 10.1016/0140-6736(90)91241-2. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Dyrberg T., Lernmark A. Autoantibodies to a 64-kilodalton islet cell protein precede the onset of spontaneous diabetes in the BB rat. Science. 1984 Jun 22;224(4655):1348–1350. doi: 10.1126/science.6374896. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Bieg S., Seissler J., Herberg L., Northemann W., Scherbaum W. A. GAD65 is recognized by T-cells, but not by antibodies from NOD-mice. Autoimmunity. 1994;17(3):189–194. doi: 10.3109/08916939409010653. [DOI] [PubMed] [Google Scholar]
- Bingley P. J., Bonifacio E., Shattock M., Gillmor H. A., Sawtell P. A., Dunger D. B., Scott R. D., Bottazzo G. F., Gale E. A. Can islet cell antibodies predict IDDM in the general population? Diabetes Care. 1993 Jan;16(1):45–50. doi: 10.2337/diacare.16.1.45. [DOI] [PubMed] [Google Scholar]
- Björk E., Velloso L. A., Kämpe O., Karlsson F. A. GAD autoantibodies in IDDM, stiff-man syndrome, and autoimmune polyendocrine syndrome type I recognize different epitopes. Diabetes. 1994 Jan;43(1):161–165. doi: 10.2337/diab.43.1.161. [DOI] [PubMed] [Google Scholar]
- Butler M. H., Solimena M., Dirkx R., Jr, Hayday A., De Camilli P. Identification of a dominant epitope of glutamic acid decarboxylase (GAD-65) recognized by autoantibodies in stiff-man syndrome. J Exp Med. 1993 Dec 1;178(6):2097–2106. doi: 10.1084/jem.178.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Gottlieb D. I. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988 Jun;8(6):2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christgau S., Schierbeck H., Aanstoot H. J., Aagaard L., Begley K., Kofod H., Hejnaes K., Baekkeskov S. Pancreatic beta cells express two autoantigenic forms of glutamic acid decarboxylase, a 65-kDa hydrophilic form and a 64-kDa amphiphilic form which can be both membrane-bound and soluble. J Biol Chem. 1991 Nov 5;266(31):21257–21264. [PubMed] [Google Scholar]
- Christie M. R., Hollands J. A., Brown T. J., Michelsen B. K., Delovitch T. L. Detection of pancreatic islet 64,000 M(r) autoantigens in insulin-dependent diabetes distinct from glutamate decarboxylase. J Clin Invest. 1993 Jul;92(1):240–248. doi: 10.1172/JCI116556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisá L., Mordes J. P., Rossini A. A. Autoimmune diabetes mellitus in the BB rat. Diabetes Metab Rev. 1992 Apr;8(1):4–37. [PubMed] [Google Scholar]
- Dyrberg T., Poussier P., Nakhooda A. F., Marliss E. B., Lernmark A. Humoral immunity in the spontaneously diabetic BB rat. Metabolism. 1983 Jul;32(7 Suppl 1):87–91. doi: 10.1016/s0026-0495(83)80018-3. [DOI] [PubMed] [Google Scholar]
- Dyrberg T., Poussier P., Nakhooda F., Marliss E. B., Lernmark A. Islet cell surface and lymphocyte antibodies often precede the spontaneous diabetes in the BB rat. Diabetologia. 1984 Feb;26(2):159–165. doi: 10.1007/BF00281126. [DOI] [PubMed] [Google Scholar]
- Elder M., Maclaren N., Riley W., McConnell T. Gastric parietal cell and other autoantibodies in the BB rat. Diabetes. 1982 Apr;31(4 Pt 1):313–318. doi: 10.2337/diab.31.4.313. [DOI] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese S., Bonifacio E., McNally J. M., Dean B. M., Wagner R., Bosi E., Gale E. A., Bottazzo G. F. Distinct cytoplasmic islet cell antibodies with different risks for type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992 Apr;35(4):385–388. doi: 10.1007/BF00401207. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Honeyman M. C., DeAizpurua H. J., Schmidli R. S., Colman P. G., Tait B. D., Cram D. S. Inverse relation between humoral and cellular immunity to glutamic acid decarboxylase in subjects at risk of insulin-dependent diabetes. Lancet. 1993 May 29;341(8857):1365–1369. doi: 10.1016/0140-6736(93)90940-i. [DOI] [PubMed] [Google Scholar]
- Kaufman D. L., Houser C. R., Tobin A. J. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991 Feb;56(2):720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborie C., Sai P., Feutren G., Debray-Sachs M., Quiniou-Debrie M. C., Poussier P., Marliss E. B., Assan R. Time course of islet cell antibodies in diabetic and nondiabetic BB rats. Diabetes. 1985 Sep;34(9):904–910. doi: 10.2337/diab.34.9.904. [DOI] [PubMed] [Google Scholar]
- Landin-Olsson M., Palmer J. P., Lernmark A., Blom L., Sundkvist G., Nyström L., Dahlquist G. Predictive value of islet cell and insulin autoantibodies for type 1 (insulin-dependent) diabetes mellitus in a population-based study of newly-diagnosed diabetic and matched control children. Diabetologia. 1992 Nov;35(11):1068–1073. doi: 10.1007/BF02221683. [DOI] [PubMed] [Google Scholar]
- Laupacis A., Stiller C. R., Gardell C., Keown P., Dupre J., Wallace A. C., Thibert P. Cyclosporin prevents diabetes in BB Wistar rats. Lancet. 1983 Jan 1;1(8314-5):10–12. doi: 10.1016/s0140-6736(83)91558-1. [DOI] [PubMed] [Google Scholar]
- Like A. A., Appel M. C., Rossini A. A. Autoantibodies in the BB/W rat. Diabetes. 1982 Sep;31(9):816–820. doi: 10.2337/diab.31.9.816. [DOI] [PubMed] [Google Scholar]
- Like A. A., Kislauskis E., Williams R. R., Rossini A. A. Neonatal thymectomy prevents spontaneous diabetes mellitus in the BB/W rat. Science. 1982 May 7;216(4546):644–646. doi: 10.1126/science.7041259. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A., Guberski D. L., Appel M. C., Williams R. M. Spontaneous diabetes mellitus: reversal and prevention in the BB/W rat with antiserum to rat lymphocytes. Science. 1979 Dec 21;206(4425):1421–1423. doi: 10.1126/science.388619. [DOI] [PubMed] [Google Scholar]
- Métroz-Dayer M. D., Mouland A., Brideau C., Duhamel D., Poussier P. Adoptive transfer of diabetes in BB rats induced by CD4 T lymphocytes. Diabetes. 1990 Aug;39(8):928–932. doi: 10.2337/diab.39.8.928. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Schmechel D. E., Tappaz M. L., Kopin I. J. Production of a specific antiserum to rat brain glutamic acid decarboxylase by injection of an antigen-antibody complex. Neuroscience. 1981;6(12):2689–2700. doi: 10.1016/0306-4522(81)90113-5. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Asplin C. M., Clemons P., Lyen K., Tatpati O., Raghu P. K., Paquette T. L. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983 Dec 23;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- Pontesilli O., Carotenuto P., Gazda L. S., Pratt P. F., Prowse S. J. Circulating lymphocyte populations and autoantibodies in non-obese diabetic (NOD) mice: a longitudinal study. Clin Exp Immunol. 1987 Oct;70(1):84–93. [PMC free article] [PubMed] [Google Scholar]
- Richter W., Shi Y., Baekkeskov S. Autoreactive epitopes defined by diabetes-associated human monoclonal antibodies are localized in the middle and C-terminal domains of the smaller form of glutamate decarboxylase. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2832–2836. doi: 10.1073/pnas.90.7.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley W. J., Maclaren N. K., Krischer J., Spillar R. P., Silverstein J. H., Schatz D. A., Schwartz S., Malone J., Shah S., Vadheim C. A prospective study of the development of diabetes in relatives of patients with insulin-dependent diabetes. N Engl J Med. 1990 Oct 25;323(17):1167–1172. doi: 10.1056/NEJM199010253231704. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Solimena M., Folli F., Aparisi R., Pozza G., De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med. 1990 May 31;322(22):1555–1560. doi: 10.1056/NEJM199005313222202. [DOI] [PubMed] [Google Scholar]
- Solimena M., Folli F., Denis-Donini S., Comi G. C., Pozza G., De Camilli P., Vicari A. M. Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. N Engl J Med. 1988 Apr 21;318(16):1012–1020. doi: 10.1056/NEJM198804213181602. [DOI] [PubMed] [Google Scholar]
- Tisch R., Yang X. D., Singer S. M., Liblau R. S., Fugger L., McDevitt H. O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993 Nov 4;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- Velloso L. A., Eizirik D. L., Karlsson F. A., Kämpe O. Absence of autoantibodies against glutamate decarboxylase (GAD) in the non-obese diabetic (NOD) mouse and low expression of the enzyme in mouse islets. Clin Exp Immunol. 1994 Apr;96(1):129–137. doi: 10.1111/j.1365-2249.1994.tb06242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velloso L. A., Kämpe O., Eizirik D. L., Hallberg A., Andersson A., Karlsson F. A. Human autoantibodies react with glutamic acid decarboxylase antigen in human and rat but not in mouse pancreatic islets. Diabetologia. 1993 Jan;36(1):39–46. doi: 10.1007/BF00399091. [DOI] [PubMed] [Google Scholar]
- Velloso L. A., Kämpe O., Hallberg A., Christmanson L., Betsholtz C., Karlsson F. A. Demonstration of GAD-65 as the main immunogenic isoform of glutamate decarboxylase in type 1 diabetes and determination of autoantibodies using a radioligand produced by eukaryotic expression. J Clin Invest. 1993 May;91(5):2084–2090. doi: 10.1172/JCI116431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velloso L. A., Kämpe O., Hallberg A., Christmanson L., Betsholtz C., Karlsson F. A. Demonstration of GAD-65 as the main immunogenic isoform of glutamate decarboxylase in type 1 diabetes and determination of autoantibodies using a radioligand produced by eukaryotic expression. J Clin Invest. 1993 May;91(5):2084–2090. doi: 10.1172/JCI116431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M., Schlosser M., Hamann J., Vieregge P., Lühder F., Klöting I., Ziegler B. Autoantibodies to glutamate decarboxylase detected in diabetes-prone BB/OK rats do not distinguish onset of diabetes. Exp Clin Endocrinol. 1994;102(2):98–103. doi: 10.1055/s-0029-1211270. [DOI] [PubMed] [Google Scholar]