Abstract
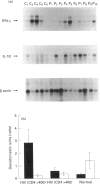
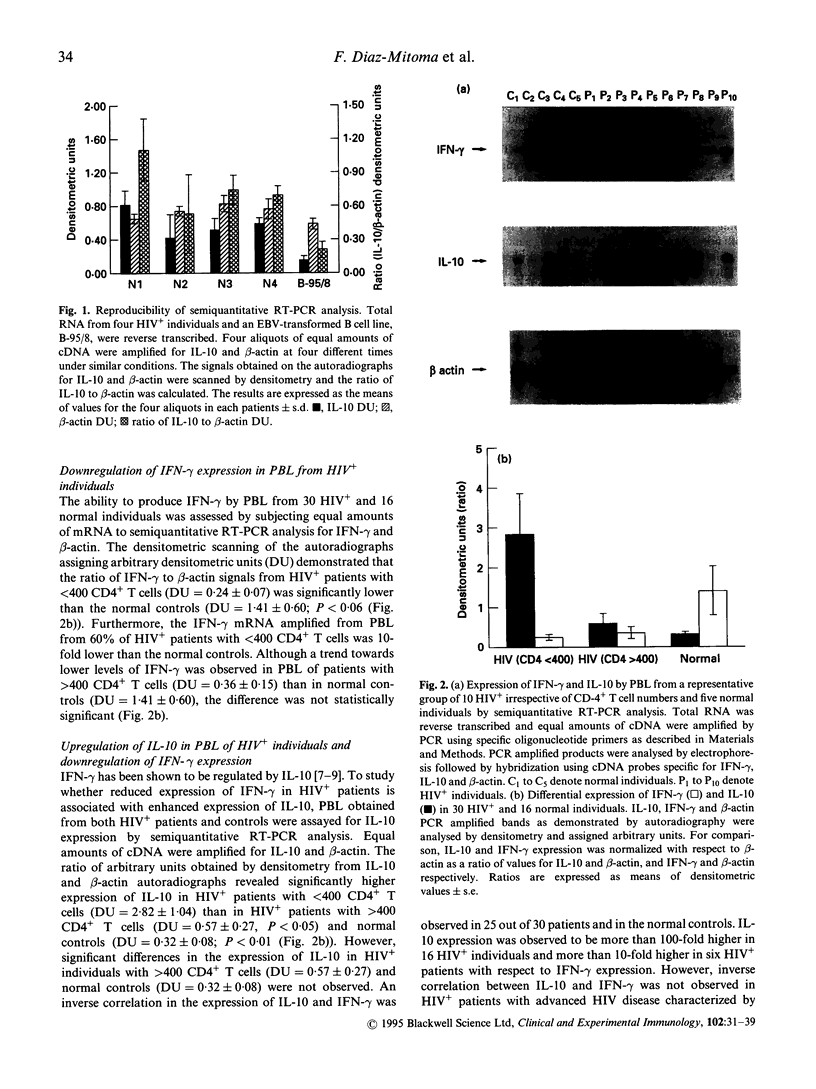
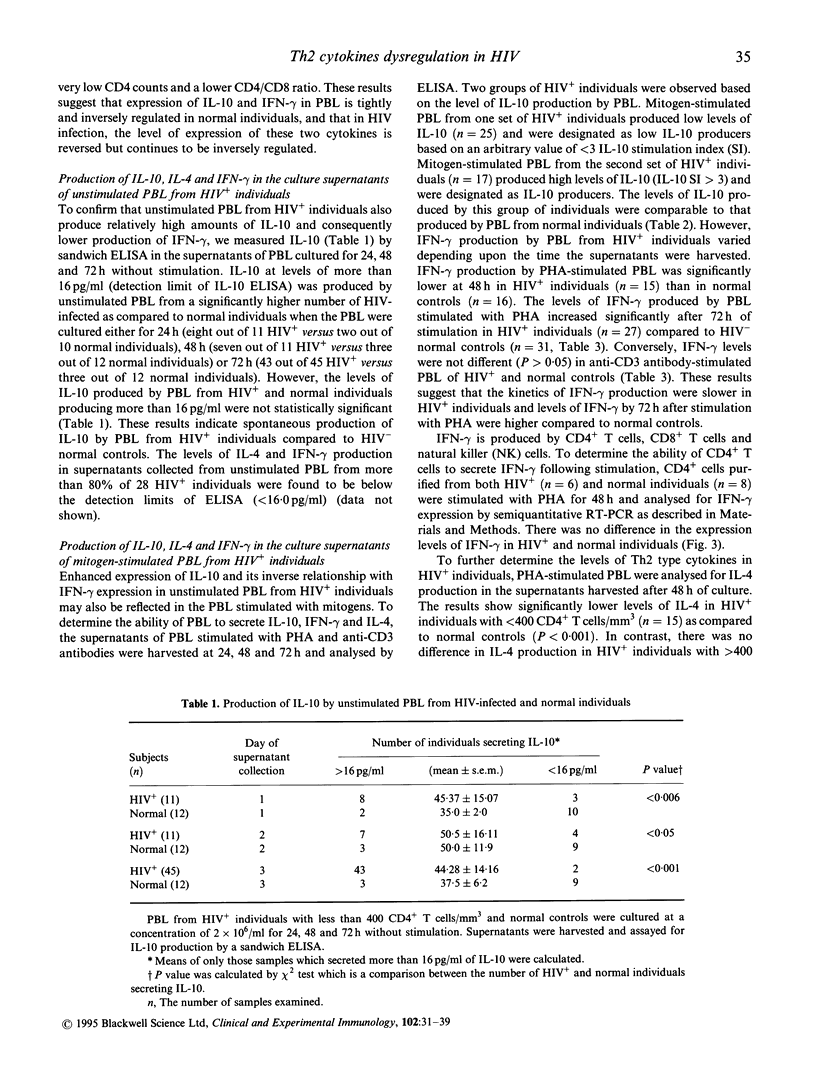
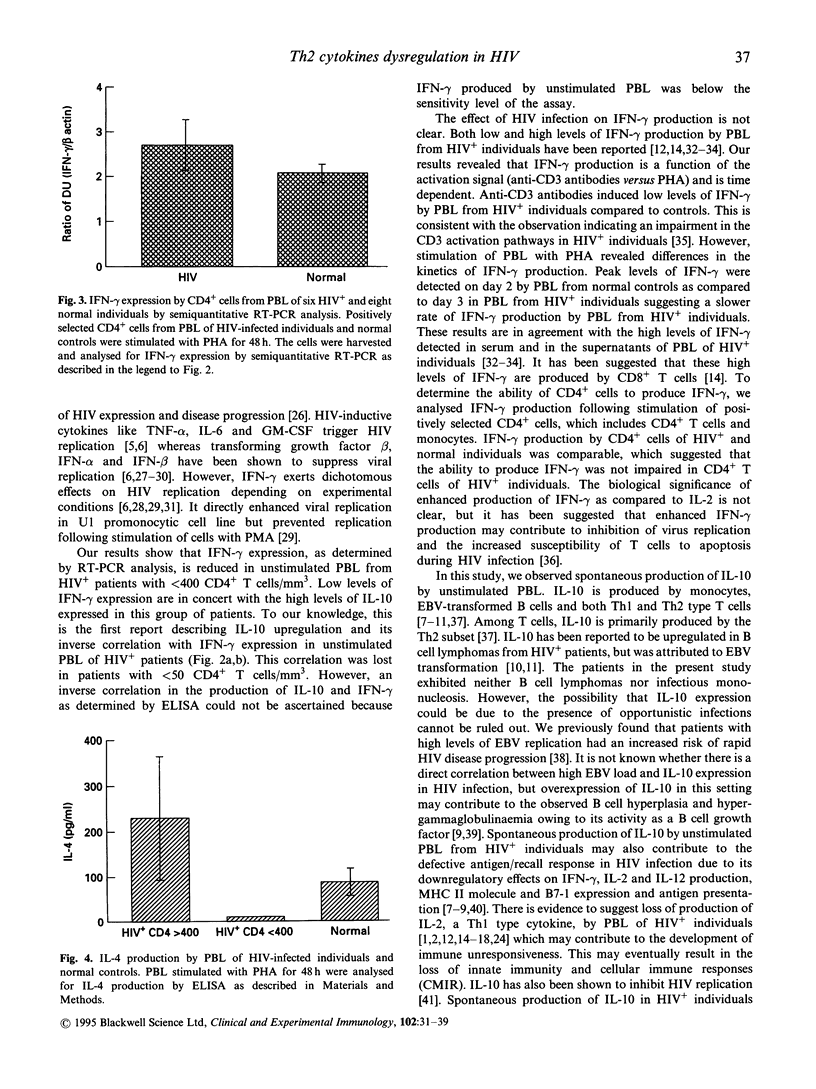
Infection of immune cells with HIV induces dysregulation of cytokines which may play a vital role in HIV pathogenesis. We analysed the expression of T helper type 1 (Th1) (interferon-gamma (IFN-gamma)) and Th2 (IL-4, IL-10) type cytokines in peripheral blood lymphocytes (PBL) from HIV+ patients. The semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) analysis revealed that IFN-gamma mRNA in unstimulated PBL was significantly decreased and IL-10 mRNA was significantly upregulated in patients with < 400 CD4+ T cells/mm3 (n = 30) as compared to patients with > 400 CD4+ T cells/mm3 (n = 6) and normal controls (n = 16). In addition, IL-10 mRNA levels were inversely associated with IFN-gamma expression. Similar results were obtained by measuring IL-10 production in the supernatants of PBL cultured in vitro without stimulation by employing an enzyme immunosorbent assay (ELISA). However, the levels of IL-4 and IFN-gamma produced by unstimulated PBL were undetectable by ELISA. Mitogen stimulation of PBL revealed two groups of HIV+ individuals based on IL-10 production. PBL from one set of individuals produced low levels of IL-10 (low IL-10 producers) whereas the other group produced IL-10 comparable to that of normal controls (IL-10 producers). Production of IL-4 was significantly reduced in HIV+ individuals with < 400 CD4+ T cells/mm3 as compared to the normal controls. However, ability to produce IFN-gamma by mitogen-stimulated total PBL and CD4+ purified cells was not impaired in HIV+ individuals. These results suggest that unstimulated and mitogen-stimulated PBL of HIV+ individuals exhibit dysregulation of Th2 type cytokines which may play a role in HIV immunopathogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Roncarolo M. G., Yssel H., Andersson U., Gleich G. J., Silver J. E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992 Jun;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Burdin N., Péronne C., Banchereau J., Rousset F. Epstein-Barr virus transformation induces B lymphocytes to produce human interleukin 10. J Exp Med. 1993 Feb 1;177(2):295–304. doi: 10.1084/jem.177.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Hakim F. T., Venzon D. J., Blatt S., Hendrix C. W., Wynn T. A., Shearer G. M. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993 Mar;91(3):759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993 Mar;14(3):107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Lucey D. R., Via C. S., Shearer G. M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989 Dec;84(6):1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Wynn T. A., Berzofsky J. A., Blatt S. P., Hendrix C. W., Sher A., Coffman R. L., Shearer G. M. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994 Feb;93(2):768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Diaz-Mitoma F., Ruiz A., Flowerdew G., Houston S., Romanowski B., Kovithavongs T., Preiksaitis J., Tyrrell D. L. High levels of Epstein-Barr virus in the oropharynx: a predictor of disease progression in human immunodeficiency virus infection. J Med Virol. 1990 Jun;31(2):69–75. doi: 10.1002/jmv.1890310202. [DOI] [PubMed] [Google Scholar]
- Ding L., Linsley P. S., Huang L. Y., Germain R. N., Shevach E. M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993 Aug 1;151(3):1224–1234. [PubMed] [Google Scholar]
- Emilie D., Fior R., Llorente L., Marfaing-Koka A., Peuchmaur M., Devergne O., Jarrousse B., Wijdenes J., Boue F., Galanaud P. Cytokines from lymphoid organs of HIV-infected patients: production and role in the immune disequilibrium of the disease and in the development of B lymphomas. Immunol Rev. 1994 Aug;140:5–34. doi: 10.1111/j.1600-065x.1994.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Emilie D., Maillot M. C., Nicolas J. F., Fior R., Galanaud P. Antagonistic effect of interferon-gamma on tat-induced transactivation of HIV long terminal repeat. J Biol Chem. 1992 Oct 15;267(29):20565–20570. [PubMed] [Google Scholar]
- Emilie D., Touitou R., Raphael M., Peuchmaur M., Devergnee O., Rea D., Coumbraras J., Crevon M. C., Edelman L., Joab I. In vivo production of interleukin-10 by malignant cells in AIDS lymphomas. Eur J Immunol. 1992 Nov;22(11):2937–2942. doi: 10.1002/eji.1830221127. [DOI] [PubMed] [Google Scholar]
- Fan J., Bass H. Z., Fahey J. L. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. J Immunol. 1993 Nov 1;151(9):5031–5040. [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., Monte D., Plouvier B., Capron A., Ameisen J. C. CD3-mediated apoptosis of human medullary thymocytes and activated peripheral T cells: respective roles of interleukin-1, interleukin-2, interferon-gamma and accessory cells. Eur J Immunol. 1993 Jul;23(7):1623–1629. doi: 10.1002/eji.1830230734. [DOI] [PubMed] [Google Scholar]
- Hammer S. M., Gillis J. M., Groopman J. E., Rose R. M. In vitro modification of human immunodeficiency virus infection by granulocyte-macrophage colony-stimulating factor and gamma interferon. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8734–8738. doi: 10.1073/pnas.83.22.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., O'Garra A., Ishida H., de Waal Malefyt R., de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992 Jul;12(4):239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- Kornbluth R. S., Oh P. S., Munis J. R., Cleveland P. H., Richman D. D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989 Mar 1;169(3):1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., O'Brien W. A., Zhao J. Q., Golde D. W., Gasson J. C., Chen I. S. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988 Sep 23;241(4873):1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- Lacroix F., Zhao D. Y., Izaguirre C. A., Filion L. G. Suppression by HIV of IL-1 and IL-6 secretion in accessory cells: AC function defect partially corrected with exogenous IL-1 and IL-6. Clin Immunol Immunopathol. 1993 May;67(2):109–116. doi: 10.1006/clin.1993.1052. [DOI] [PubMed] [Google Scholar]
- Lin R. H., Mamula M. J., Hardin J. A., Janeway C. A., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991 Jun 1;173(6):1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi E., Macchia D., Parronchi P., Mazzetti M., Ravina A., Milo D., Romagnani S. Reduced production of interleukin 2 and interferon-gamma and enhanced helper activity for IgG synthesis by cloned CD4+ T cells from patients with AIDS. Eur J Immunol. 1987 Dec;17(12):1685–1690. doi: 10.1002/eji.1830171202. [DOI] [PubMed] [Google Scholar]
- Maggi E., Mazzetti M., Ravina A., Annunziato F., de Carli M., Piccinni M. P., Manetti R., Carbonari M., Pesce A. M., del Prete G. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994 Jul 8;265(5169):244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- Masood R., Lunardi-Iskandar Y., Moudgil T., Zhang Y., Law R. E., Huang C. L., Puri R. K., Levine A. M., Gill P. S. IL-10 inhibits HIV-1 replication and is induced by tat. Biochem Biophys Res Commun. 1994 Jul 15;202(1):374–383. doi: 10.1006/bbrc.1994.1938. [DOI] [PubMed] [Google Scholar]
- Miedema F., Tersmette M., van Lier R. A. AIDS pathogenesis: a dynamic interaction between HIV and the immune system. Immunol Today. 1990 Aug;11(8):293–297. doi: 10.1016/0167-5699(90)90116-q. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Schindler R., Ferriani R., Sakaguchi M., Vannier E., Dinarello C. A., Groopman J. E. Production of cytokines by peripheral blood monocytes/macrophages infected with human immunodeficiency virus type 1 (HIV-1). J Infect Dis. 1990 May;161(5):888–893. doi: 10.1093/infdis/161.5.888. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Martínez-Maza O., Hirano T., Breen E. C., Nishanian P. G., Salazar-Gonzalez J. F., Fahey J. L., Kishimoto T. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol. 1989 Jan 15;142(2):531–536. [PubMed] [Google Scholar]
- Phair J. P. Estimating prognosis in HIV-1 infection. Ann Intern Med. 1993 May 1;118(9):742–744. doi: 10.7326/0003-4819-118-9-199305010-00015. [DOI] [PubMed] [Google Scholar]
- Poli G., Bressler P., Kinter A., Duh E., Timmer W. C., Rabson A., Justement J. S., Stanley S., Fauci A. S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990 Jul 1;172(1):151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Kinter A. L., Justement J. S., Bressler P., Kehrl J. H., Fauci A. S. Transforming growth factor beta suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J Exp Med. 1991 Mar 1;173(3):589–597. doi: 10.1084/jem.173.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Del Prete G., Manetti R., Ravina A., Annunziato F., De Carli M., Mazzetti M., Piccinni M. P., D'Elios M. M., Parronchi P. Role of TH1/TH2 cytokines in HIV infection. Immunol Rev. 1994 Aug;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Maggi E. Th1 versus Th2 responses in AIDS. Curr Opin Immunol. 1994 Aug;6(4):616–622. doi: 10.1016/0952-7915(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Immunopathogenic mechanisms of HIV infection: cytokine induction of HIV expression. Immunol Today. 1990 May;11(5):176–180. doi: 10.1016/0167-5699(90)90070-p. [DOI] [PubMed] [Google Scholar]
- Saville M. W., Taga K., Foli A., Broder S., Tosato G., Yarchoan R. Interleukin-10 suppresses human immunodeficiency virus-1 replication in vitro in cells of the monocyte/macrophage lineage. Blood. 1994 Jun 15;83(12):3591–3599. [PubMed] [Google Scholar]
- Vyakarnam A., Matear P., Meager A., Kelly G., Stanley B., Weller I., Beverley P. Altered production of tumour necrosis factors alpha and beta and interferon gamma by HIV-infected individuals. Clin Exp Immunol. 1991 Apr;84(1):109–115. doi: 10.1111/j.1365-2249.1991.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanidworanun C., Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993 Dec 15;151(12):6853–6861. [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]