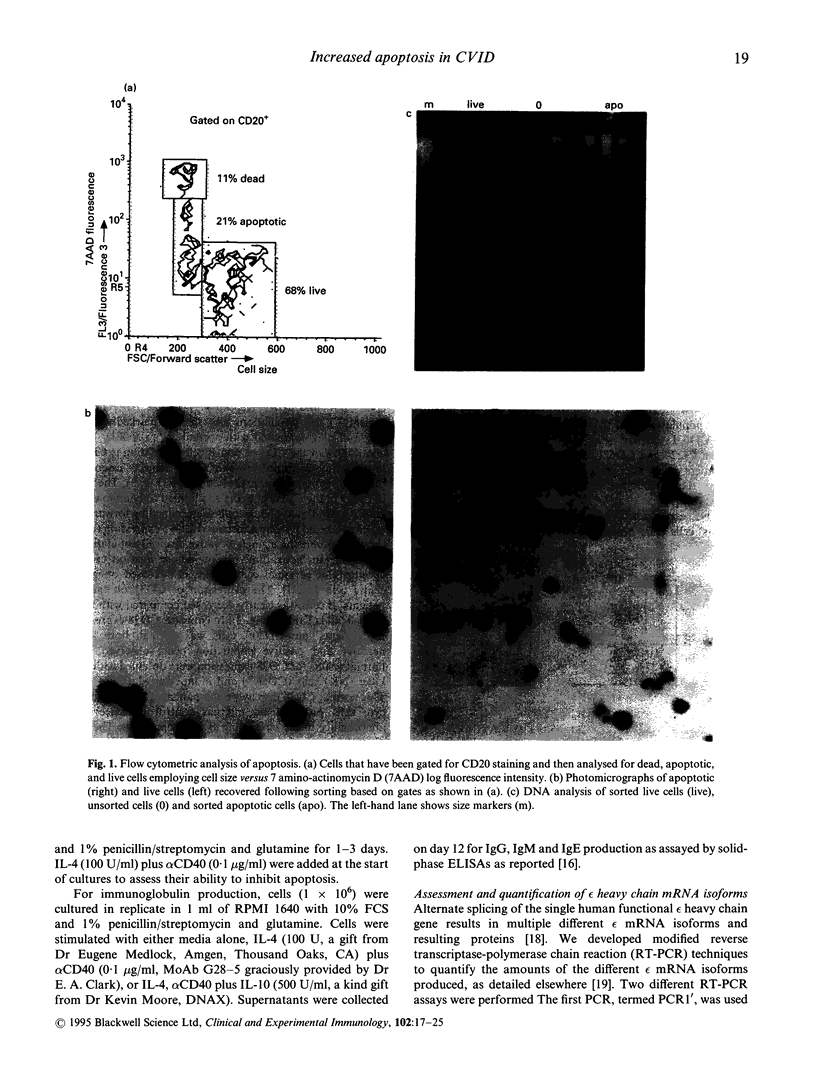
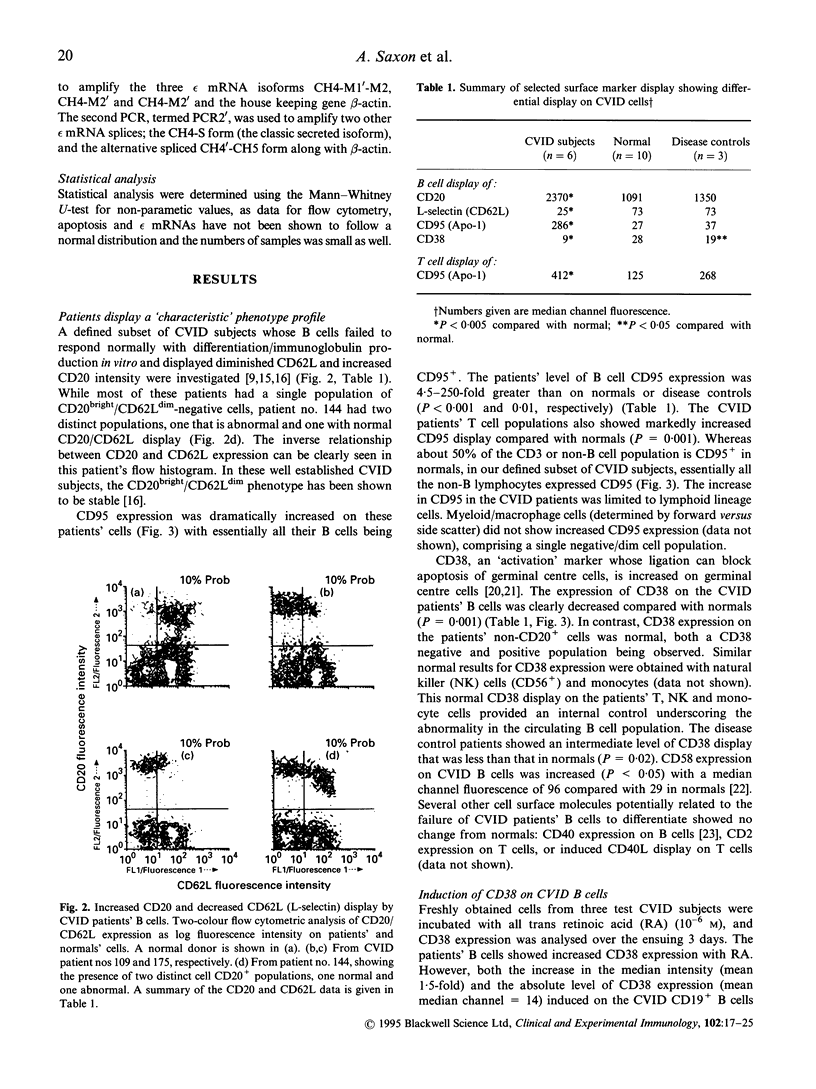
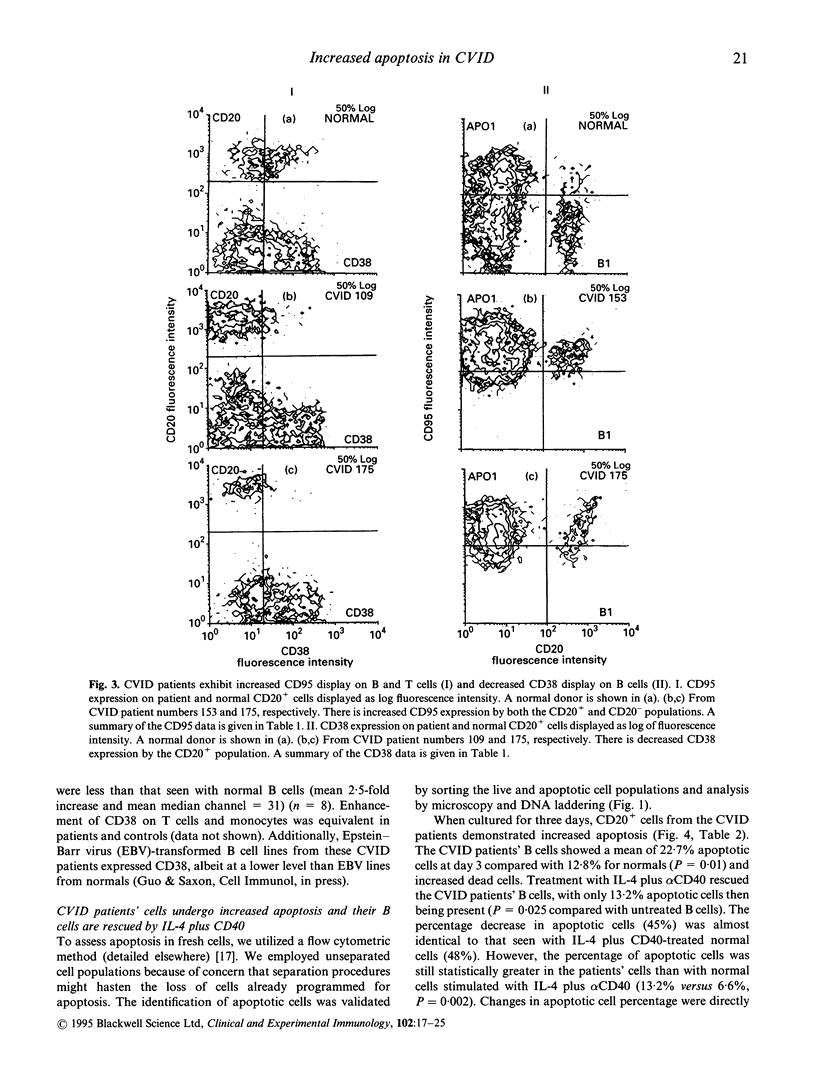
Abstract
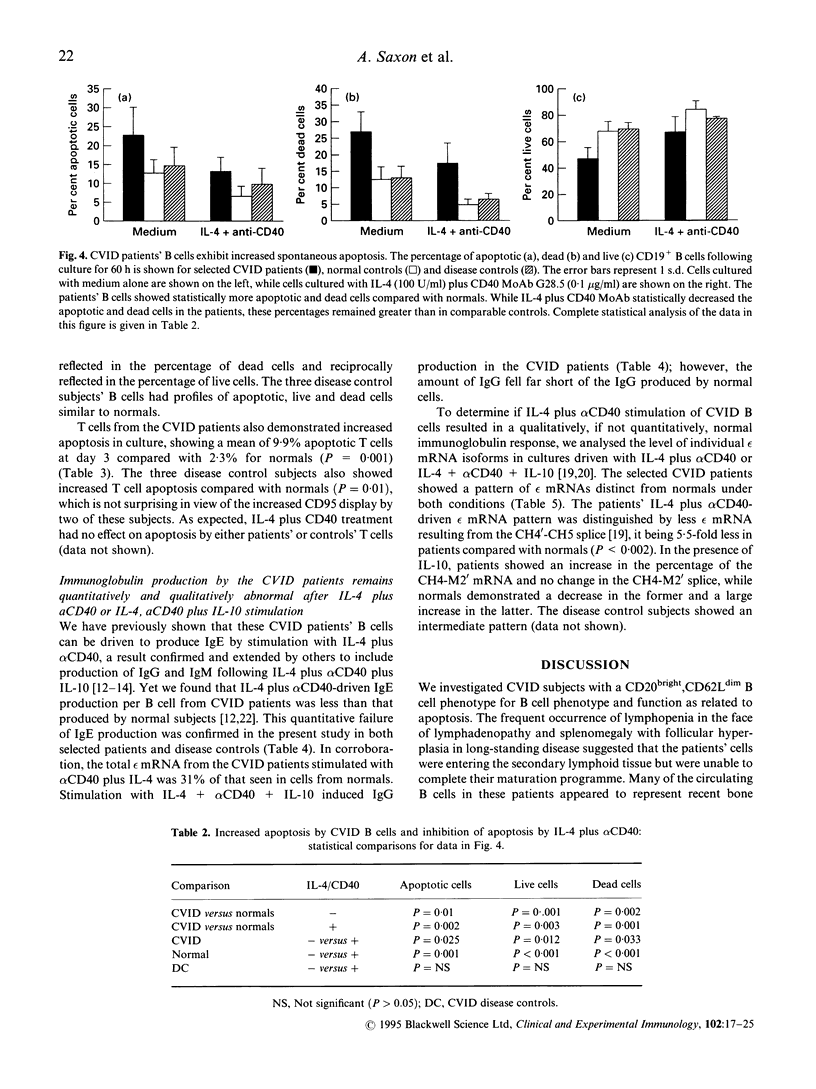
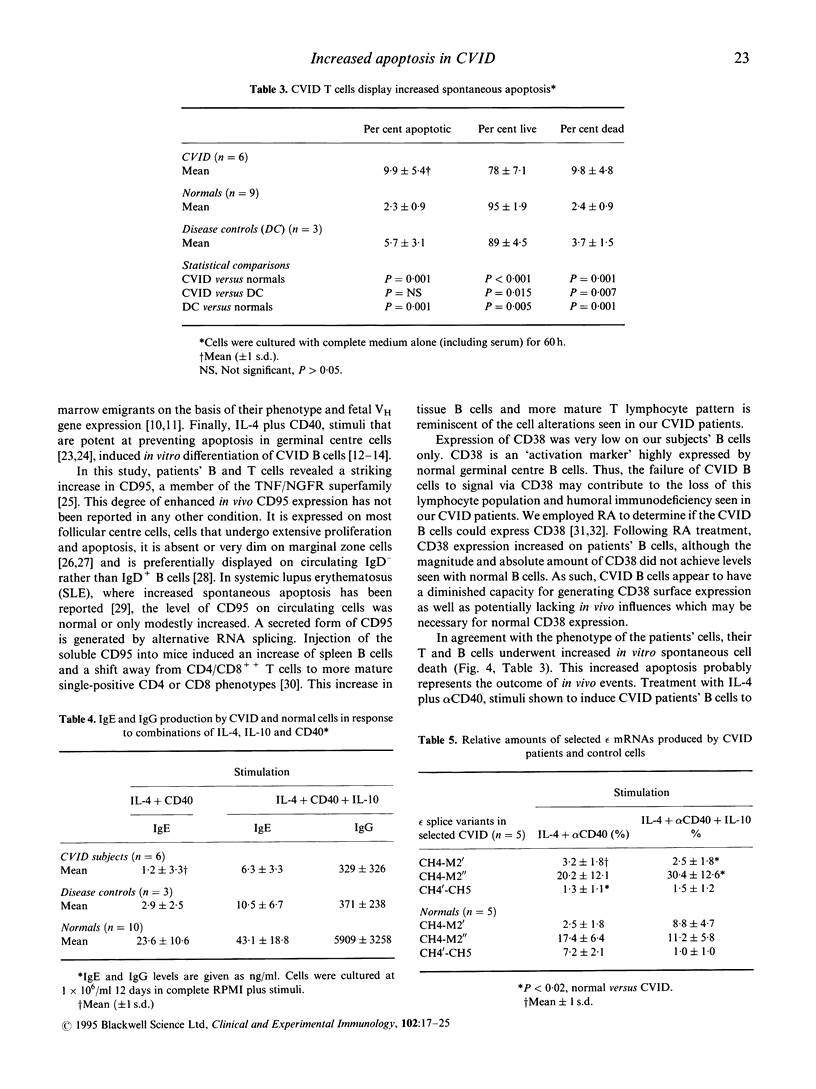
We investigated the role of apoptosis in the differentiation failure of B cells from a selected subpopulation of patients with CVID delineated by B cell surface marker analysis, in vitro IgE response, and molecular markers of B cell VH gene repertoire. These patients had altered display of B cell surface molecules that play a role in apoptosis. The patients' B cells had a 4.5-250-fold increase in CD95 (Apo-1, fas) expression and increased CD95 display on their T cells. CD38, a molecule important in preventing germinal centre B cell apoptosis, was reduced on the patients' B cells. The expression of this molecule was inducible on the CVID lymphocytes with retinoic acid. Increased spontaneous apoptosis in vitro was observed with the patients' B (23%) and T cells (10%) compared with normal cells (13% and 3%, respectively). Stimulation in vitro with IL-4 and CD40 rescued the B cells from apoptosis and allowed for their differentiation. However, IL-4 plus alpha CD40-driven immunoglobulin production was not quantitatively or qualitatively normal. Failure to overcome apoptosis, a normal step in germinal centre B cell development, may be involved in the lack of differentiation seen in this subset of CVID patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman D. C., Matsuda T., Hirano T., Kishimoto T., Saxon A. Elevated serum interleukin-6 associated with a failure in B cell differentiation in common variable immunodeficiency. J Allergy Clin Immunol. 1990 Oct;86(4 Pt 1):512–521. doi: 10.1016/s0091-6749(05)80207-6. [DOI] [PubMed] [Google Scholar]
- Adelman D. C., Yen T. Y., Cumberland W. G., Sidell N., Saxon A. 13-cis retinoic acid enhances in vivo B-lymphocyte differentiation in patients with common variable immunodeficiency. J Allergy Clin Immunol. 1991 Nov;88(5):705–712. doi: 10.1016/0091-6749(91)90176-o. [DOI] [PubMed] [Google Scholar]
- Baumert E., Wolff-Vorbeck G., Schlesier M., Peter H. H. Immunophenotypical alterations in a subset of patients with common variable immunodeficiency (CVID). Clin Exp Immunol. 1992 Oct;90(1):25–30. doi: 10.1111/j.1365-2249.1992.tb05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Berberian L., King L., Sanz I., Govan H. L., 3rd Restricted use of fetal VH3 immunoglobulin genes by unselected B cells in the adult. Predominance of 56p1-like VH genes in common variable immunodeficiency. J Clin Invest. 1992 May;89(5):1395–1402. doi: 10.1172/JCI115728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Galbraith L., Valles-Ayoub Y., Saxon A. Human immunodeficiency resulting from a maturational arrest of germinal center B cells. Immunol Lett. 1991 Mar;27(3):205–208. doi: 10.1016/0165-2478(91)90152-z. [DOI] [PubMed] [Google Scholar]
- Cheng J., Zhou T., Liu C., Shapiro J. P., Brauer M. J., Kiefer M. C., Barr P. J., Mountz J. D. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994 Mar 25;263(5154):1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol. 1989 Jan;9(1):22–33. doi: 10.1007/BF00917124. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Chegini S., Zhang K., Saxon A. CD58 (LFA-3) stimulation provides a signal for human isotype switching and IgE production distinct from CD40. J Immunol. 1994 Jul 1;153(1):10–20. [PubMed] [Google Scholar]
- Diaz-Sanchez D., Dotson A. R., Takenaka H., Saxon A. Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms. J Clin Invest. 1994 Oct;94(4):1417–1425. doi: 10.1172/JCI117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drach J., Zhao S., Malavasi F., Mehta K. Rapid induction of CD38 antigen on myeloid leukemia cells by all trans-retinoic acid. Biochem Biophys Res Commun. 1993 Sep 15;195(2):545–550. doi: 10.1006/bbrc.1993.2080. [DOI] [PubMed] [Google Scholar]
- Eisenstein E. M., Chua K., Strober W. B cell differentiation defects in common variable immunodeficiency are ameliorated after stimulation with anti-CD40 antibody and IL-10. J Immunol. 1994 Jun 15;152(12):5957–5968. [PubMed] [Google Scholar]
- Emlen W., Niebur J., Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994 Apr 1;152(7):3685–3692. [PubMed] [Google Scholar]
- Fischer M. B., Wolf H. M., Eggenbauer H., Thon V., Vogel E., Lokaj J., Litzman J., Mannhalter J. W., Eibl M. M. The costimulatory signal CD28 is fully functional but cannot correct the impaired antigen response in T cells of patients with common variable immunodeficiency. Clin Exp Immunol. 1994 Feb;95(2):209–214. doi: 10.1111/j.1365-2249.1994.tb06512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder M. J., Wang H., Milner A. E., Casamayor M., Armitage R., Spriggs M. K., Fanslow W. C., MacLennan I. C., Gregory C. D., Gordon J. Suppression of apoptosis in normal and neoplastic human B lymphocytes by CD40 ligand is independent of Bc1-2 induction. Eur J Immunol. 1993 Sep;23(9):2368–2371. doi: 10.1002/eji.1830230948. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Iwai K., Miyawaki T., Takizawa T., Konno A., Ohta K., Yachie A., Seki H., Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994 Aug 15;84(4):1201–1208. [PubMed] [Google Scholar]
- Jaffe J. S., Strober W., Sneller M. C. Functional abnormalities of CD8+ T cells define a unique subset of patients with common variable immunodeficiency. Blood. 1993 Jul 1;82(1):192–201. [PubMed] [Google Scholar]
- Kontani K., Nishina H., Ohoka Y., Takahashi K., Katada T. NAD glycohydrolase specifically induced by retinoic acid in human leukemic HL-60 cells. Identification of the NAD glycohydrolase as leukocyte cell surface antigen CD38. J Biol Chem. 1993 Aug 15;268(23):16895–16898. [PubMed] [Google Scholar]
- Liu Y. J., Johnson G. D., Gordon J., MacLennan I. C. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992 Jan;13(1):17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Uehara T., Nibu R., Tsuji T., Yachie A., Yonehara S., Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992 Dec 1;149(11):3753–3758. [PubMed] [Google Scholar]
- Möller P., Henne C., Leithäuser F., Eichelmann A., Schmidt A., Brüderlein S., Dhein J., Krammer P. H. Coregulation of the APO-1 antigen with intercellular adhesion molecule-1 (CD54) in tonsillar B cells and coordinate expression in follicular center B cells and in follicle center and mediastinal B-cell lymphomas. Blood. 1993 Apr 15;81(8):2067–2075. [PubMed] [Google Scholar]
- Nonoyama S., Farrington M., Ishida H., Howard M., Ochs H. D. Activated B cells from patients with common variable immunodeficiency proliferate and synthesize immunoglobulin. J Clin Invest. 1993 Sep;92(3):1282–1287. doi: 10.1172/JCI116701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North M. E., Akbar A. N., Borthwick N., Sagawa K., Funauchi M., Webster A. D., Farrant J. Co-stimulation with anti-CD28 (Kolt-2) enhances DNA synthesis by defective T cells in common variable immunodeficiency. Clin Exp Immunol. 1994 Feb;95(2):204–208. doi: 10.1111/j.1365-2249.1994.tb06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Yonehara S., Crump W. L., 3rd, Grimm E. A. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992 Mar;140(1):197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- Pascual V., Liu Y. J., Magalski A., de Bouteiller O., Banchereau J., Capra J. D. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994 Jul 1;180(1):329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S. J., Scott C. S., Cole J. C., Gooi H. C. Abnormal CD45R expression in patients with common variable immunodeficiency and X-linked agammaglobulinaemia. Br J Haematol. 1992 Jun;81(2):160–166. doi: 10.1111/j.1365-2141.1992.tb08201.x. [DOI] [PubMed] [Google Scholar]
- Santos-Argumedo L., Teixeira C., Preece G., Kirkham P. A., Parkhouse R. M. A B lymphocyte surface molecule mediating activation and protection from apoptosis via calcium channels. J Immunol. 1993 Sep 15;151(6):3119–3130. [PubMed] [Google Scholar]
- Saxon A., Giorgi J. V., Sherr E. H., Kagan J. M. Failure of B cells in common variable immunodeficiency to transit from proliferation to differentiation is associated with altered B cell surface-molecule display. J Allergy Clin Immunol. 1989 Jul;84(1):44–55. doi: 10.1016/0091-6749(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Saxon A., Keld B., Braun J., Dotson A., Sidell N. Long-term administration of 13-cis retinoic acid in common variable immunodeficiency: circulating interleukin-6 levels, B-cell surface molecule display, and in vitro and in vivo B-cell antibody production. Immunology. 1993 Nov;80(3):477–487. [PMC free article] [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C. H., Keld B., Giorgi J. V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994 Apr 15;170(2):145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Sneller M. C., Strober W., Eisenstein E., Jaffe J. S., Cunningham-Rundles C. NIH conference. New insights into common variable immunodeficiency. Ann Intern Med. 1993 May 1;118(9):720–730. doi: 10.7326/0003-4819-118-9-199305010-00011. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Matamoros N., Farrant J. Lymphocyte surface phenotype in common variable immunodeficiency. Dis Markers. 1992 Mar-Apr;10(2):67–80. [PubMed] [Google Scholar]
- Spickett G. P., Webster A. D., Farrant J. Cellular abnormalities in common variable immunodeficiency. Immunodefic Rev. 1990;2(3):199–219. [PubMed] [Google Scholar]
- Stagg A. J., Funauchi M., Knight S. C., Webster A. D., Farrant J. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID). Clin Exp Immunol. 1994 Apr;96(1):48–53. doi: 10.1111/j.1365-2249.1994.tb06228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. J., Wagner D. K., Blaese R. M., Hagengruber C., Waldmann T. A., Fleisher T. A. Characterization of common variable immunodeficiency: identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood. 1990 Nov 15;76(10):2046–2051. [PubMed] [Google Scholar]
- Yoshino T., Kondo E., Cao L., Takahashi K., Hayashi K., Nomura S., Akagi T. Inverse expression of bcl-2 protein and Fas antigen in lymphoblasts in peripheral lymph nodes and activated peripheral blood T and B lymphocytes. Blood. 1994 Apr 1;83(7):1856–1861. [PubMed] [Google Scholar]
- Zhang K., Max E. E., Cheah H. K., Saxon A. Complex alternative RNA splicing of epsilon-immunoglobulin transcripts produces mRNAs encoding four potential secreted protein isoforms. J Biol Chem. 1994 Jan 7;269(1):456–462. [PubMed] [Google Scholar]