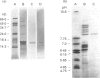
Abstract
Some cysteine proteases such as papain and those of mites and schistosomes have potent allergenic properties. To clarify the allergenicity of nematode cysteine proteases, the enzyme was purified from the intestinal nematode Nippostrongylus brasiliensis using cation exchange chromatography and gel filtration chromatography. The purified protease, of 16 kD and pI 8.5, showed maximum enzyme activity at pH 5.5 and substrate preference for Z-Phe-Arg-MCA. The specific inhibitors of cysteine protease leupeptin, iodoacetic acid, and E-64, completely suppressed the activity, indicating that the purified enzyme belongs to the cysteine protease family. Cysteine protease activity was found not only in somatic extract, but also in the excretory-secretory (ES) product of the nematode. When anti-cysteine protease immunoglobulin isotypes were examined in sera from rats infected with N. brasiliensis, a high level of IgG1 and a lower level of IgE antibody were detected. Depletion of IgG antibodies from the sera using protein G affinity columns resulted in a marked increase in reactivity of anti-cysteine protease IgE with the antigen, possibly due to the removal of competing IgG antibodies. In contrast to IgE and IgG1, production of anti-cysteine protease IgG2a was negligible. These results indicate that the nematode cysteine protease preferentially evokes an IgE/IgG1 antibody response.
Full text
PDF

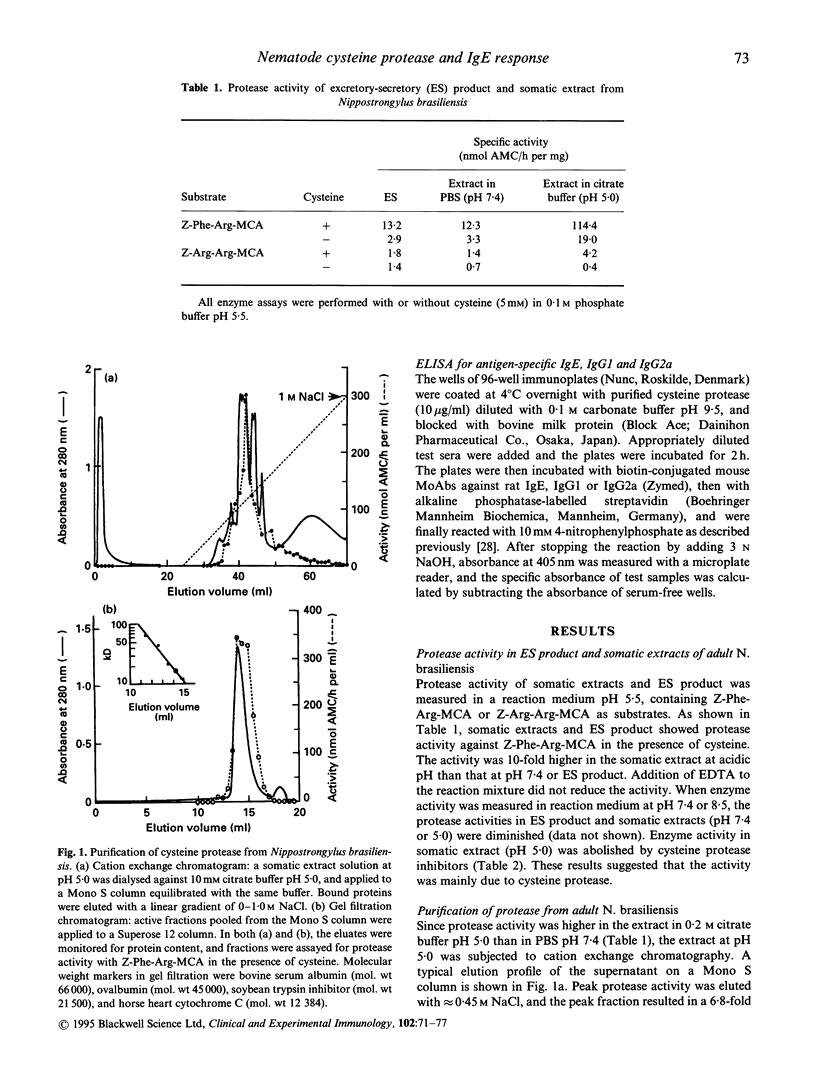

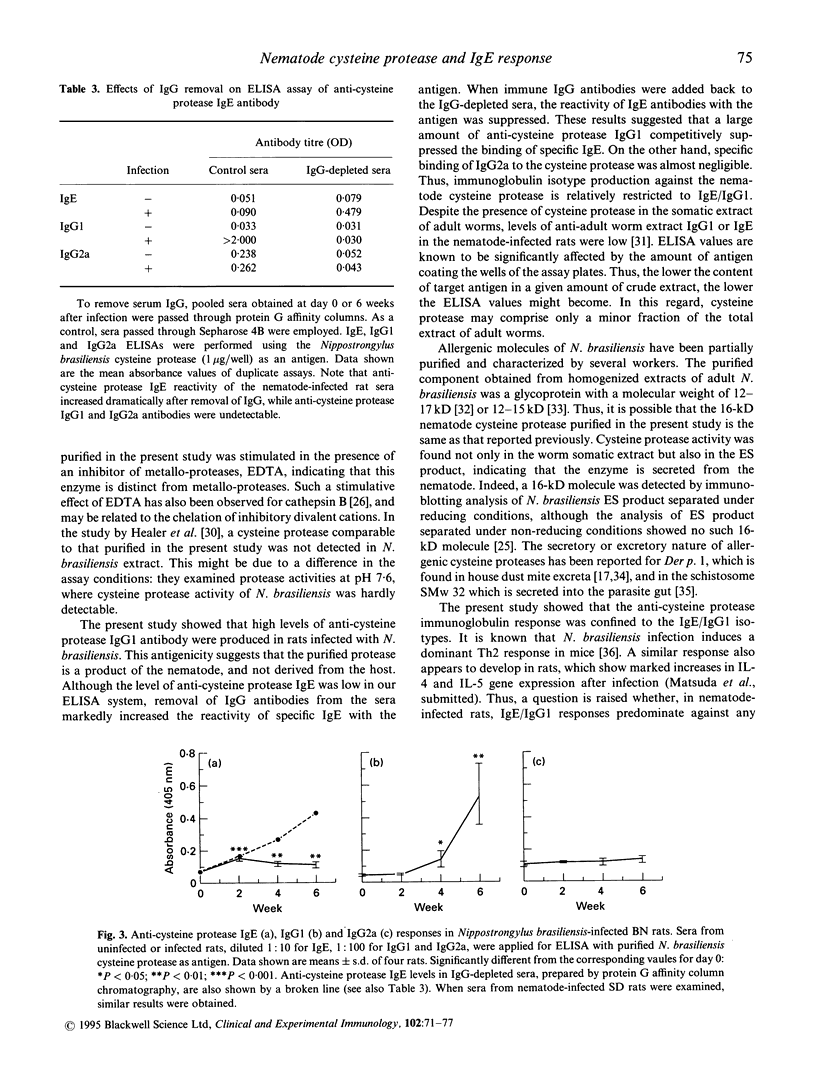


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell C. L., Dresden M. H. Purification of cysteine proteinases from adult Schistosoma mansoni. Arch Biochem Biophys. 1987 Aug 1;256(2):560–568. doi: 10.1016/0003-9861(87)90613-8. [DOI] [PubMed] [Google Scholar]
- Chappell C. L., Kalter D. C., Dresden M. H. The hypersensitivity response to the adult worm proteinase, SMw32, in Schistosoma mansoni infected mice. Am J Trop Med Hyg. 1988 Nov;39(5):463–468. doi: 10.4269/ajtmh.1988.39.463. [DOI] [PubMed] [Google Scholar]
- Chua K. Y., Stewart G. A., Thomas W. R., Simpson R. J., Dilworth R. J., Plozza T. M., Turner K. J. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J Exp Med. 1988 Jan 1;167(1):175–182. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Pratt D., Hageman R., Boisvenue R. J. Molecular cloning and primary sequence of a cysteine protease expressed by Haemonchus contortus adult worms. Mol Biochem Parasitol. 1990 Jun;41(1):25–34. doi: 10.1016/0166-6851(90)90093-2. [DOI] [PubMed] [Google Scholar]
- Culpepper J., Grieve R. B., Friedman L., Mika-Grieve M., Frank G. R., Dale B. Molecular characterization of a Dirofilaria immitis cDNA encoding a highly immunoreactive antigen. Mol Biochem Parasitol. 1992 Aug;54(1):51–62. doi: 10.1016/0166-6851(92)90094-z. [DOI] [PubMed] [Google Scholar]
- Dresden M. H., Payne D. C., Basch P. F. Acidic thiol proteinase activity of Schistosoma mansoni cultured in vitro. Mol Biochem Parasitol. 1982 Oct;6(4):203–208. doi: 10.1016/0166-6851(82)90054-8. [DOI] [PubMed] [Google Scholar]
- Dresden M. H., Rege A. A., Murrell K. D. Strongyloides ransomi: proteolytic enzymes from larvae. Exp Parasitol. 1985 Apr;59(2):257–263. doi: 10.1016/0014-4894(85)90080-3. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Urban J. F., Jr Cytokines: making the right choice. Parasitol Today. 1992 Sep;8(9):311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- Healer J., Ashall F., Maizels R. M. Characterization of proteolytic enzymes from larval and adult Nippostrongylus brasiliensis. Parasitology. 1991 Oct;103(Pt 2):305–314. doi: 10.1017/s0031182000059588. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- Jones V. E., Ogilvie B. M. Reaginic antibodies and immunity to Nippostrongylus brasiliensis in the rat. II. Some properties of the antibodies and antigens. Immunology. 1967 May;12(5):583–597. [PMC free article] [PubMed] [Google Scholar]
- King C. L., Low C. C., Nutman T. B. IgE production in human helminth infection. Reciprocal interrelationship between IL-4 and IFN-gamma. J Immunol. 1993 Mar 1;150(5):1873–1880. [PubMed] [Google Scholar]
- Novey H. S., Marchioli L. E., Sokol W. N., Wells I. D. Papain-induced asthma--physiological and immunological features. J Allergy Clin Immunol. 1979 Feb;63(2):98–103. doi: 10.1016/0091-6749(79)90198-2. [DOI] [PubMed] [Google Scholar]
- Paxton W. A., Yazdanbakhsh M., Kurniawan A., Partono F., Maizels R. M., Selkirk M. E. Primary structure of and immunoglobulin E response to the repeat subunit of gp15/400 from human lymphatic filarial parasites. Infect Immun. 1993 Jul;61(7):2827–2833. doi: 10.1128/iai.61.7.2827-2833.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond L., Wassom D. L., Hayes C. E. Evidence for differential induction of helper T cell subsets during Trichinella spiralis infection. J Immunol. 1989 Dec 15;143(12):4232–4237. [PubMed] [Google Scholar]
- Poole C. B., Grandea A. G., 3rd, Maina C. V., Jenkins R. E., Selkirk M. E., McReynolds L. A. Cloning of a cuticular antigen that contains multiple tandem repeats from the filarial parasite Dirofilaria immitis. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5986–5990. doi: 10.1073/pnas.89.13.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege A. A., Herrera P. R., Lopez M., Dresden M. H. Isolation and characterization of a cysteine proteinase from Fasciola hepatica adult worms. Mol Biochem Parasitol. 1989 Jun 1;35(1):89–95. doi: 10.1016/0166-6851(89)90146-1. [DOI] [PubMed] [Google Scholar]
- Rege A. A., Wang W., Dresden M. H. Cysteine proteinases from Schistosoma haematobium adult worms. J Parasitol. 1992 Feb;78(1):16–23. [PubMed] [Google Scholar]
- Sarkis G. J., Kurpiewski M. R., Ashcom J. D., Jen-Jacobson L., Jacobson L. A. Proteases of the nematode Caenorhabditis elegans. Arch Biochem Biophys. 1988 Feb 15;261(1):80–90. doi: 10.1016/0003-9861(88)90106-3. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Cheever A. W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990 Dec 1;145(11):3911–3916. [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Soloway P., Fish S., Passmore H., Gefter M., Coffee R., Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or major histocompatibility complex haplotype. J Exp Med. 1991 Oct 1;174(4):847–858. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence H. J., Moore J., Brass A., Kennedy M. W. A cDNA encoding repeating units of the ABA-1 allergen of Ascaris. Mol Biochem Parasitol. 1993 Feb;57(2):339–343. doi: 10.1016/0166-6851(93)90210-o. [DOI] [PubMed] [Google Scholar]
- Stewart G. A., Thompson P. J., Simpson R. J. Protease antigens from house dust mite. Lancet. 1989 Jul 15;2(8655):154–155. doi: 10.1016/s0140-6736(89)90203-1. [DOI] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Svetić A., Madden K. B., Zhou X. D., Lu P., Katona I. M., Finkelman F. D., Urban J. F., Jr, Gause W. C. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J Immunol. 1993 Apr 15;150(8 Pt 1):3434–3441. [PubMed] [Google Scholar]
- Tovey E. R., Chapman M. D., Platts-Mills T. A. Mite faeces are a major source of house dust allergens. Nature. 1981 Feb 12;289(5798):592–593. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- Tweedie S., Paxton W. A., Ingram L., Maizels R. M., McReynolds L. A., Selkirk M. E. Brugia pahangi and Brugia malayi: a surface-associated glycoprotein (gp15/400) is composed of multiple tandemly repeated units and processed from a 400-kDa precursor. Exp Parasitol. 1993 Mar;76(2):156–164. doi: 10.1006/expr.1993.1018. [DOI] [PubMed] [Google Scholar]
- Urban J. F., Jr, Katona I. M., Paul W. E., Finkelman F. D. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. J. Homocytotropic antibody response to the nematode Nippostrongylus brasiliensis in the rat--studies on the worm antigen. J Parasitol. 1967 Aug;53(4):752–762. [PubMed] [Google Scholar]
- Yamada M., Nakazawa M., Arizono N. IgE and IgG2a antibody responses are induced by different antigen groups of the nematode Nippostrongylus brasiliensis in rats. Immunology. 1993 Feb;78(2):298–302. [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Nakazawa M., Matsumoto Y., Arizono N. IgE antibody production in rats against multiple components of excretory-secretory products of the nematode Nippostrongylus brasiliensis. Immunology. 1991 Jan;72(1):104–108. [PMC free article] [PubMed] [Google Scholar]
- Zerda K. S., Dresden M. H., Chappell C. L. Schistosoma mansoni: expression and role of cysteine proteinases in developing schistosomula. Exp Parasitol. 1988 Dec;67(2):238–246. doi: 10.1016/0014-4894(88)90071-9. [DOI] [PubMed] [Google Scholar]
- Zhang L., Mohapatra S. S. Antigen- and isotype-specific immune responses to a recombinant antigen-allergen chimeric (RAAC) protein. J Immunol. 1993 Jul 15;151(2):791–799. [PubMed] [Google Scholar]