Abstract
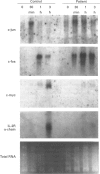
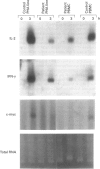
Cartilage-hair hypoplasia (CHH) is an autosomal recessive disease of unknown etiology characterized by metaphyseal dysostosis, unpigmented hair, and defective cellular immunity. We studied peripheral blood mononuclear cells (PBMC) of a boy with CHH and combined immunodeficiency in an attempt to characterize further the immune defect in this disease. Stimulation of his PBMC with mitogens was associated with severely depressed IL-2 and interferon-gamma (IFN-gamma) synthesis and IL-2 receptor alpha-chain (IL-2R alpha) expression and resulted in poor lymphocyte proliferation that was only modestly upregulated by the addition of recombinant IL-2 (rIL-2). The defective proliferation and lymphokine synthesis were not corrected by the addition of phorbol myristate acetate (PMA) and ionomycin, agents that bypass receptor-mediated signalling, indicative of a distal abnormality. Importantly, the levels of mRNA encoding c-myc, IL-2R alpha, IL-2 and IFN-gamma were markedly decreased in patient lymphocytes stimulated with PMA+ionomycin as compared to control lymphocytes. The defect in the expression of these early activation genes was selective in that induction by mitogens of mRNA encoding other early activation gene products such as c-fos and c-jun was not impaired. These results suggest that the underlying defect in this patient and perhaps others with CHH may be an abnormality in a component of intracellular signalling pathways or in a trans-acting factor which regulates the expression of a selected number of early activation genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatila T., Castigli E., Pahwa R., Pahwa S., Chirmule N., Oyaizu N., Good R. A., Geha R. S. Primary combined immunodeficiency resulting from defective transcription of multiple T-cell lymphokine genes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10033–10037. doi: 10.1073/pnas.87.24.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Keever C. A., Small T. N., Nicols G. L., O'Reilly R. J., Flomenberg N. Absence of interleukin 2 production in a severe combined immunodeficiency disease syndrome with T cells. J Exp Med. 1990 May 1;171(5):1697–1704. doi: 10.1084/jem.171.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyao M. P., Buckler A. J., Sonenshein G. E. Interaction of an NF-kappa B-like factor with a site upstream of the c-myc promoter. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4727–4731. doi: 10.1073/pnas.87.12.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyao M. P., Kessler D. J., Spicer D. B., Bartholomew C., Cleveland J. L., Siekevitz M., Sonenshein G. E. Transactivation of the c-myc promoter by human T cell leukemia virus type 1 tax is mediated by NF kappa B. J Biol Chem. 1992 Aug 15;267(23):16288–16291. [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Iwamoto M., Yagami K., Lu Valle P., Olsen B. R., Petropoulos C. J., Ewert D. L., Pacifici M. Expression and role of c-myc in chondrocytes undergoing endochondral ossification. J Biol Chem. 1993 May 5;268(13):9645–9652. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Böhnlein E., Greene W. C. Tumor necrosis factor alpha induces proteins that bind specifically to kappa B-like enhancer elements and regulate interleukin 2 receptor alpha-chain gene expression in primary human T lymphocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2331–2335. doi: 10.1073/pnas.86.7.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKUSICK V. A., ELDRIDGE R., HOSTETLER J. A., RUANGWIT U., EGELAND J. A. DWARFISM IN THE AMISH. II. CARTILAGE-HAIR HYPOPLASIA. Bull Johns Hopkins Hosp. 1965 May;116:285–326. [PubMed] [Google Scholar]
- Mäkitie O., Kaitila I. Cartilage-hair hypoplasia--clinical manifestations in 108 Finnish patients. Eur J Pediatr. 1993 Mar;152(3):211–217. doi: 10.1007/BF01956147. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Polmar S. H. Lymphocyte dysfunction in cartilage-hair hypoplasia: evidence for an intrinsic defect in cellular proliferation. J Immunol. 1982 Aug;129(2):570–575. [PubMed] [Google Scholar]
- Polmar S. H., Pierce G. F. Cartilage hair hypoplasia: immunological aspects and their clinical implications. Clin Immunol Immunopathol. 1986 Jul;40(1):87–93. doi: 10.1016/0090-1229(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Taniguchi T. Involvement of a common transcription factor in the regulated expression of IL-2 and IL-2 receptor genes. Int Immunol. 1989;1(1):43–49. doi: 10.1093/intimm/1.1.43. [DOI] [PubMed] [Google Scholar]
- Sulisalo T., Sistonen P., Hästbacka J., Wadelius C., Mäkitie O., de la Chapelle A., Kaitila I. Cartilage-hair hypoplasia gene assigned to chromosome 9 by linkage analysis. Nat Genet. 1993 Apr;3(4):338–341. doi: 10.1038/ng0493-338. [DOI] [PubMed] [Google Scholar]
- Weinberg K., Parkman R. Severe combined immunodeficiency due to a specific defect in the production of interleukin-2. N Engl J Med. 1990 Jun 14;322(24):1718–1723. doi: 10.1056/NEJM199006143222406. [DOI] [PubMed] [Google Scholar]