Abstract
Hyperacute rejection triggered by activation of the recipient's complement system represents the major barrier to successful xenotransplantation. Transfer of human membrane-associated complement regulators to donor organs has been suggested as one strategy to interfere with complement-mediated hyperacute xenograft rejection. Pigs are discussed as potential organ donors. We therefore investigated a putative protective function of the membrane-bound complement inhibitor CD59 in a pig-to-human in vitro model of hyperacute xenograft rejection. Aortic porcine endothelial cells were transfected with human CD59 cDNA. Expression of human CD59 was demonstrated by cytofluorimetric and RNA analysis. Removal of CD59 from the cell surface by phosphatidylinositol-specific phospholipase C (PI-PLC) demonstrated its production as a glycosyl phosphatidylinositol (GPI)-anchored protein. Functional activity of the transfected CD59 was tested by a lactate dehydrogenase (LDH) release assay for complement-mediated lysis. Porcine endothelial cells expressing human CD59 were significantly protected from lysis by human serum complement compared with CD59- cells. The protective effect was abolished by preincubating the cells with anti-CD59 antibodies or PI-PLC. We calculated by Scatchard analysis that the established CD59+ cell line expressed a CD59 level comparable to that of human endothelial cells. Our results recommend the production of pigs transgenic for CD59.
Full text
PDF

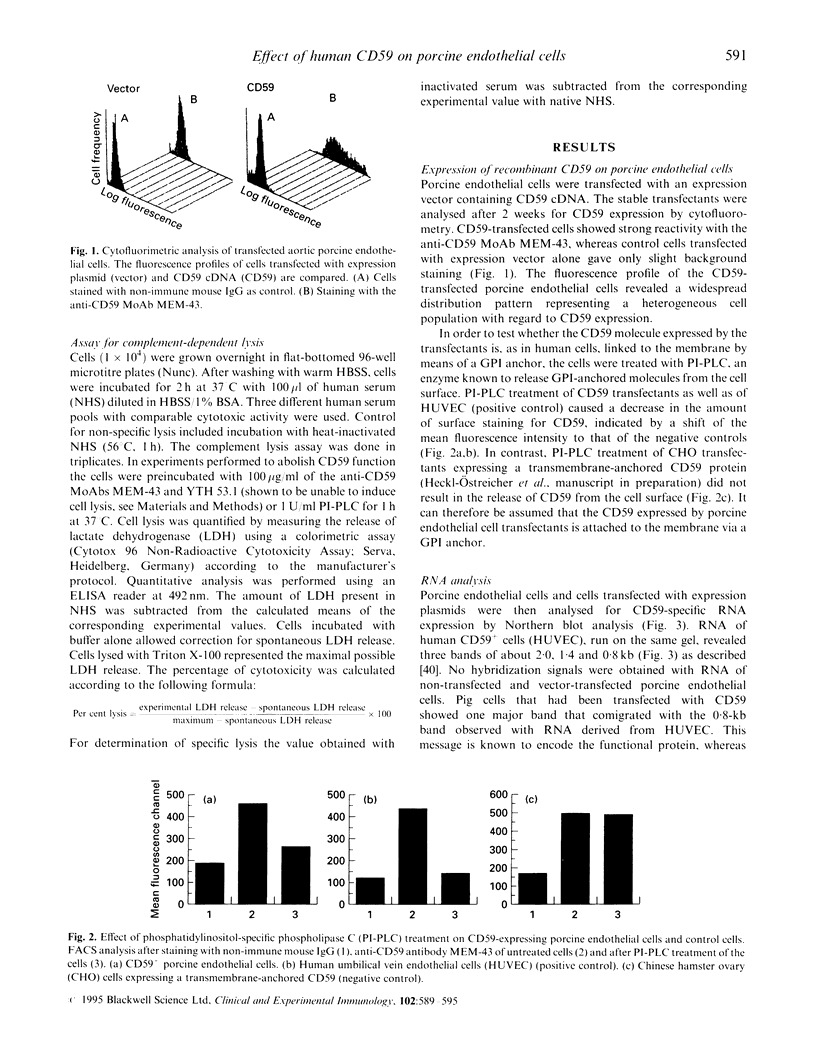
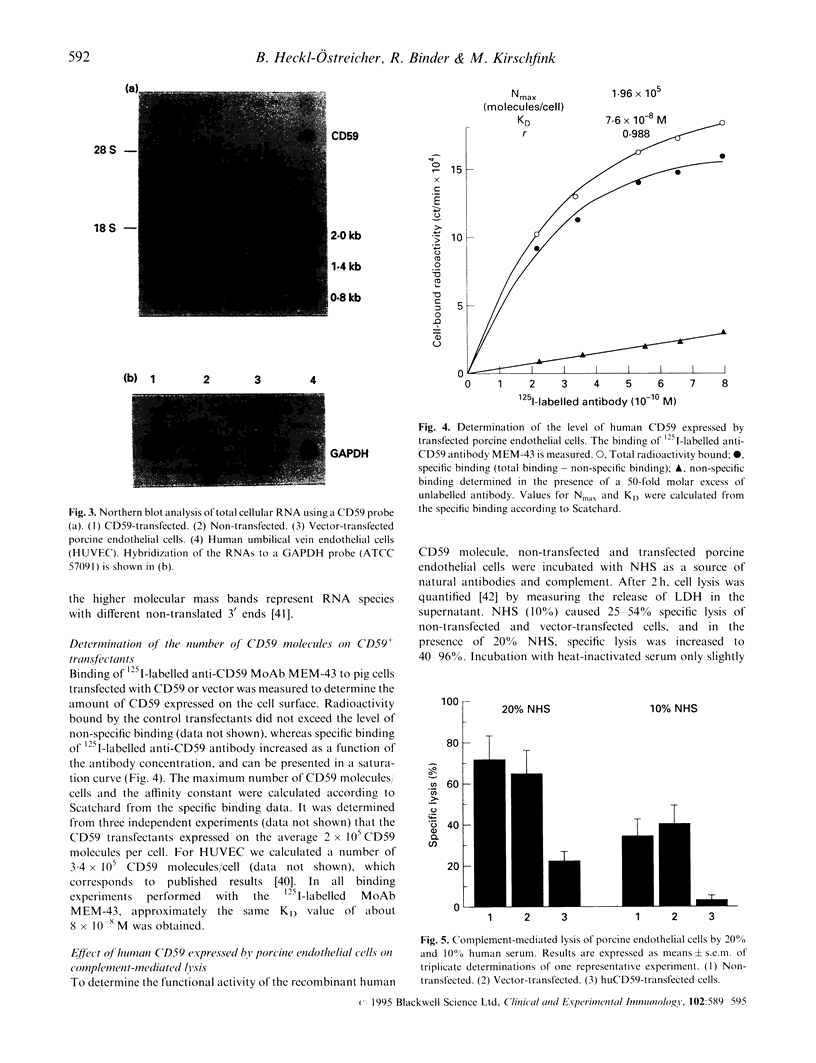
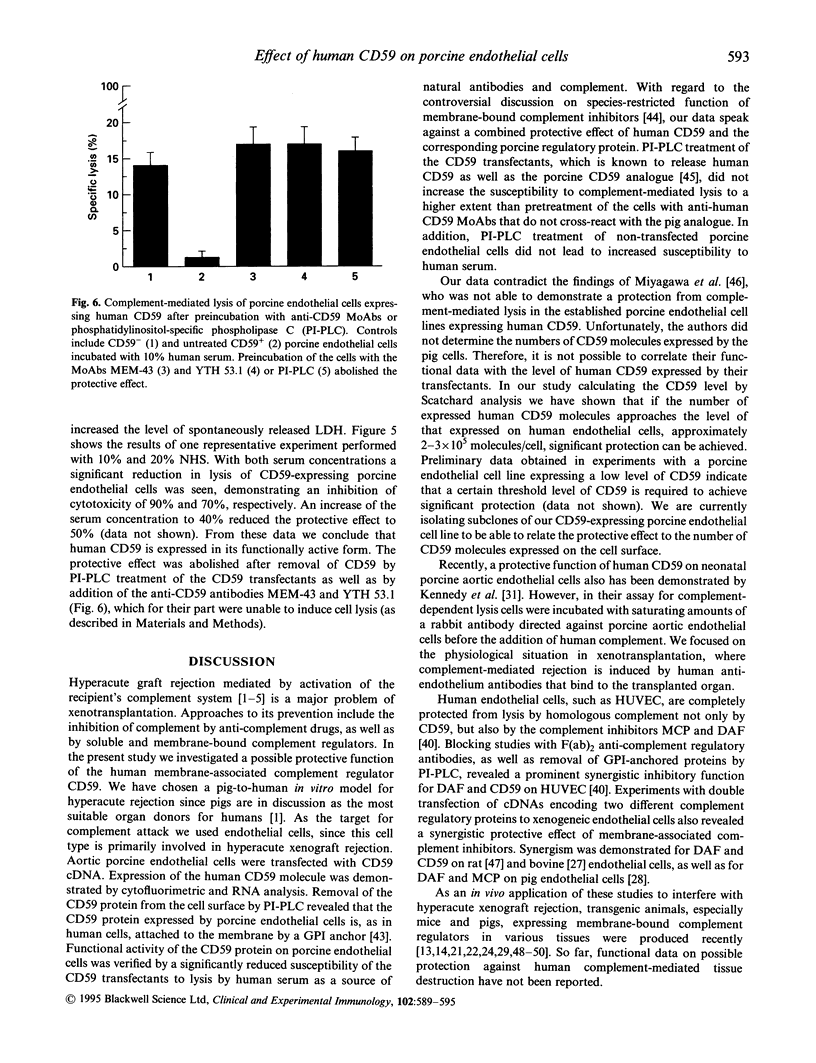


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akami T., Arakawa K., Okamoto M., Akioka K., Fujiwara I., Nakai I., Mitsuo M., Tomita N., Kaneda Y., Tanaka K. Introduction and expression of human CD59 gene in the canine kidney. Transplant Proc. 1994 Jun;26(3):1315–1317. [PubMed] [Google Scholar]
- Auchincloss H., Jr Xenogeneic transplantation. A review. Transplantation. 1988 Jul;46(1):1–20. doi: 10.1097/00007890-198807000-00001. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Platt J. L. Xenotransplantation: a view of issues. Transplant Proc. 1992 Aug;24(4 Suppl 2):49–52. [PubMed] [Google Scholar]
- Brooimans R. A., Van der Ark A. A., Tomita M., Van Es L. A., Daha M. R. CD59 expressed by human endothelial cells functions as a protective molecule against complement-mediated lysis. Eur J Immunol. 1992 Mar;22(3):791–797. doi: 10.1002/eji.1830220324. [DOI] [PubMed] [Google Scholar]
- Charreau B., Cassard A., Tesson L., Le Mauff B., Blanchard D., Lublin D., Soulillou J. P., Anegon I. Permanent expression of human CD59 and/or decay-accelerating factor by rat endothelial cells confers protection from human complement-mediated lysis. Transplant Proc. 1995 Feb;27(1):336–337. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cozzi E., Langford G. A., Richards A., Elsome K., Lancaster R., Chen P., Yannoutsos N., White D. J. Expression of human decay accelerating factor in transgenic pigs. Transplant Proc. 1994 Jun;26(3):1402–1403. [PubMed] [Google Scholar]
- Dalmasso A. P., Vercellotti G. M., Platt J. L., Bach F. H. Inhibition of complement-mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation. 1991 Sep;52(3):530–533. doi: 10.1097/00007890-199109000-00029. [DOI] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. E., Oldham E. R., Platt J. L., Waldmann H., Tone M., Walsh L. A., Logan J. S. Cell- and tissue-specific expression of a human CD59 minigene in transgenic mice. Transplant Proc. 1994 Jun;26(3):1239–1239. [PubMed] [Google Scholar]
- Grumach A. S., Kirschfink M. Complement and IgG subclasses in agammaglobulinemic patients. Exp Clin Immunogenet. 1995;12(1):10–15. [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R. C., Logan J. S., Kooyman D., Byrne G. W., Platt J. L. Ex vivo perfusion of mouse hearts expressing the human complement regulatory protein CD59. Transplant Proc. 1994 Jun;26(3):1245–1245. [PubMed] [Google Scholar]
- Hayashi S., Isobe K., Emi N., Kasai Y., Yokoyama I., Takagi H. Protection from complement-mediated cytolysis in cross-species hepatocytes transfected with complement regulatory factor (HRF20) using retroviral vector. Transplant Proc. 1994 Jun;26(3):1252–1252. [PubMed] [Google Scholar]
- Hayashi S., Isobe K., Emi N., Yokoyama I., Takagi H. Inhibition of species-specific complement-mediated cytolysis in xenoendothelial cells transfected with GPI-anchored complement regulatory factor (DAF, HRF20) gene using retroviral vector: comparison between single (DAF or HRF20) and double (DAF and HRF20) transfected xenoendothelial cells. Transplant Proc. 1994 Jun;26(3):1243–1244. [PubMed] [Google Scholar]
- Heckl-Ostreicher B., Ragg S., Drechsler M., Scherthan H., Royer-Pokora B. Localization of the human CD59 gene by fluorescence in situ hybridization and pulsed-field gel electrophoresis. Cytogenet Cell Genet. 1993;63(3):144–146. doi: 10.1159/000133522. [DOI] [PubMed] [Google Scholar]
- Kagan D., Platt J., Logan J., Byrne G. W. Expression of complement regulatory factors using heterologous promoters in transgenic mice. Transplant Proc. 1994 Jun;26(3):1242–1242. [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. P., Rollins S. A., Burton W. V., Sims P. J., Bothwell A. L., Squinto S. P., Zavoico G. B. Protection of porcine aortic endothelial cells from complement-mediated cell lysis and activation by recombinant human CD59. Transplantation. 1994 May 27;57(10):1494–1501. [PubMed] [Google Scholar]
- Kooyman D., Byrne G., McClellan S., Nielsen D., Kagan D., Coffman T., Masahide T., Waldmann H., Platt J., Logan J. Erythroid-specific expression of human CD59 and transfer to vascular endothelial cells. Transplant Proc. 1994 Jun;26(3):1241–1241. [PubMed] [Google Scholar]
- Korzeniewski C., Callewaert D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983 Nov 25;64(3):313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Langford G. A., Yannoutsos N., Cozzi E., Lancaster R., Elsome K., Chen P., Richards A., White D. J. Production of pigs transgenic for human decay accelerating factor. Transplant Proc. 1994 Jun;26(3):1400–1401. [PubMed] [Google Scholar]
- Liszewski M. K., Atkinson J. P. Membrane cofactor protein. Curr Top Microbiol Immunol. 1992;178:45–60. doi: 10.1007/978-3-642-77014-2_4. [DOI] [PubMed] [Google Scholar]
- McClellan S., Kagan D., Byrne G., Platt J., Logan J., Kooyman D. Demonstration of intermembrane transfer of CD59 during coculture of human erythrocytes with porcine and bovine aortic endothelial cells. Transplant Proc. 1994 Jun;26(3):1240–1240. [PubMed] [Google Scholar]
- McCurry K. R., Kooyman D. L., Diamond L. E., Byrne G. W., Martin M. J., Logan J. S., Platt J. L. Human complement regulatory proteins in transgenic animals regulate complement activation in xenoperfused organs. Transplant Proc. 1995 Feb;27(1):317–318. [PubMed] [Google Scholar]
- Miyagawa S., Hirose H., Shirakura R., Naka Y., Nakata S., Kawashima Y., Seya T., Matsumoto M., Uenaka A., Kitamura H. The mechanism of discordant xenograft rejection. Transplantation. 1988 Dec;46(6):825–830. doi: 10.1097/00007890-198812000-00007. [DOI] [PubMed] [Google Scholar]
- Miyagawa S., Hirose H., Shirakura R., Nakata S., Naka Y., Kitagawa S., Nakano S., Matsumoto M., Seya T., Kitamura H. The mechanism of discordant xenograft rejection. Transplant Proc. 1989 Feb;21(1 Pt 1):520–521. [PubMed] [Google Scholar]
- Miyagawa S., Shirakura R., Izutani H., Matsumiya G., Nakata S., Matsuda H., Iwata K., Nagasawa S., Terado A., Matsumoto M. Effect of transfectant molecules, MCP, DAF, and MCP/DAF hybrid on xenogeneic vascular endothelium. Transplant Proc. 1994 Jun;26(3):1253–1254. [PubMed] [Google Scholar]
- Miyagawa S., Shirakura R., Matsumiya G., Fukushima N., Nakata S., Matsuda H., Matsumoto M., Kitamura H., Seya T. Prolonging discordant xenograft survival with anticomplement reagents K76COOH and FUT175. Transplantation. 1993 Apr;55(4):709–713. doi: 10.1097/00007890-199304000-00004. [DOI] [PubMed] [Google Scholar]
- Miyagawa S., Shirakura R., Matsumiya G., Nakata S., Matsuda H., Hatanaka M., Matsumoto M., Seya T. Possibility of prevention of hyperacute rejection by DAF and CD59 in xenotransplantation. Transplant Proc. 1994 Jun;26(3):1235–1238. [PubMed] [Google Scholar]
- Miyagawa S., Shirakura R., Nakata S., Izutani H., Matsuda H., Iwata K., Nagasawa S., Terado A., Hatanaka M., Matsumoto M. Effect of transfected MACIF (CD59) on complement-mediated swine endothelial cell lysis, compared with those of membrane cofactor protein (CD46) and decay-accelerating factor (CD55). Transplant Proc. 1995 Feb;27(1):328–329. [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Platt J. L., Bach F. H. The barrier to xenotransplantation. Transplantation. 1991 Dec;52(6):937–947. doi: 10.1097/00007890-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Vercellotti G. M., Dalmasso A. P., Matas A. J., Bolman R. M., Najarian J. S., Bach F. H. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990 Dec;11(12):450–457. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- Pruitt S. K., Baldwin W. M., 3rd, Marsh H. C., Jr, Lin S. S., Yeh C. G., Bollinger R. R. The effect of soluble complement receptor type 1 on hyperacute xenograft rejection. Transplantation. 1991 Nov;52(5):868–873. doi: 10.1097/00007890-199111000-00022. [DOI] [PubMed] [Google Scholar]
- Ratnoff W. D., Knez J. J., Prince G. M., Okada H., Lachmann P. J., Medof M. E. Structural properties of the glycoplasmanylinositol anchor phospholipid of the complement membrane attack complex inhibitor CD59. Clin Exp Immunol. 1992 Mar;87(3):415–421. doi: 10.1111/j.1365-2249.1992.tb03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönermark S., Rauterberg E. W., Shin M. L., Löke S., Roelcke D., Hänsch G. M. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. 1986 Mar 1;136(5):1772–1776. [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem. 1988 Oct;104(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122524. [DOI] [PubMed] [Google Scholar]
- Tone M., Walsh L. A., Waldmann H. Gene structure of human CD59 and demonstration that discrete mRNAs are generated by alternative polyadenylation. J Mol Biol. 1992 Oct 5;227(3):971–976. doi: 10.1016/0022-2836(92)90239-g. [DOI] [PubMed] [Google Scholar]
- Yannoutsos N., Langford G. A., Cozzi E., Lancaster R., Elsome K., Chen P., White D. J. Production of pigs transgenic for human regulators of complement activation. Transplant Proc. 1995 Feb;27(1):324–325. [PubMed] [Google Scholar]
- Zalman L. S., Wood L. M., Müller-Eberhard H. J. Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6975–6979. doi: 10.1073/pnas.83.18.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg C. W., Harrison R. A., Morgan B. P. A rapid method for the isolation of analogues of human CD59 by preparative SDS-PAGE: application to pig CD59. J Immunol Methods. 1995 Feb 27;179(2):223–231. doi: 10.1016/0022-1759(94)00288-8. [DOI] [PubMed] [Google Scholar]
- van den Berg C. W., Morgan B. P. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J Immunol. 1994 Apr 15;152(8):4095–4101. [PubMed] [Google Scholar]



