Abstract
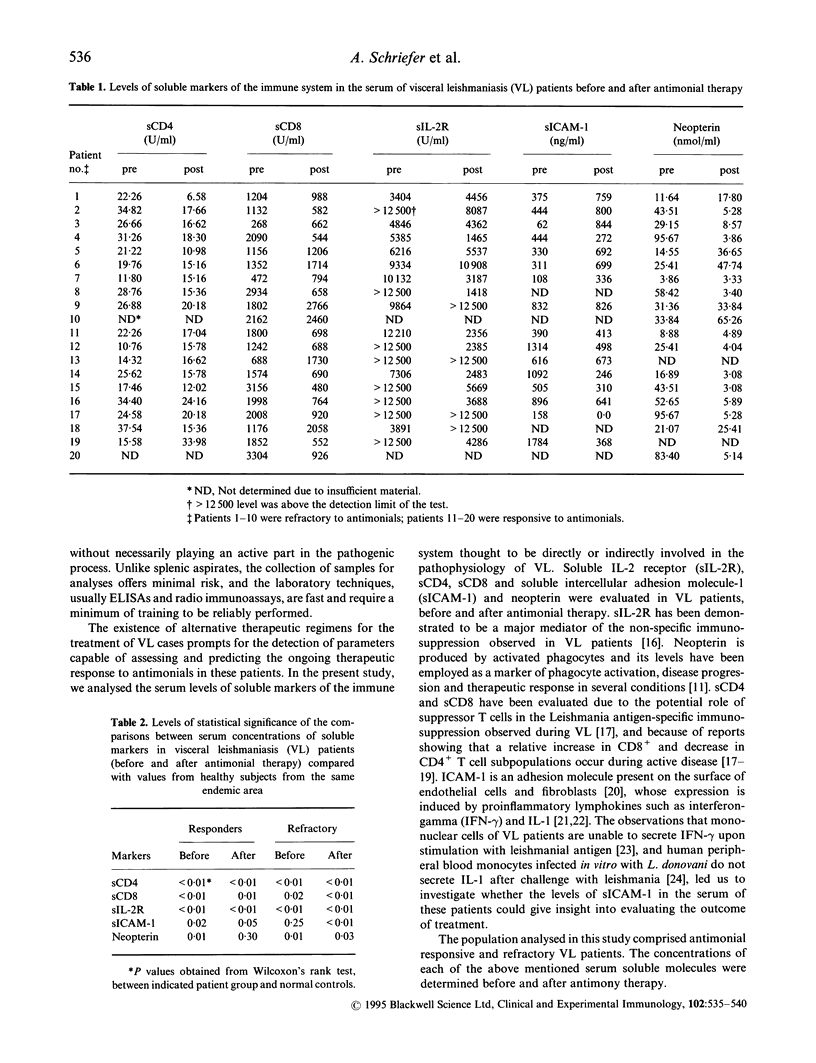
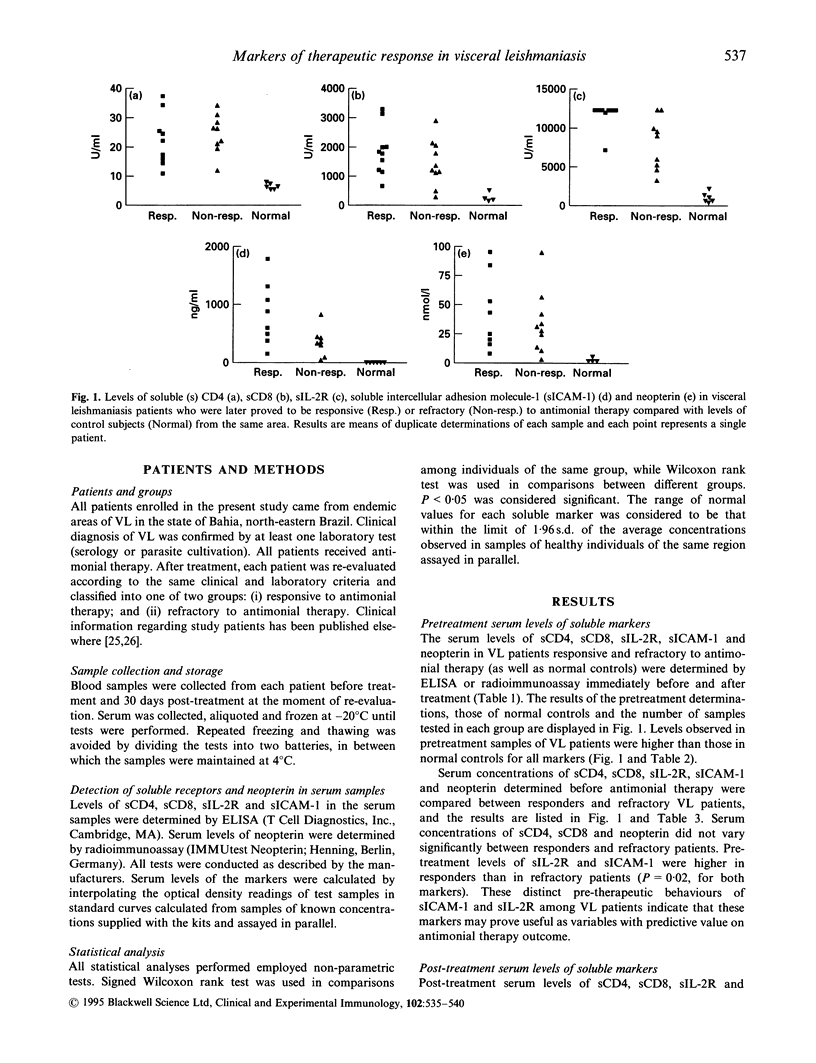
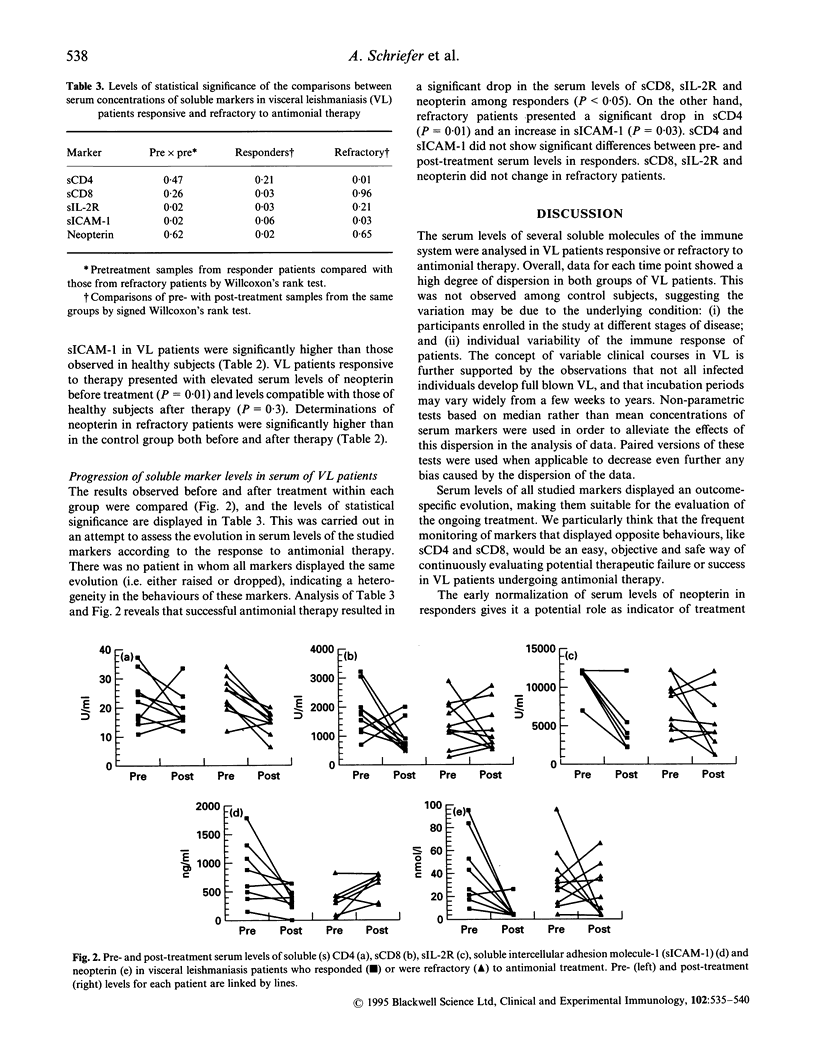
Visceral leishmaniasis (VL) has a fatal course if not properly treated. Recovery from VL is linked to cellular immune response. Unresponsiveness to antimonial therapy reinforces the importance of determining parameters for treatment assessment. We analysed the pre- and post-treatment serum levels of soluble CD4 (sCD4), sCD8, sIL-2R, soluble intercellular adhesion molecule-1 (sICAM-1) and neopterin in groups of VL patients either responsive or not to standard antimonial therapy. Pretreatment serum levels of all markers except for sICAM-1 were significantly higher in VL patients than in healthy subjects from the same area (P < 0.05). sICAM-1 levels were similar in healthy controls and in VL patients refractory to antimonial therapy (P = 0.25), but significantly higher in patients responsive to treatment (P = 0.02). The comparison of pre- and post-treatment concentrations showed that all markers, except sCD4 and sICAM-1, presented a significant fall (P < 0.05) in patients responsive to antimonial therapy. However, only neopterin presented with levels compatible with those of healthy subjects at the end of treatment (P = 0.30). In refractory patients sICAM-1 presented with post-treatment levels significantly higher than the pretreatment determinations (P = 0.03), while sCD4 experienced a significant drop (P = 0.01). All markers displayed clearly distinct behaviour according to the patient's response to therapy. This makes all soluble molecules studied suitable for use as indicators of antimonial therapy response. Additionally the comparison of pretreatment levels of the markers between responders and refractory patients to antimonial therapy showed that serum concentrations of sIL-2R and sICAM-1 significantly differed among these two groups (P = 0.02 in each case), suggesting that they may be used in future as predictors of antimonial therapy response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barral-Netto M., Badaró R., Barral A., Almeida R. P., Santos S. B., Badaró F., Pedral-Sampaio D., Carvalho E. M., Falcoff E., Falcoff R. Tumor necrosis factor (cachectin) in human visceral leishmaniasis. J Infect Dis. 1991 Apr;163(4):853–857. doi: 10.1093/infdis/163.4.853. [DOI] [PubMed] [Google Scholar]
- Barral-Netto M., Barral A., Santos S. B., Carvalho E. M., Badaro R., Rocha H., Reed S. G., Johnson W. D., Jr Soluble IL-2 receptor as an agent of serum-mediated suppression in human visceral leishmaniasis. J Immunol. 1991 Jul 1;147(1):281–284. [PubMed] [Google Scholar]
- Beckham J. C., Caldwell D. S., Peterson B. L., Pippen A. M., Currie M. S., Keefe F. J., Weinberg J. B. Disease severity in rheumatoid arthritis: relationships of plasma tumor necrosis factor-alpha, soluble interleukin 2-receptor, soluble CD4/CD8 ratio, neopterin, and fibrin D-dimer to traditional severity and functional measures. J Clin Immunol. 1992 Sep;12(5):353–361. doi: 10.1007/BF00920793. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D., Chulay J. D., Ho M., Mugambii M., Were J. B., Muigai R., Chunge C., Gachihi G., Meme J., Anabwani G. Visceral leishmaniasis unresponsive to antimonial drugs. I. Clinical and immunological studies. Trans R Soc Trop Med Hyg. 1985;79(5):700–704. doi: 10.1016/0035-9203(85)90197-x. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D., Chulay J. D., Mugambi M., Were J. B., Gachihi G., Chunge C. N., Muigai R., Bhatt S. M., Ho M., Spencer H. C. Visceral leishmaniasis unresponsive to antimonial drugs. II. Response to high dosage sodium stibogluconate or prolonged treatment with pentamidine. Trans R Soc Trop Med Hyg. 1985;79(5):705–714. doi: 10.1016/0035-9203(85)90199-3. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Bacellar O., Barral A., Badaro R., Johnson W. D., Jr Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. J Clin Invest. 1989 Mar;83(3):860–864. doi: 10.1172/JCI113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Barral A., Pedral-Sampaio D., Barral-Netto M., Badaró R., Rocha H., Johnson W. D., Jr Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J Infect Dis. 1992 Mar;165(3):535–540. doi: 10.1093/infdis/165.3.535. [DOI] [PubMed] [Google Scholar]
- Cenini P., Berhe N., Hailu A., McGinnes K., Frommel D. Mononuclear cell subpopulations and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J Infect Dis. 1993 Oct;168(4):986–993. doi: 10.1093/infdis/168.4.986. [DOI] [PubMed] [Google Scholar]
- Chulay J. D., Bryceson A. D. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg. 1983 May;32(3):475–479. doi: 10.4269/ajtmh.1983.32.475. [DOI] [PubMed] [Google Scholar]
- Chunge C. N., Gachihi G., Muigai R., Wasunna K., Rashid J. R., Chulay J. D., Anabwani G., Oster C. N., Bryceson A. D. Visceral leishmaniasis unresponsive to antimonial drugs. III. Successful treatment using a combination of sodium stibogluconate plus allopurinol. Trans R Soc Trop Med Hyg. 1985;79(5):715–718. doi: 10.1016/0035-9203(85)90200-7. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Ho A. D., Grossmann M., Knauf W., Martin H., Trümper L., Zwingers T., Sonnen R., Pralle H., Hunstein W. Plasma levels of soluble CD8 antigen and interleukin-2 receptor antigen in patients with hairy cell leukemia, relationship with splenectomy and with clinical response to therapy. Leukemia. 1989 Oct;3(10):718–723. [PubMed] [Google Scholar]
- Koech D. K. Subpopulations of T lymphocytes in Kenyan patients with visceral leishmaniasis. Am J Trop Med Hyg. 1987 May;36(3):497–500. doi: 10.4269/ajtmh.1987.36.497. [DOI] [PubMed] [Google Scholar]
- Pampiglione S., La Placa M., Schlick G. Studies on mediterranean Leishmaniasis. I. An outbreak of visceral leishmaniasis in Northern Italy. Trans R Soc Trop Med Hyg. 1974;68(5):349–359. doi: 10.1016/0035-9203(74)90148-5. [DOI] [PubMed] [Google Scholar]
- Pui C. H., Ip S. H., Thompson E., Dodge R. K., Brown M., Wilimas J., Carrabis S., Kung P., Berard C. W., Crist W. M. Increased serum CD8 antigen level in childhood Hodgkin's disease relates to advanced stage and poor treatment outcome. Blood. 1989 Jan;73(1):209–213. [PubMed] [Google Scholar]
- Reiner N. E., Ng W., Wilson C. B., McMaster W. R., Burchett S. K. Modulation of in vitro monocyte cytokine responses to Leishmania donovani. Interferon-gamma prevents parasite-induced inhibition of interleukin 1 production and primes monocytes to respond to Leishmania by producing both tumor necrosis factor-alpha and interleukin 1. J Clin Invest. 1990 Jun;85(6):1914–1924. doi: 10.1172/JCI114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Rubin L. A., Nelson D. L. The soluble interleukin-2 receptor: biology, function, and clinical application. Ann Intern Med. 1990 Oct 15;113(8):619–627. doi: 10.7326/0003-4819-113-8-619. [DOI] [PubMed] [Google Scholar]
- Sciotto A., Russo-Mancuso G., Zinna C. M., Comisi F. F., Sciannaca R. M., Schiliro G. Lymphocyte subsets in kala-azar. Ann Allergy. 1989 Oct;63(4):343–346. [PubMed] [Google Scholar]
- Sfikakis P. P., Tesar J., Baraf H., Lipnick R., Klipple G., Tsokos G. C. Circulating intercellular adhesion molecule-1 in patients with systemic sclerosis. Clin Immunol Immunopathol. 1993 Jul;68(1):88–92. doi: 10.1006/clin.1993.1100. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Vitale G., Reina G., Mansueto S., Malta R., Gambino G., Mocciaro C., D'Agostino R., Dieli M., Cillari E. The significance of serum soluble IL-2 receptor as a marker for active visceral leishmaniasis in Sicilian patients. Clin Exp Immunol. 1992 Nov;90(2):219–222. doi: 10.1111/j.1365-2249.1992.tb07932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter H., Fuchs D., Hausen A., Reibnegger G., Werner E. R. Neopterin as marker for activation of cellular immunity: immunologic basis and clinical application. Adv Clin Chem. 1989;27:81–141. doi: 10.1016/s0065-2423(08)60182-1. [DOI] [PubMed] [Google Scholar]
- Wijers D. J. A 10 years' study of kala-azar in Tharaka (Meru district, Kenya). II. Relapses. East Afr Med J. 1971 Oct;48(10):551–558. [PubMed] [Google Scholar]


