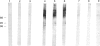Abstract
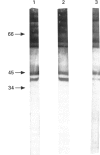
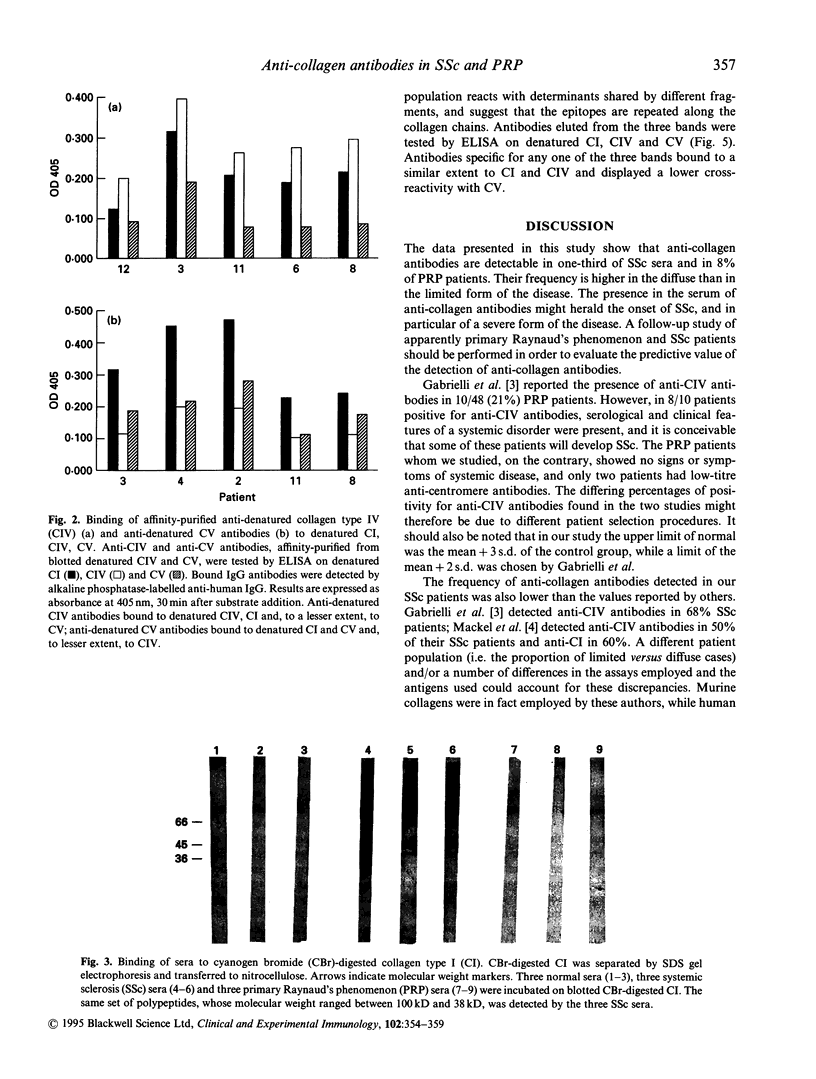
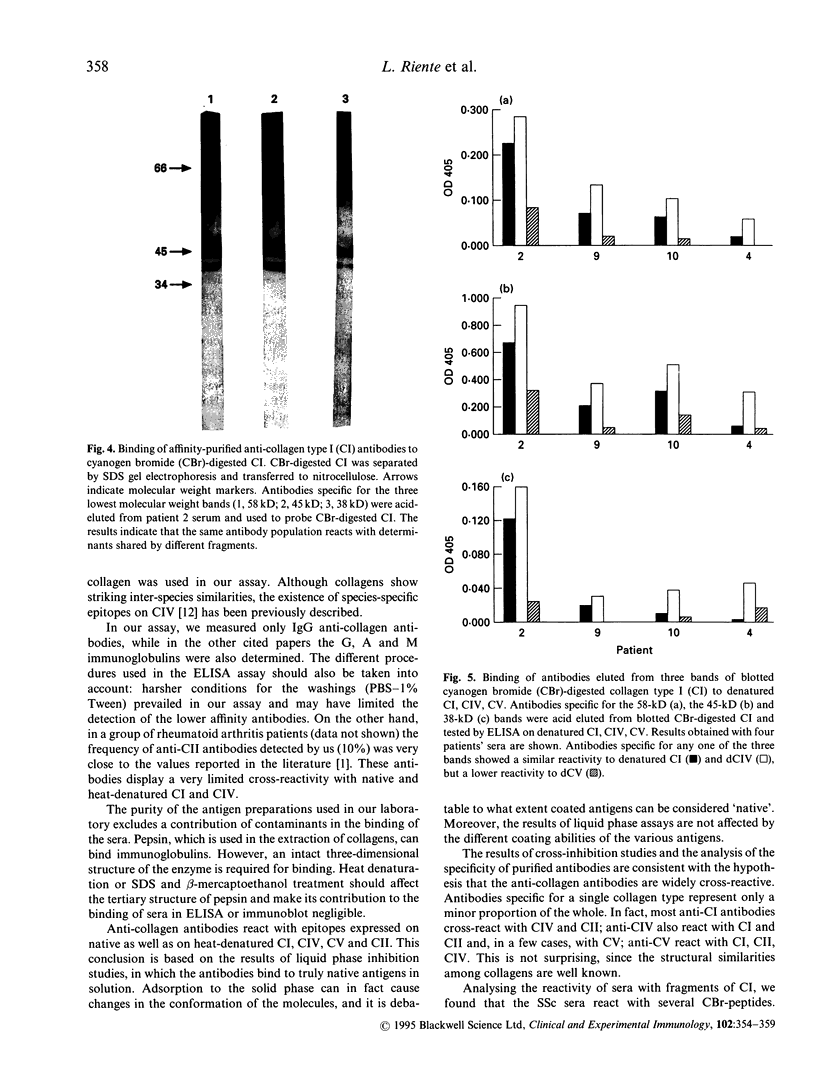
The frequency and specificity of antibodies to native and denatured collagens were evaluated in systemic sclerosis (SSc) and in primary Raynaud's phenomenon (PRP) by direct and competitive ELISA. Antibodies reactive with denatured collagen type I (CI) were found in 43% of the SSc sera, and anti-CIV and anti-CV in 31%. In PRP, anti-CI, anti-CIV and anti-CV antibodies were detected in 8% of patient sera. Anti-CI, anti-CIV and anti-CV antibodies reacted with determinants expressed on the native as well as on the denatured molecule. Anti-CI and anti-CIV were cross-reactive; a reactivity with CII and a lower one with CV were detected. Anti-CV antibodies also reacted with CI and CII and, in a smaller proportion of cases, with CIV. Anti-collagen antibodies, affinity-purified from blotted collagen IV and V and cyanogen bromide (CBr)-digested CI, displayed the cross-reactivities shown by inhibition studies on sera. Moreover, antibodies eluted from a CBr fragment of CI reacted with the other CBr fragments as well. These data show that one-third of SSc sera contain antibodies that react with epitopes expressed on native as well as on heat-denatured CI, CII, CIV and CV, and therefore have the potential to bind collagens in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Buckee C., Morgan K., Ayad S., Collins I., Clague R. B., Holt P. J. Diversity of antibodies to type II collagen in patients with rheumatoid arthritis: detection by binding to alpha-chains and to cyanogen bromide peptides. Br J Rheumatol. 1990 Aug;29(4):254–258. doi: 10.1093/rheumatology/29.4.254. [DOI] [PubMed] [Google Scholar]
- Gabrielli A., Montroni M., Rupoli S., Caniglia M. L., DeLustro F., Danieli G. A retrospective study of antibodies against basement membrane antigens (type IV collagen and laminin) in patients with primary and secondary Raynaud's phenomenon. Arthritis Rheum. 1988 Nov;31(11):1432–1436. doi: 10.1002/art.1780311114. [DOI] [PubMed] [Google Scholar]
- Huffstutter J. E., DeLustro F. A., LeRoy E. C. Cellular immunity to collagen and laminin in scleroderma. Arthritis Rheum. 1985 Jul;28(7):775–780. doi: 10.1002/art.1780280708. [DOI] [PubMed] [Google Scholar]
- Iribe H., Kabashima H., Ishii Y., Koga T. Epitope specificity of antibody response against human type II collagen in the mouse susceptible to collagen-induced arthritis and patients with rheumatoid arthritis. Clin Exp Immunol. 1988 Sep;73(3):443–448. [PMC free article] [PubMed] [Google Scholar]
- Mackel A. M., DeLustro F., Harper F. E., LeRoy E. C. Antibodies to collagen in scleroderma. Arthritis Rheum. 1982 May;25(5):522–531. doi: 10.1002/art.1780250505. [DOI] [PubMed] [Google Scholar]
- Migliorini P., Ardman B., Kaburaki J., Schwartz R. S. Parallel sets of autoantibodies in MRL-lpr/lpr mice. An anti-DNA, anti-SmRNP, anti-gp70 network. J Exp Med. 1987 Feb 1;165(2):483–499. doi: 10.1084/jem.165.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Gay S. Collagen: an overview. Methods Enzymol. 1982;82(Pt A):3–32. doi: 10.1016/0076-6879(82)82058-2. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Morgan K. What do anti-collagen antibodies mean? Ann Rheum Dis. 1990 Jan;49(1):62–65. doi: 10.1136/ard.49.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty R. E., Hunt D. W., Rosenberg A. M. Antibodies to type IV collagen in rheumatic diseases. J Rheumatol. 1986 Apr;13(2):246–253. [PubMed] [Google Scholar]
- Rowley M. J., Cook A. D., Teuber S. S., Gershwin M. E. Antibodies to collagen: comparative epitope mapping in women with silicone breast implants, systemic lupus erythematosus and rheumatoid arthritis. J Autoimmun. 1994 Dec;7(6):775–789. doi: 10.1006/jaut.1994.1061. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Mackay I. R., Brand C. A., Bateman J. F., Chan D. Epitope specificity of antibodies to type II collagen in rheumatoid arthritis and systemic lupus erythematosus. Rheumatol Int. 1992;12(2):65–69. doi: 10.1007/BF00300979. [DOI] [PubMed] [Google Scholar]
- Sabbatini A., Bombardieri S., Migliorini P. Autoantibodies from patients with systemic lupus erythematosus bind a shared sequence of SmD and Epstein-Barr virus-encoded nuclear antigen EBNA I. Eur J Immunol. 1993 May;23(5):1146–1152. doi: 10.1002/eji.1830230525. [DOI] [PubMed] [Google Scholar]
- Terato K., Arai H., Shimozuru Y., Fukuda T., Tanaka H., Watanabe H., Nagai Y., Fujimoto K., Okubo F., Cho F. Sex-linked differences in susceptibility of cynomolgus monkeys to type II collagen-induced arthritis. Evidence that epitope-specific immune suppression is involved in the regulation of type II collagen autoantibody formation. Arthritis Rheum. 1989 Jun;32(6):748–758. doi: 10.1002/anr.1780320613. [DOI] [PubMed] [Google Scholar]
- Terato K., Hasty K. A., Cremer M. A., Stuart J. M., Townes A. S., Kang A. H. Collagen-induced arthritis in mice. Localization of an arthritogenic determinant to a fragment of the type II collagen molecule. J Exp Med. 1985 Aug 1;162(2):637–646. doi: 10.1084/jem.162.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]