Abstract
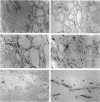
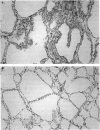
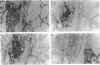
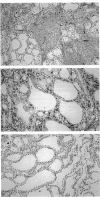
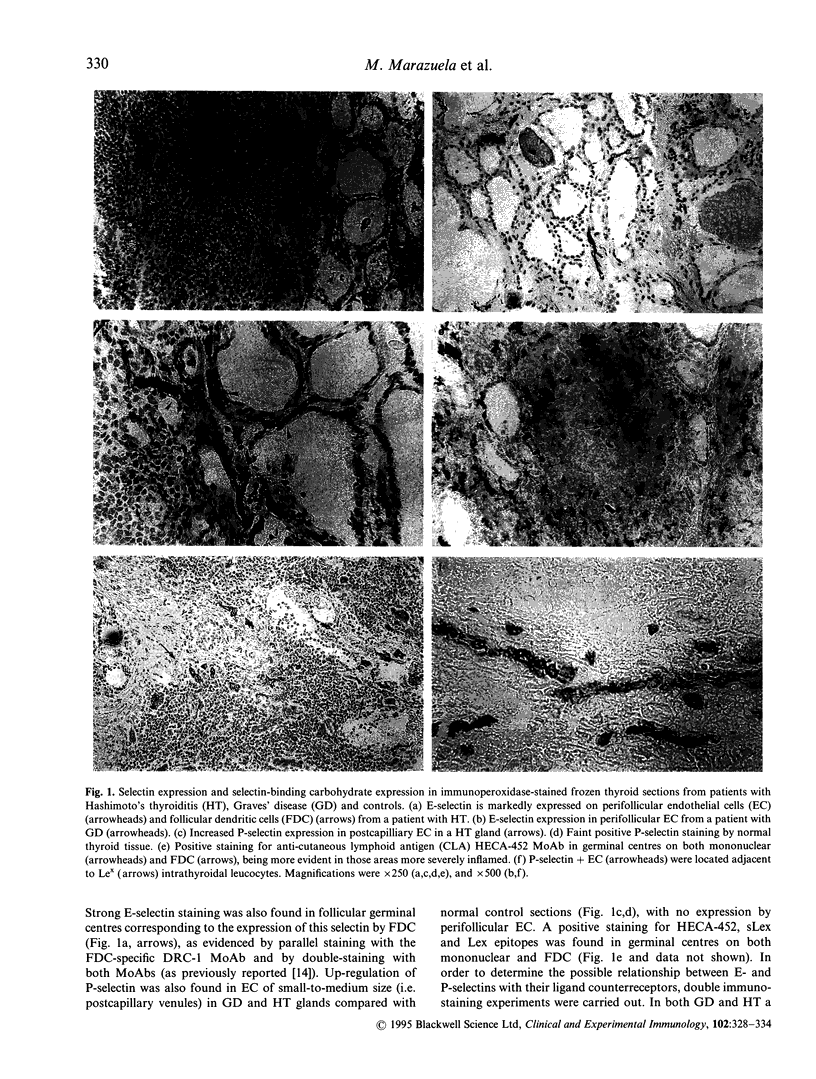
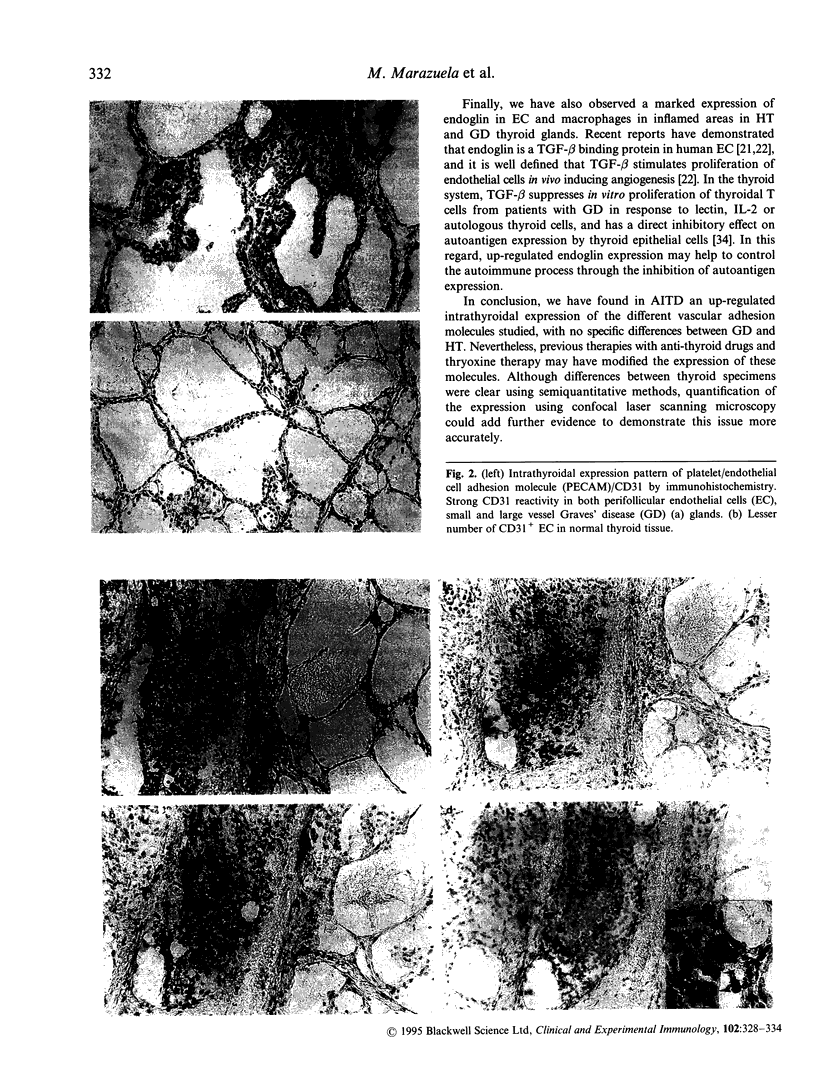
Cellular activation and expression of certain adhesion molecules within vascular endothelium is a critical event in leucocyte recruitment and emigration. A wide array of different adhesion receptors has been identified to mediate the interaction between endothelial cells (EC) and leucocyte subpopulations. In this study, the tissue expression of E-selectin, P-selectin, CD31, and endoglin endothelial cell adhesion molecules was studied on thyroid tissue from patients with Graves' disease (GD) and Hashimoto's thyroiditis (HT). We found an up-regulated expression of E-selectin in EC in GD and HT thyroids, specifically in those areas more severely inflamed, with no reactivity in control thyroids. P-selectin was basally expressed in postcapillary venules in control glands, with an increased expression in HT and GD glands. On the other hand, increased CD31 expression was found on perifollicular, small and large venule EC from GD and HT glands, that correlated with the severity of mononuclear infiltration. In addition, CD31 expression was observed in some intrathyroidal macrophages and T cells in close proximity to CD31+ EC. Furthermore, a markedly enhanced expression of endoglin, a transforming growth factor-beta binding protein, was mainly located on perifollicular EC and EC from small venules as well as in adjacent macrophages from GD and HT thyroid glands. This enhanced expression of E- and P-selectins, CD31 and endoglin by thyroid EC in GD and HT may reflect their ability to regulate leucocyte trafficking and activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman L. K., Aylett G. W. Expression of CD31 epitopes on human lymphocytes: CD31 monoclonal antibodies differentiate between naive (CD45RA+) and memory (CD45RA-) CD4-positive T cells. Tissue Antigens. 1991 Nov;38(5):208–212. doi: 10.1111/j.1399-0039.1991.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991 Dec 1;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Champion B. R., Cooke A., Rayner D. C. Thyroid autoimmunity. Curr Opin Immunol. 1992 Dec;4(6):770–778. doi: 10.1016/0952-7915(92)90060-r. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Bellón T., Calés C., Vera S., Bernabeu C., Massagué J., Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992 Sep 25;267(27):19027–19030. [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Aruffo A. Vascular cell adhesion molecule 1 induces T-cell antigen receptor-dependent activation of CD4+T lymphocytes. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6403–6407. doi: 10.1073/pnas.88.15.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser H. M., Newman P. J., Albelda S. M. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994 Oct;15(10):490–495. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- García-Monzón C., Sánchez-Madrid F., García-Buey L., García-Arroyo A., García-Sánchez A., Moreno-Otero R. Vascular adhesion molecule expression in viral chronic hepatitis: evidence of neoangiogenesis in portal tracts. Gastroenterology. 1995 Jan;108(1):231–241. doi: 10.1016/0016-5085(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Gotsch U., Jäger U., Dominis M., Vestweber D. Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-alpha in vivo. Cell Adhes Commun. 1994 Apr;2(1):7–14. doi: 10.3109/15419069409014198. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iwatani Y., Gerstein H. C., Iitaka M., Row V. V., Volpé R. Thyrocyte HLA-DR expression and interferon-gamma production in autoimmune thyroid disease. J Clin Endocrinol Metab. 1986 Sep;63(3):695–708. doi: 10.1210/jcem-63-3-695. [DOI] [PubMed] [Google Scholar]
- Larsen E., Palabrica T., Sajer S., Gilbert G. E., Wagner D. D., Furie B. C., Furie B. PADGEM-dependent adhesion of platelets to monocytes and neutrophils is mediated by a lineage-specific carbohydrate, LNF III (CD15). Cell. 1990 Nov 2;63(3):467–474. doi: 10.1016/0092-8674(90)90443-i. [DOI] [PubMed] [Google Scholar]
- Lastres P., Bellon T., Cabañas C., Sanchez-Madrid F., Acevedo A., Gougos A., Letarte M., Bernabeu C. Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp-containing surface antigen. Eur J Immunol. 1992 Feb;22(2):393–397. doi: 10.1002/eji.1830220216. [DOI] [PubMed] [Google Scholar]
- Marazuela M., Postigo A. A., Acevedo A., Díaz-González F., Sanchez-Madrid F., de Landázuri M. O. Adhesion molecules from the LFA-1/ICAM-1,3 and VLA-4/VCAM-1 pathways on T lymphocytes and vascular endothelium in Graves' and Hashimoto's thyroid glands. Eur J Immunol. 1994 Oct;24(10):2483–2490. doi: 10.1002/eji.1830241034. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Leukocyte-endothelial cell interactions. Curr Opin Cell Biol. 1992 Oct;4(5):840–849. doi: 10.1016/0955-0674(92)90109-p. [DOI] [PubMed] [Google Scholar]
- Miyazaki A., Mirakian R., Bottazzo G. F. Adhesion molecule expression in Graves' thyroid glands; potential relevance of granule membrane protein (GMP-140) and intercellular adhesion molecule-1 (ICAM-1) in the homing and antigen presentation processes. Clin Exp Immunol. 1992 Jul;89(1):52–57. doi: 10.1111/j.1365-2249.1992.tb06876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Berman M. E., Newman P. J., DeLisser H. M., Albelda S. M. A heterophilic adhesion mechanism for platelet/endothelial cell adhesion molecule 1 (CD31). J Exp Med. 1992 May 1;175(5):1401–1404. doi: 10.1084/jem.175.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Postigo A. A., Garcia-Vicuña R., Diaz-Gonzalez F., Arroyo A. G., De Landázuri M. O., Chi-Rosso G., Lobb R. R., Laffon A., Sánchez-Madrid F. Increased binding of synovial T lymphocytes from rheumatoid arthritis to endothelial-leukocyte adhesion molecule-1 (ELAM-1) and vascular cell adhesion molecule-1 (VCAM-1). J Clin Invest. 1992 May;89(5):1445–1452. doi: 10.1172/JCI115734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo A. A., Marazuela M., Sánchez-Madrid F., de Landázuri M. O. B lymphocyte binding to E- and P-selectins is mediated through the de novo expression of carbohydrates on in vitro and in vivo activated human B cells. J Clin Invest. 1994 Oct;94(4):1585–1596. doi: 10.1172/JCI117500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako D., Chang X. J., Barone K. M., Vachino G., White H. M., Shaw G., Veldman G. M., Bean K. M., Ahern T. J., Furie B. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993 Dec 17;75(6):1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Albelda S. M., Horgan K. J., van Seventer G. A., Shimizu Y., Newman W., Hallam J., Newman P. J., Buck C. A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992 Jul 1;176(1):245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder J., Dorfinger K., Wilfing A., Trieb K., Pirich K., Loebenstein R., Niederle B., Gessl A., Spitzauer S., Grubeck-Loebenstein B. The immunoregulatory influence of transforming growth factor beta in thyroid autoimmunity: TGF beta inhibits autoreactivity in Graves' disease. J Autoimmun. 1991 Aug;4(4):689–701. doi: 10.1016/0896-8411(91)90186-g. [DOI] [PubMed] [Google Scholar]
- Zheng R. Q., Abney E. R., Chu C. Q., Field M., Maini R. N., Lamb J. R., Feldmann M. Detection of in vivo production of tumour necrosis factor-alpha by human thyroid epithelial cells. Immunology. 1992 Mar;75(3):456–462. [PMC free article] [PubMed] [Google Scholar]
- Zheng R. Q., Abney E., Chu C. Q., Field M., Grubeck-Loebenstein B., Maini R. N., Feldmann M. Detection of interleukin-6 and interleukin-1 production in human thyroid epithelial cells by non-radioactive in situ hybridization and immunohistochemical methods. Clin Exp Immunol. 1991 Feb;83(2):314–319. doi: 10.1111/j.1365-2249.1991.tb05634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]