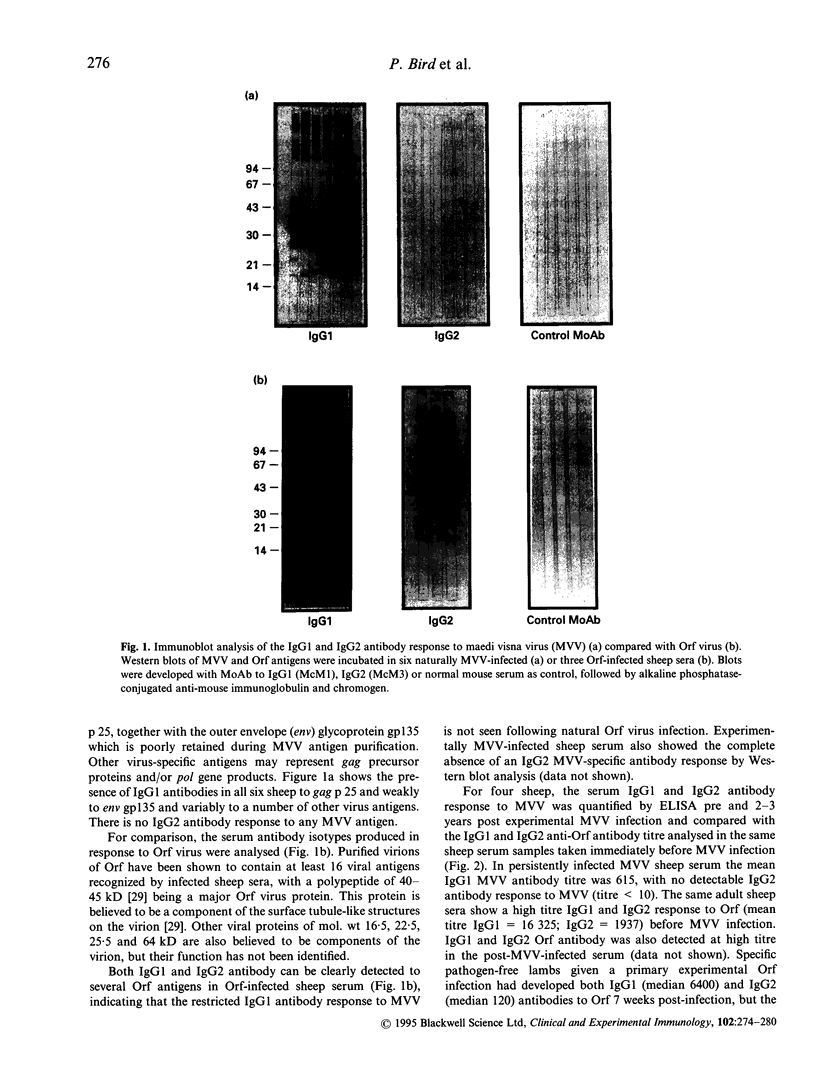
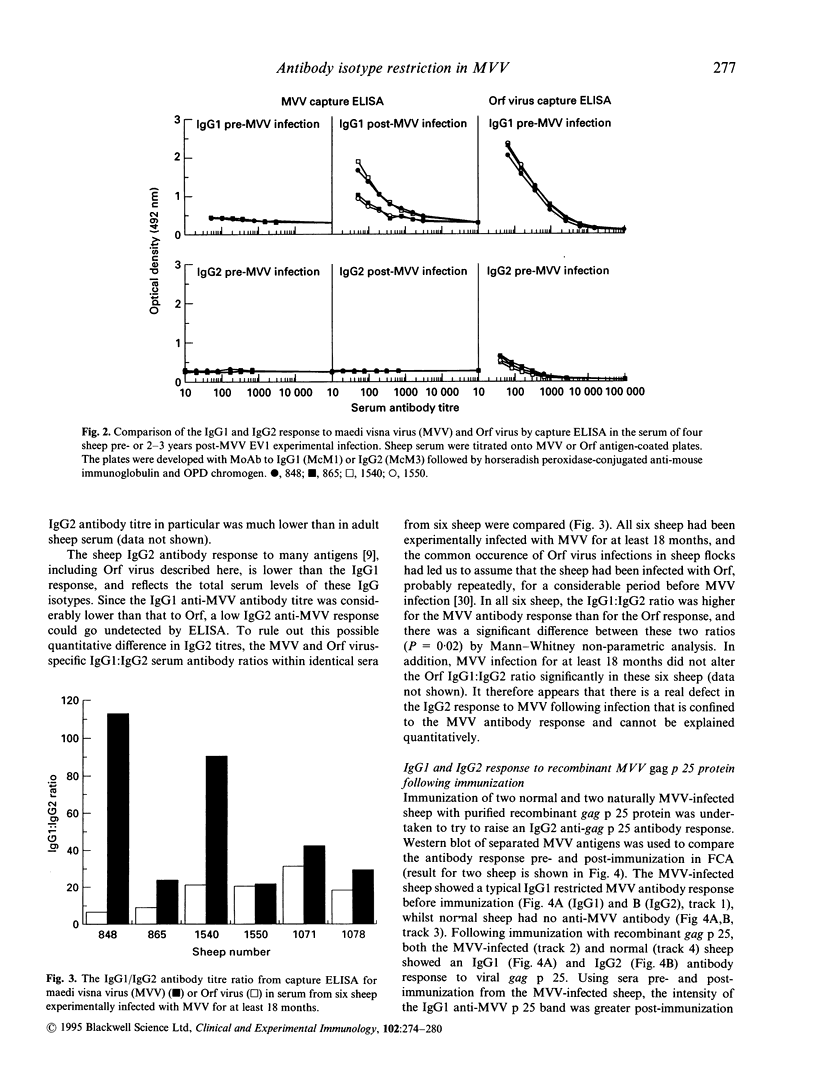
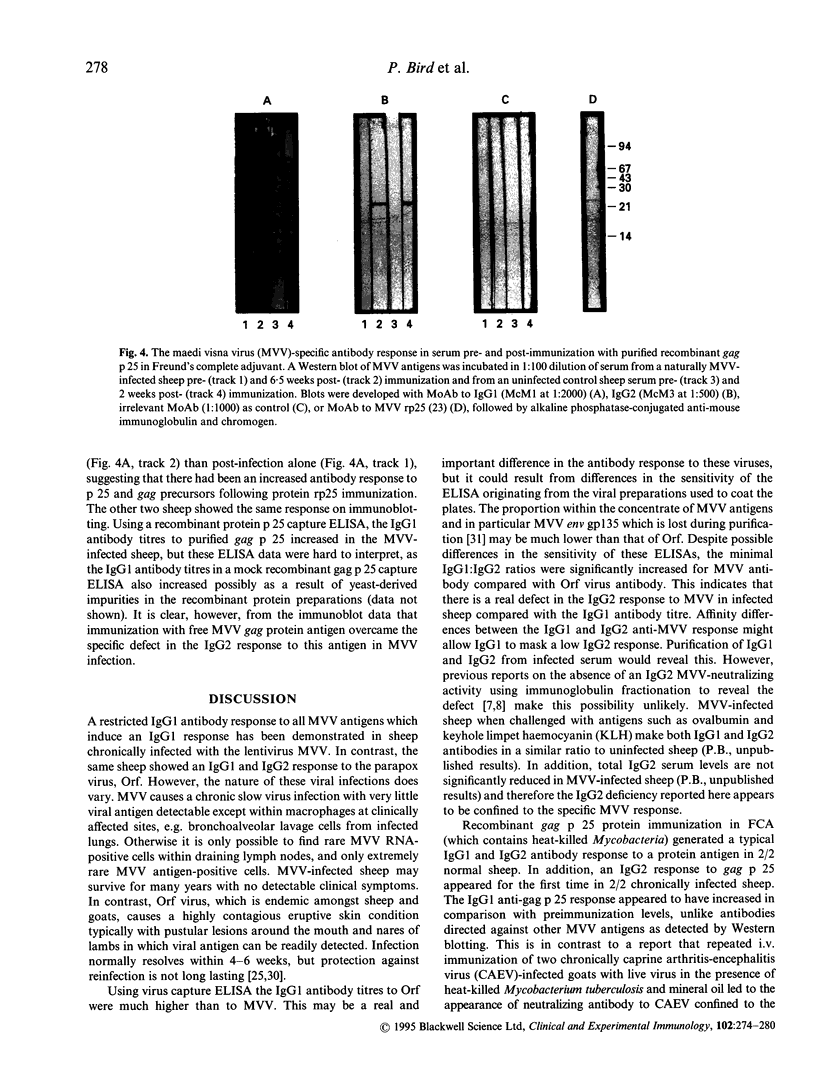
Abstract
Maedi-visna (MVV) is a retrovirus of the subfamily lentivirinae which includes HIV, simian immunodeficiency virus (SIV) and feline immunodeficiency virus (FIV). Infection of its natural host, the sheep, does not cause overt immunodeficiency, but rather a chronic inflammatory disease. However, subtle immunological changes following infection have been reported including a sheep IgG1 subclass-restricted MVV-neutralizing antibody. Here we demonstrate by Western blotting that there is no IgG2 serum antibody response to any MVV antigen after MVV infection, in contrast to infection with the parapox virus Orf, when serum IgG2 anti-Orf antibody is readily detected. By ELISA, the IgG1 antibody titres to Orf are higher than to MVV, but the minimum MVV serum antibody IgG1/IgG2 ratio is significantly raised compared with that for Orf virus antibody in the same sheep, indicating that the IgG2 defect in MVV infection cannot be accounted for by differences in the sensitivity of the Orf and MVV ELISA. Serum IgG2 anti-MVV gag p. 25 can be detected in both normal and MVV-infected sheep following immunization with purified recombinant MVV gag p 25 protein in Freund's complete adjuvant. The failure to make an IgG2 MVV-specific antibody indicates that immunological dysfunction can arise with macrophage tropic lentiviruses, and it may aid viral persistence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beh K. J. Production and characterization of monoclonal antibodies specific for sheep IgG subclasses IgG1 or IgG2. Vet Immunol Immunopathol. 1987 Feb;14(2):187–196. doi: 10.1016/0165-2427(87)90053-5. [DOI] [PubMed] [Google Scholar]
- Bird P., Blacklaws B., Reyburn H. T., Allen D., Hopkins J., Sargan D., McConnell I. Early events in immune evasion by the lentivirus maedi-visna occurring within infected lymphoid tissue. J Virol. 1993 Sep;67(9):5187–5197. doi: 10.1128/jvi.67.9.5187-5197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns M., Frenzel B. Isolation of a glycoprotein and two structural proteins of Maedi-Visna virus. Virology. 1979 Aug;97(1):207–211. doi: 10.1016/0042-6822(79)90389-1. [DOI] [PubMed] [Google Scholar]
- Butler J. E. Biochemistry and biology of ruminant immunoglobulins. Prog Vet Microbiol Immunol. 1986;2:1–53. [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994 Dec;15(12):575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Lebman D. A., Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–270. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- Emery D. L., Rothel J. S., Wood P. R. Influence of antigens and adjuvants on the production of gamma-interferon and antibody by ovine lymphocytes. Immunol Cell Biol. 1990 Apr;68(Pt 2):127–136. doi: 10.1038/icb.1990.18. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Hobart M. J. Structural relationship and complement fixing activity of sheep and other ruminant immunoglobulin G subclasses. Nature. 1969 Aug 30;223(5209):950–952. doi: 10.1038/223950a0. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrell M. D., Brandon M. R., Sheffer D., Adams R. J., Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992 May;66(5):2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal A. S., Rouse B. T. Characterization of bovine leukocytes involved in antibody-dependent cell cytotoxicity. Int Arch Allergy Appl Immunol. 1979;60(2):169–177. doi: 10.1159/000232339. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Taylor G., Brownlie J. Surface receptors for immunoglobulin on bovine polymorphonuclear neutrophils and macrophages. Res Vet Sci. 1980 Jul;29(1):128–130. [PubMed] [Google Scholar]
- Johnson G. C., Adams D. S., McGuire T. C. Pronounced production of polyclonal immunoglobulin G1 in the synovial fluid of goats with caprine arthritis-encephalitis virus infection. Infect Immun. 1983 Aug;41(2):805–815. doi: 10.1128/iai.41.2.805-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. C., Barbet A. F., Klevjer-Anderson P., McGuire T. C. Preferential immune response to virion surface glycoproteins by caprine arthritis-encephalitis virus-infected goats. Infect Immun. 1983 Aug;41(2):657–665. doi: 10.1128/iai.41.2.657-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge J. P., Cheville N. F., Peery T. M. Ultrastructural studies of contagious ecthyma in sheep. Am J Vet Res. 1972 Jun;33(6):1191–1200. [PubMed] [Google Scholar]
- Knight K. L., Suter M., Becker R. S. Genetic engineering of bovine Ig. Construction and characterization of hapten-binding bovine/murine chimeric IgE, IgA, IgG1, IgG2, and IgG3 molecules. J Immunol. 1988 May 15;140(10):3654–3659. [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Musoke A. J., Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils and skin. Immunology. 1979 Oct;38(2):249–256. [PMC free article] [PubMed] [Google Scholar]
- McKeever D. J., Reid H. W., Inglis N. F., Herring A. J. A qualitative and quantitative assessment of the humoral antibody response of the sheep to orf virus infection. Vet Microbiol. 1987 Nov;15(3):229–241. doi: 10.1016/0378-1135(87)90077-0. [DOI] [PubMed] [Google Scholar]
- Mehta P. D., Thormar H. Neutralizing activity in isolated serum antibody fractions from visna-infected sheep. Infect Immun. 1974 Sep;10(3):678–680. doi: 10.1128/iai.10.3.678-680.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micusan V. V., Borduas A. G. Biological properties of goat immunoglobulins G. Immunology. 1977 Apr;32(4):373–381. [PMC free article] [PubMed] [Google Scholar]
- Nansen P. Selective immunoglobulin deficiency in cattle and susceptibility to infection. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(1):49–54. doi: 10.1111/j.1699-0463.1972.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Narayan O., Sheffer D., Griffin D. E., Clements J., Hess J. Lack of neutralizing antibodies to caprine arthritis-encephalitis lentivirus in persistently infected goats can be overcome by immunization with inactivated Mycobacterium tuberculosis. J Virol. 1984 Feb;49(2):349–355. doi: 10.1128/jvi.49.2.349-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyburn H. T., Roy D. J., Blacklaws B. A., Sargan D. R., McConnell I. Expression of maedi-visna virus major core protein, p25: development of a sensitive p25 antigen detection assay. J Virol Methods. 1992 Jun;37(3):305–320. doi: 10.1016/0166-0934(92)90031-8. [DOI] [PubMed] [Google Scholar]
- Ruegg C. L., Clements J. E., Strand M. Inhibition of lymphoproliferation and protein kinase C by synthetic peptides with sequence identity to the transmembrane and Q proteins of visna virus. J Virol. 1990 May;64(5):2175–2180. doi: 10.1128/jvi.64.5.2175-2180.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargan D. R., Bennet I. D., Cousens C., Roy D. J., Blacklaws B. A., Dalziel R. G., Watt N. J., McConnell I. Nucleotide sequence of EV1, a British isolate of maedi-visna virus. J Gen Virol. 1991 Aug;72(Pt 8):1893–1903. doi: 10.1099/0022-1317-72-8-1893. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Watson D. L. Cytophilic attachment of ovine IgG2 to autologous polymorphonuclear leucocytes. Aust J Exp Biol Med Sci. 1975 Dec;53(6):527–529. doi: 10.1038/icb.1975.57. [DOI] [PubMed] [Google Scholar]
- Watson D. L. Vaccination against experimental staphylococcal mastitis in ewes. Res Vet Sci. 1988 Jul;45(1):16–21. [PubMed] [Google Scholar]
- Watt N. J., King T. J., Collie D., McIntyre N., Sargan D., McConnell I. Clinicopathological investigation of primary, uncomplicated maedi-visna virus infection. Vet Rec. 1992 Nov 14;131(20):455–461. doi: 10.1136/vr.131.20.455. [DOI] [PubMed] [Google Scholar]
- Yasmeen D. Antigen-specific cytophilic activity of sheep IgG1 and IgG2 antibodies. Aust J Exp Biol Med Sci. 1981 Jun;59(Pt 3):297–302. doi: 10.1038/icb.1981.22. [DOI] [PubMed] [Google Scholar]
- Yirrell D. L., Reid H. W., Norval M., Howie S. E. Immune response of lambs to experimental infection with Orf virus. Vet Immunol Immunopathol. 1989 Nov 15;22(4):321–332. doi: 10.1016/0165-2427(89)90168-2. [DOI] [PubMed] [Google Scholar]