Abstract
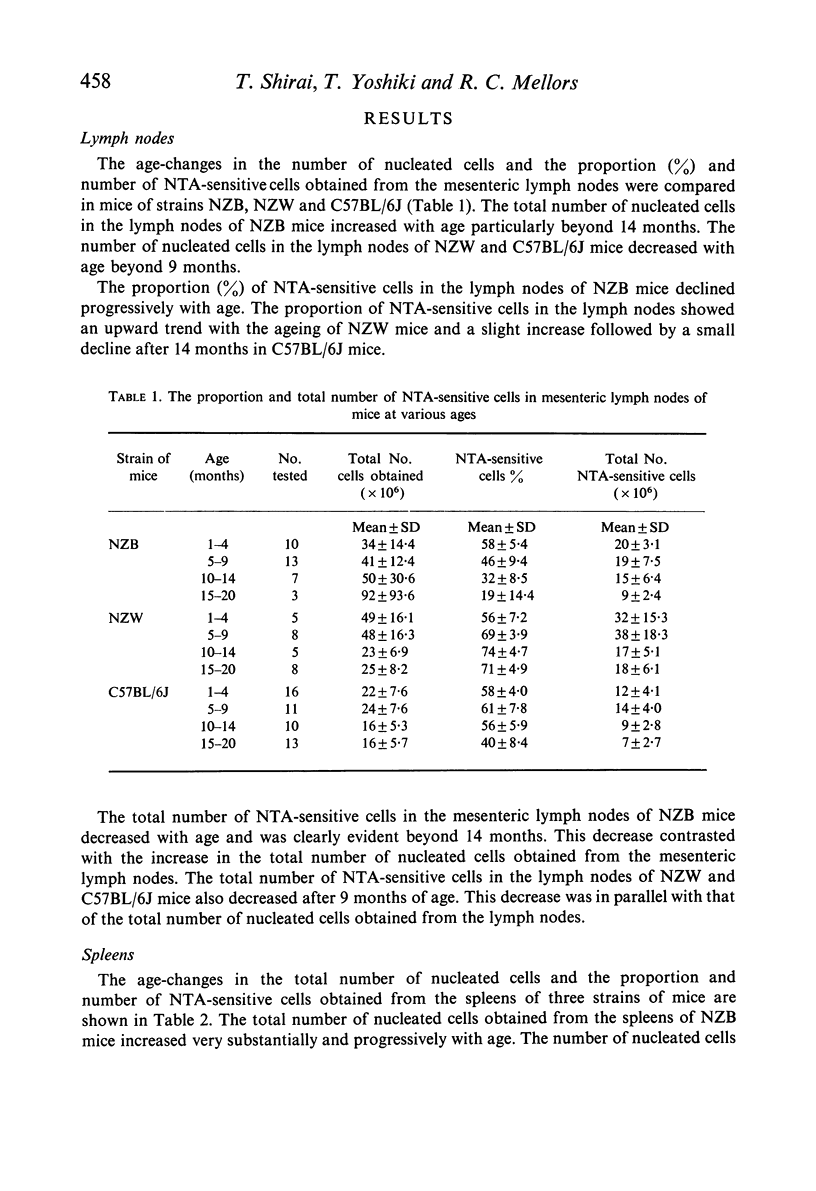
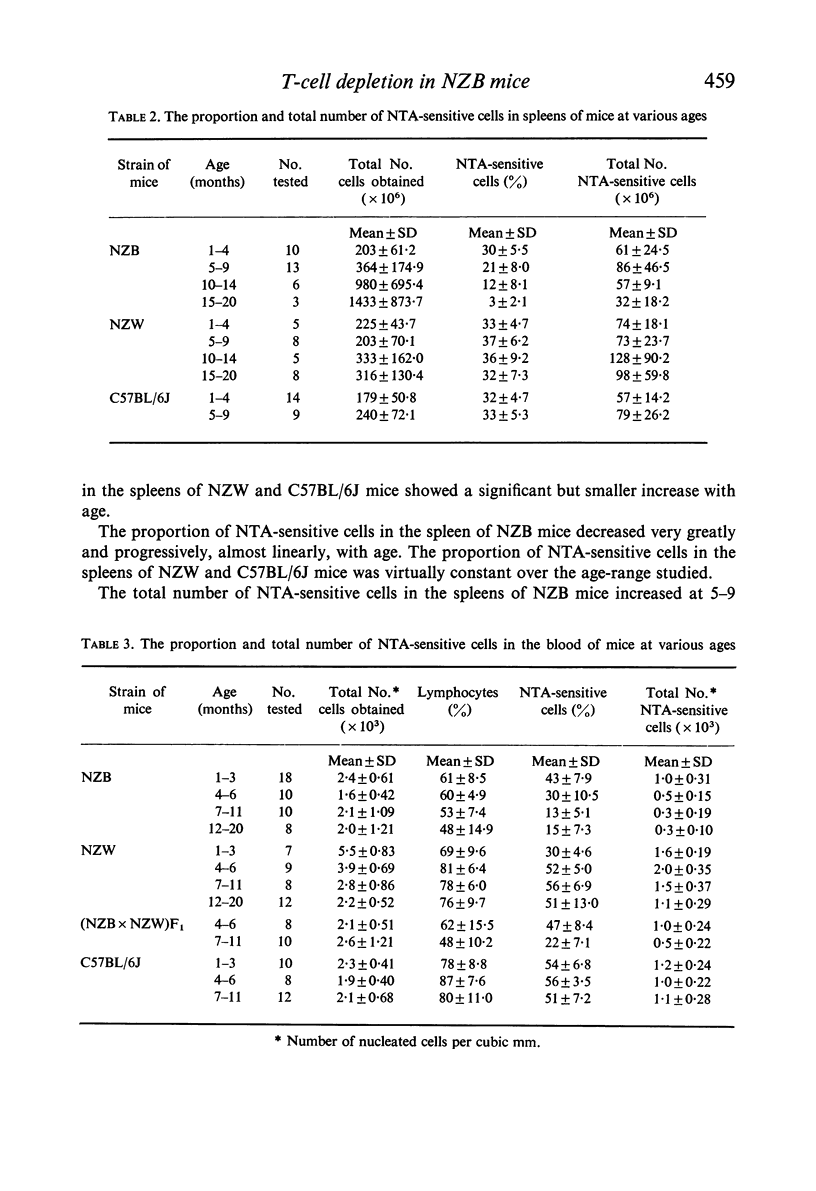
NZB mice naturally produce an autoantibody which in the presence of complement is specifically cytotoxic for thymocytes and thymus-dependent lymphocytes (T-cells) in the peripheral lymphoid tissues (lymph nodes and spleen) and the circulation of mice. Using a direct cytotoxicity test with a NZB mouse serum pool which contained the high titred autoantibody, a progressive decrease was observed with age in the proportion of the autoantibody-sensitive cells in mesenteric lymph node, spleen, and blood of NZB mice in comparison with mice of other strains (C57BL/6J and NZW). The numerical decrease in the population of autoantibody-sensitive cells was evident at younger age and greater degree in the peripheral blood than in the lymph node and spleen. The age-decrease in the number of autoantibody-sensitive cells in lymph node and spleen contrasted with the numerical increase in nucleated cells in these organs. The age-decrease in the proportion and number of the autoantibody-sensitive cells in the blood exceeded the decrease in the blood lymphocyte count. This finding indicated that T-cells in the blood are selectively depleted with the ageing of NZB mice. A similar observation was made on the blood lymphocytes of (NZB × NZW)F1 hybrid mice. The depletion of T-cells in the blood in association with the production of natural thymocytotoxic autoantibody is termed autoimmune thymus-dependent lymphocytopenia.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Bankhurst A. D., Warner N. L., Sprent J. Surface immunoglobulins on thymus and thymus-derived lymphoid cells. J Exp Med. 1971 Oct 1;134(4):1005–1015. doi: 10.1084/jem.134.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoopalam N., Yakulis V. J., Costea N., Heller P. Membrane immunoglobulins of lymphocytes in NZB mice. J Immunol. 1971 Nov;107(5):1501–1503. [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. 3. Evidence for interaction between two types of thymus-derived cells. J Exp Med. 1972 Apr 1;135(4):764–779. doi: 10.1084/jem.135.4.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Denman A. M., Denman E. J. Depletion of long-lived lymphocytes in old New Zealand black mice. Clin Exp Immunol. 1970 Apr;6(4):457–472. [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Beitzel W., Talal N. The age related responses of New Zealand mice to a murine sarcoma virus. Clin Exp Immunol. 1971 Mar;8(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- HELYER B. J., HOWIE J. B. Spontaneous auto-immune disease in NZB/BL mice. Br J Haematol. 1963 Apr;9:119–131. doi: 10.1111/j.1365-2141.1963.tb05450.x. [DOI] [PubMed] [Google Scholar]
- HOLMES M. C., BURNET F. M. THE NATURAL HISTORY OF AUTOIMMUNE DISEASE IN NZB MICE. A COMPARISON WITH THE PATTERN OF HUMAN AUTOIMMUNE MANIFESTATIONS. Ann Intern Med. 1963 Sep;59:265–276. doi: 10.7326/0003-4819-59-3-265. [DOI] [PubMed] [Google Scholar]
- Lance E. M. The mechanism of action of anti-lymphocyte serum. Studies of antibody eluate. J Exp Med. 1969 Jul 1;130(1):49–76. doi: 10.1084/jem.130.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Miller J. F. Cell to cell interaction in the immune response. IV. Site of action of antilymphocyte globulin. J Exp Med. 1968 Oct 1;128(4):855–874. doi: 10.1084/jem.128.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors R. C., Aoki T., Huebner R. J. Further implication of murine leukemia-like virs in the disorders of NZB mice. J Exp Med. 1969 May 1;129(5):1045–1062. doi: 10.1084/jem.129.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors R. C. Autoimmune and immunoproliferative diseases of NZB/Bl mice and hybrids. Int Rev Exp Pathol. 1966;5:217–252. [PubMed] [Google Scholar]
- Mellors R. C., Shirai T., Aoki T., Huebner R. J., Krawczynski K. Wild-type Gross leukemia virus and the pathogenesis of the glomerulonephritis of New Zealand mice. J Exp Med. 1971 Jan 1;133(1):113–132. doi: 10.1084/jem.133.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Genetics of immunoglobulins in the mouse. Adv Immunol. 1967;7:91–145. doi: 10.1016/s0065-2776(08)60127-3. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970 Jun 27;226(5252):1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M., Yron I. Serologic demonstration of a thymus-dependent population of lymph-node cells. J Immunol. 1970 Apr;104(4):798–804. [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1412–1415. doi: 10.1073/pnas.68.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutman O., Yunis E. J., Good R. A. Deficient immunologic functions of NZB mice. Proc Soc Exp Biol Med. 1968 Apr;127(4):1204–1207. doi: 10.3181/00379727-127-32910. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., McIntire K. R., Boyse E. A. Immunoglobulin and other surface antigens of cells of the immune system. J Exp Med. 1971 Oct 1;134(4):815–832. doi: 10.1084/jem.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M. M., Mellors R. C., Lance E. M. Changes in lymphoid populations of ageing CBA and NZB mice. Clin Exp Immunol. 1971 Mar;8(3):491–500. [PMC free article] [PubMed] [Google Scholar]


