Abstract
A possible function of the thymus gland with regard to developing neuromuscular block after immunization with thymus extract is discussed.
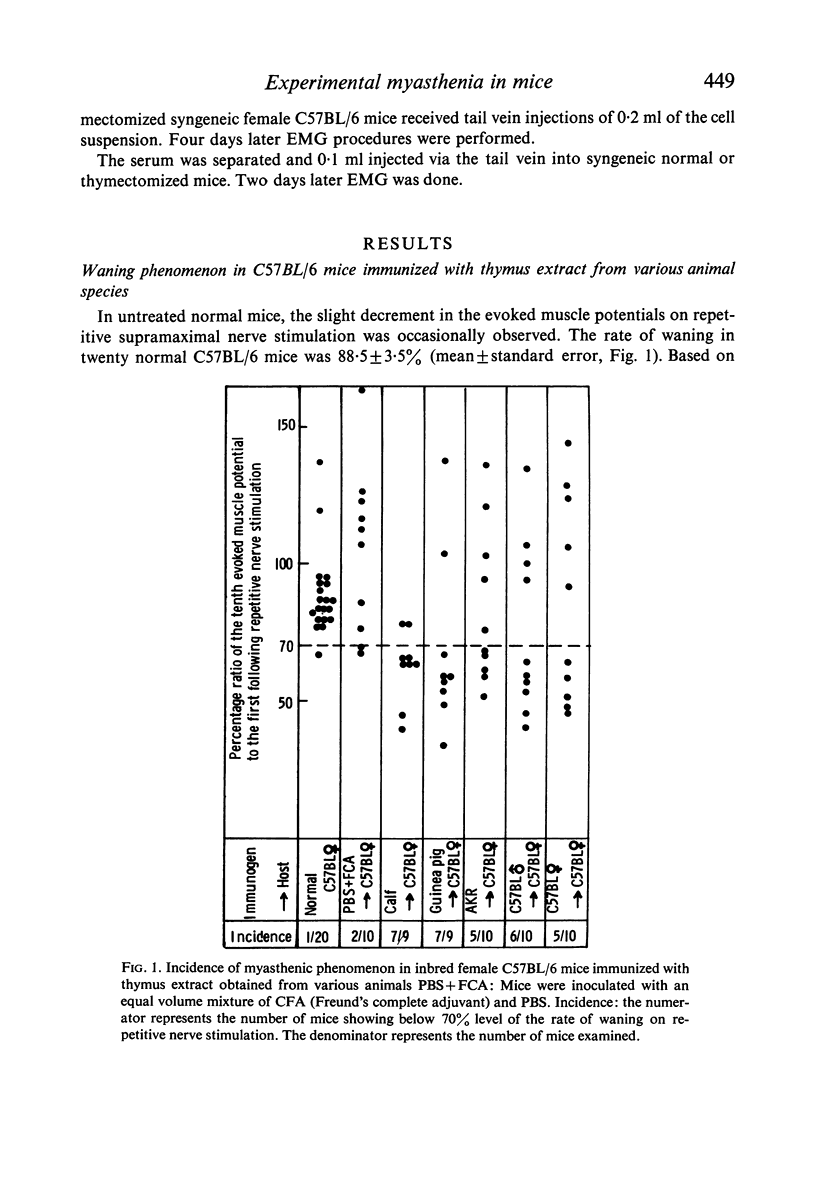
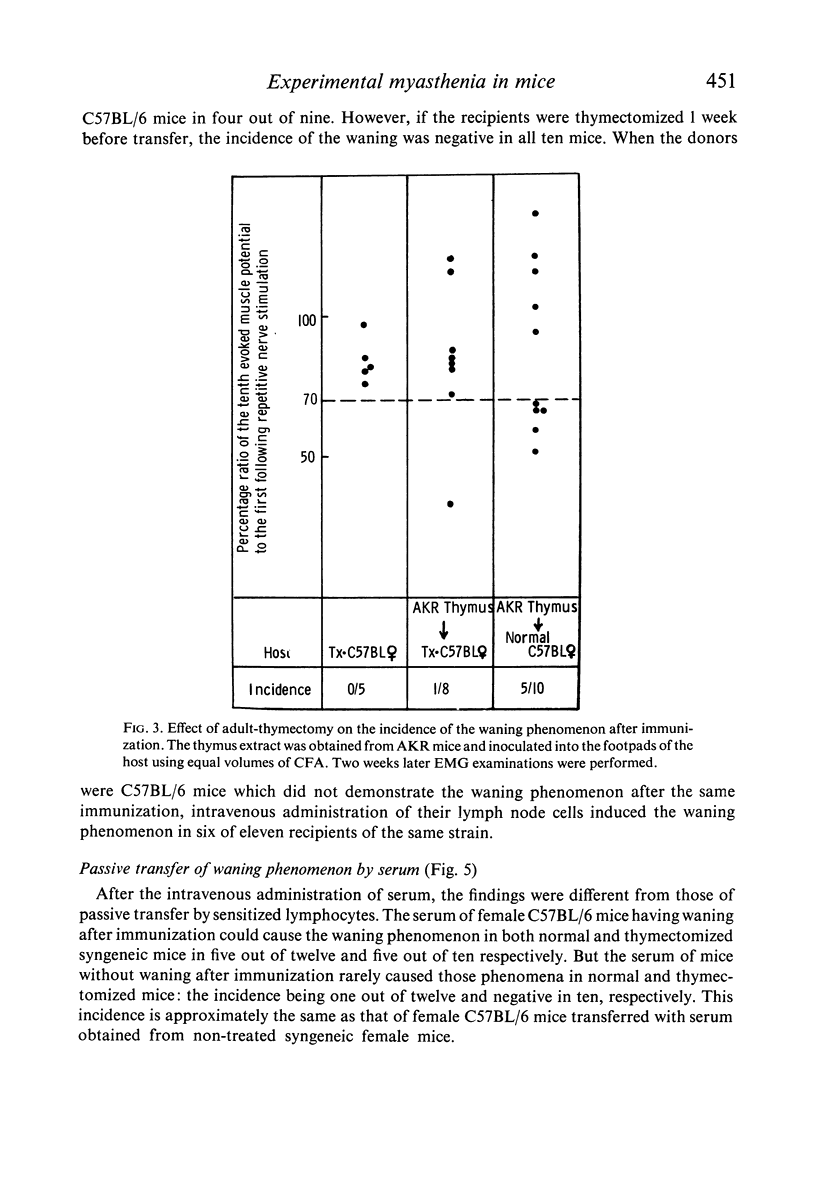
Inbred female C57BL/6 mice were immunized with thymus extract from female C57BL/6 (syngeneic), male C57BL/6 mice, AKR mice (allogeneic) and guinea-pigs and calves (xenogeneic). Two weeks later, the waning phenomenon was observed from the electromyography (EMG) in xenogeneically, allogeneically and syngeneically immunized mice. The waning reverted to a normal pattern following an intraperitoneal injection of neostigmine methylsulphate. EMG tracings revealed a decrease in the incidence of the waning phenomenon in those mice which were immunized after adult thymectomy.
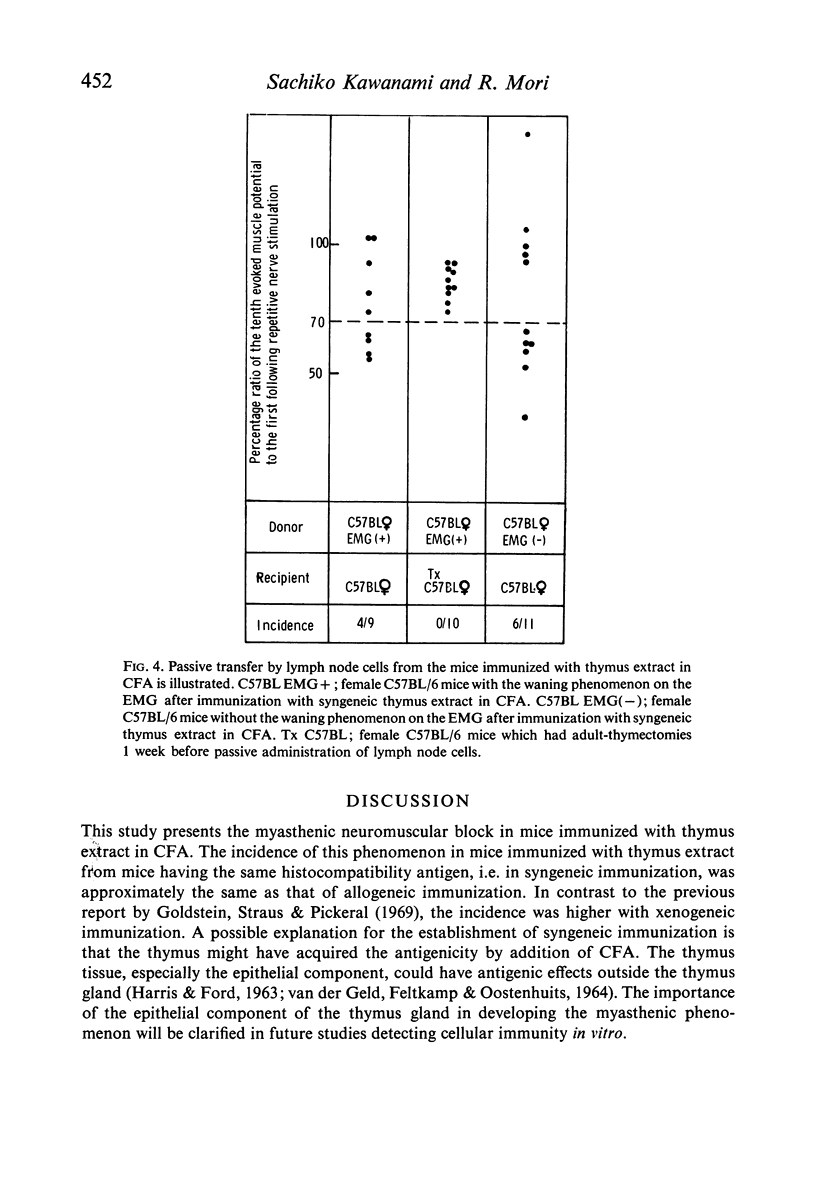
The passive transfer of the waning phenomenon to non-thymectomized recipients could be achieved by the intravenous administration of lymph node cells obtained from the mice that had developed a myasthenic neuromuscular block after immunization. This finding was also observed in the recipients when the donors of lymph node cells had not developed the block.
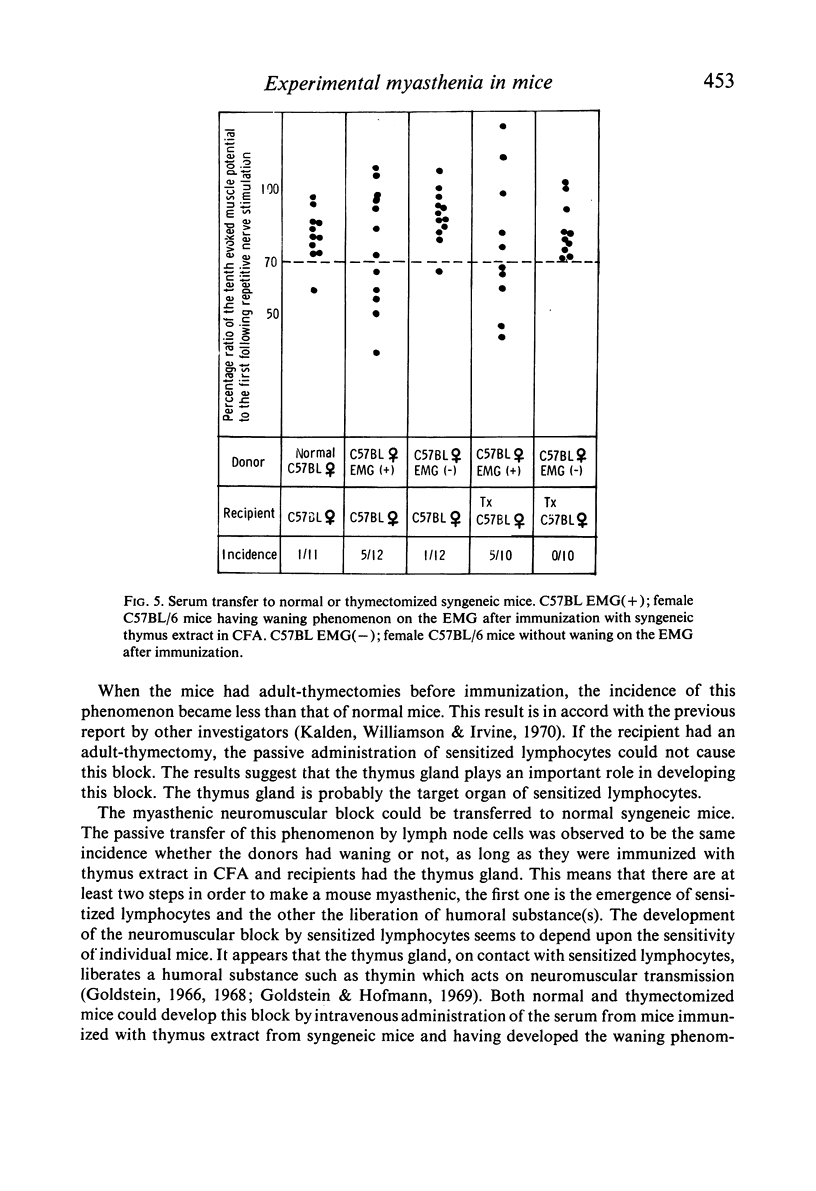
On the other hand, administration of sera from mice with the waning phenomenon after immunization resulted in a neuromuscular block in both normal and adult-thymectomized mice. However, sera obtained from mice without waning did not have this feature.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goldstein G., Hofmann W. W. Electrophysiological changes similar to those of myasthenia gravis in rats with experimental autoimmune thymitis. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):453–459. doi: 10.1136/jnnp.31.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Hofmann W. W. Endocrine function of the thymus affecting neuromuscular transmission. Clin Exp Immunol. 1969 Feb;4(2):181–189. [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Strauss A. J., Pickeral S. Antigens in thymus and muscle effective in inducing experimental autoimmune thymitis and the release of thymin. Clin Exp Immunol. 1969 Jan;4(1):3–16. [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. The thymus and neuromuscular function. A substance in thymus which causes myositis and myasthenic neuromuscular block in guineapigs. Lancet. 1968 Jul 20;2(7560):119–122. doi: 10.1016/s0140-6736(68)90414-5. [DOI] [PubMed] [Google Scholar]
- Goldstein G. Thymitis and myasthenia gravis. Lancet. 1966 Nov 26;2(7474):1164–1167. doi: 10.1016/s0140-6736(66)90479-x. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Whittingham S. Experimental autoimmune thymitis. An animal model of human myasthenia gravis. Lancet. 1966 Aug 6;2(7458):315–318. doi: 10.1016/s0140-6736(66)92599-2. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Whittingham S. Histological and serological features of experimental autoimmune thymitis in guinea-pigs. Clin Exp Immunol. 1967 May;2(3):257–268. [PMC free article] [PubMed] [Google Scholar]
- HARRIS J. E., FORD C. E. The role of the thymus: migration of cells from thymic grafts to lymph-nodes in mice. Lancet. 1963 Feb 16;1(7277):389–390. doi: 10.1016/s0140-6736(63)91418-1. [DOI] [PubMed] [Google Scholar]
- Jones S. F., Brennan J. L., McLeod J. G. An investigation of experimental myasthenia gravis. J Neurol Neurosurg Psychiatry. 1971 Aug;34(4):399–403. doi: 10.1136/jnnp.34.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalden J. R., Irvine W. J. Experimental myasthenia gravis. Lancet. 1969 Sep 20;2(7621):638–639. doi: 10.1016/s0140-6736(69)90342-0. [DOI] [PubMed] [Google Scholar]
- Kalden J. R., Williamson W. G., Irvine W. J. The effect of thymectomy, hemi-thymectomy and sham thymectomy on experimental myasthenia gravis in guinea-pigs. Clin Exp Immunol. 1970 Apr;6(4):519–530. [PMC free article] [PubMed] [Google Scholar]
- Kalden J. R., Williamson W. G., Johnston R. J., Irvine W. J. Studies on experimental autoimmune thymitis in guinea-pigs. Clin Exp Immunol. 1969 Oct;5(4):319–340. [PMC free article] [PubMed] [Google Scholar]
- Kaufman B. M., Rushworth G., Wright R. Experimental studies related to autoimmunity in myasthenia gravis. J Neurol Neurosurg Psychiatry. 1969 Aug;32(4):281–289. doi: 10.1136/jnnp.32.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER GELD H., FELTKAMP T. E., OOSTERHUIS H. J. REACTIVITY OF MYASTHENIA GRAVIS SERUM GAMMA-GLOBULIN WITH SKELETAL MUSCLE AND THYMUS DEMONSTRATED BY IMMUNOFLUORESCENCE. Proc Soc Exp Biol Med. 1964 Mar;115:782–785. doi: 10.3181/00379727-115-29037. [DOI] [PubMed] [Google Scholar]
- Vetters J. M., Simpson J. A., Folkarde A. Experimental myasthenia gravis. Lancet. 1969 Jul 5;2(7610):28–31. doi: 10.1016/s0140-6736(69)92601-4. [DOI] [PubMed] [Google Scholar]



