Abstract
The labelling of rabbit antiserum against purified and alkaline-degraded amyloid fibrils with fluorescein isothiocyanate (FITC), has enabled the detection of amyloid deposits in various tissue sections by direct immunofluorescence. There was antigenic identity between amyloid from different organs of the same patient. In addition, indirect immunofluorescence revealed individual antigenic specificity and cross-reactivity among amyloid from different individuals. A close relationship between deposits of amyloid, immunoglobulins and complement was observed when using FITC-and rhodamine-labelled antisera against various human plasma components.
Full text
PDF


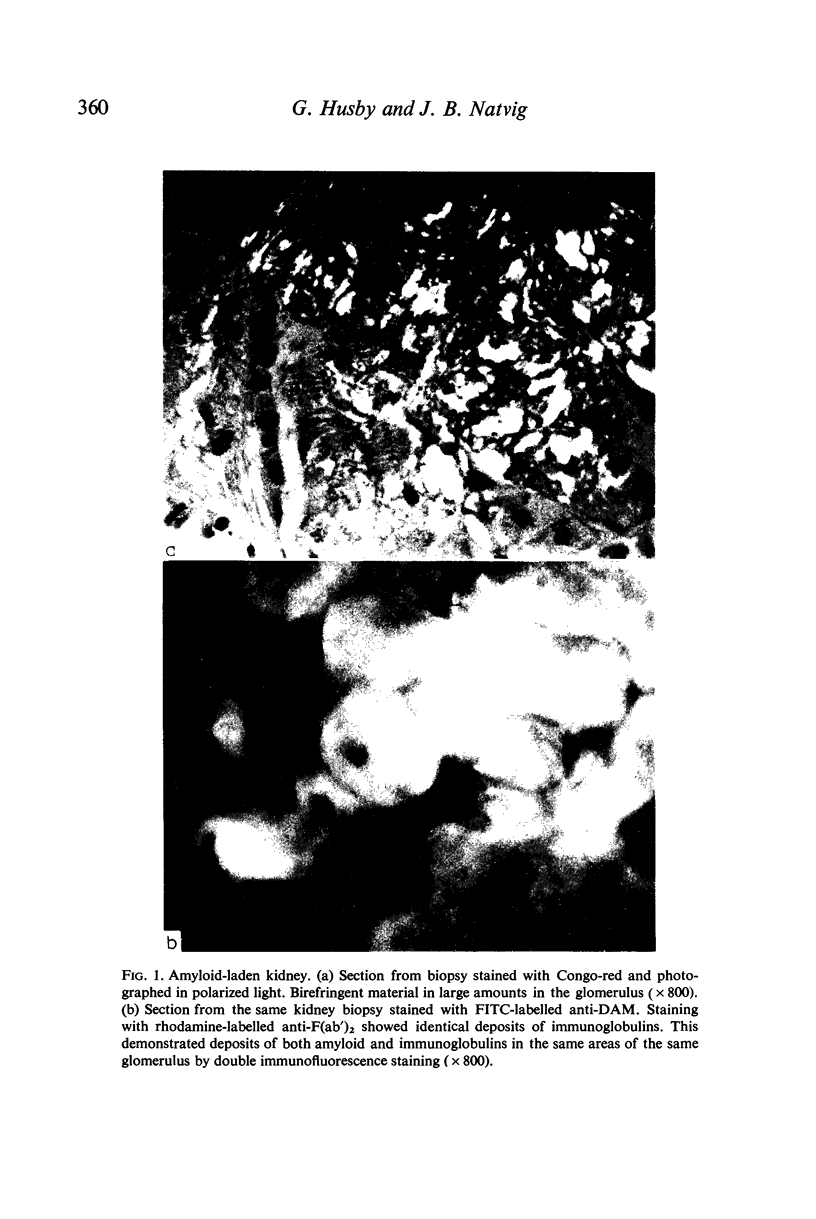
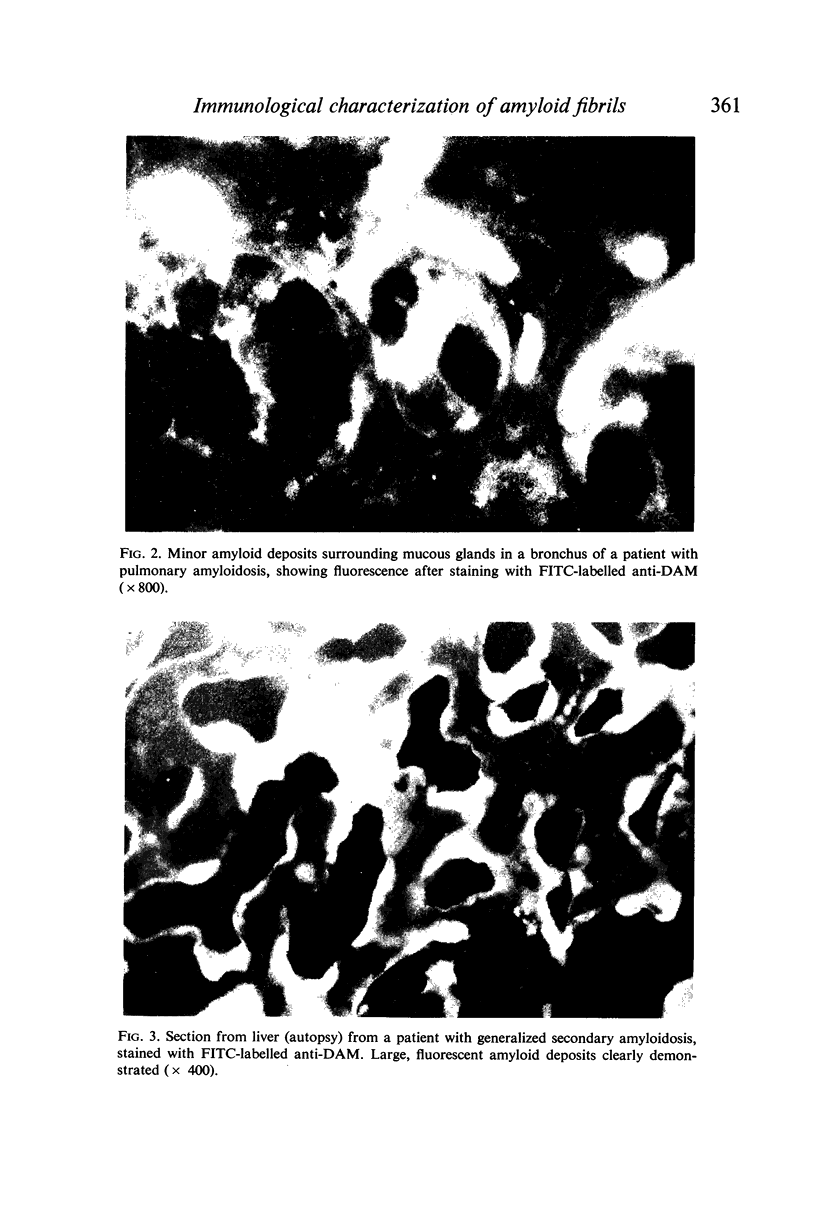

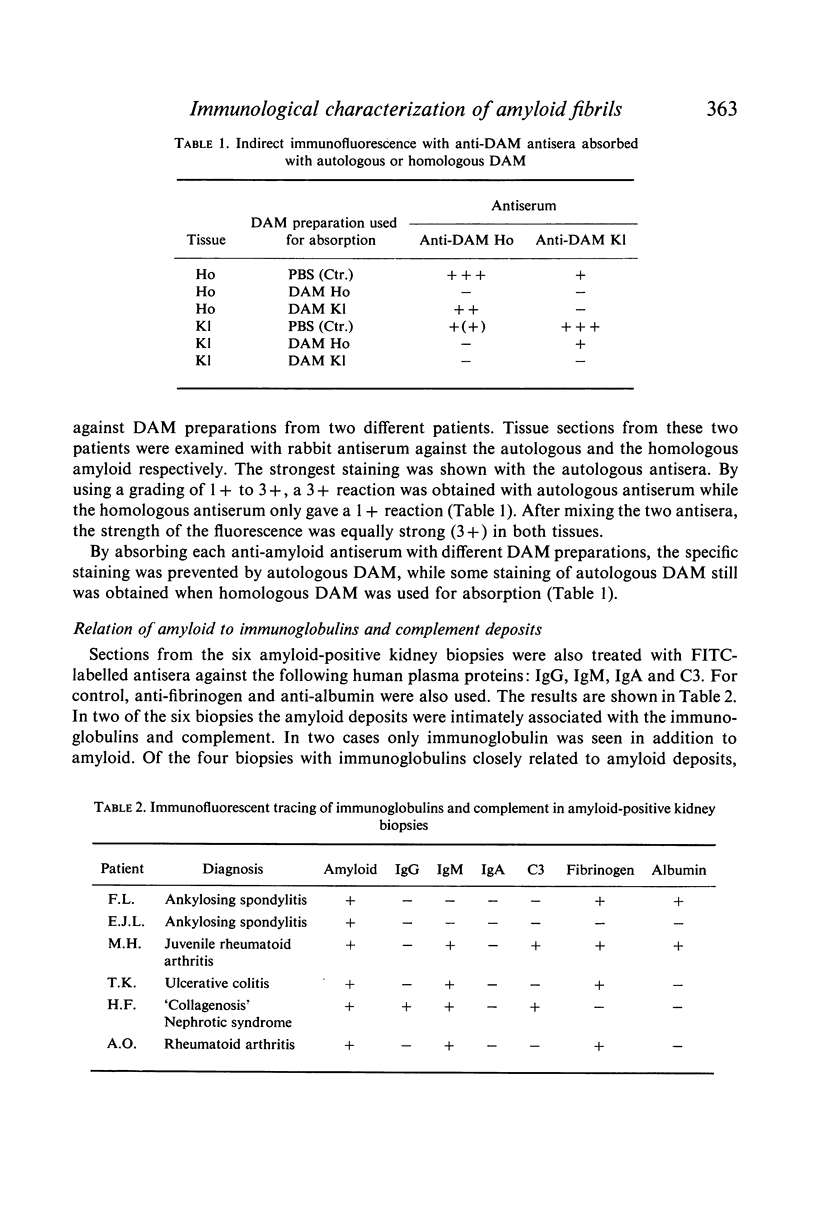



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutner E. H., Holborow E. J., Johnson G. D. Quantitative studies of immunofluorescent staining. I. Analyses of mixed immunofluorescence. Immunology. 1967 Mar;12(3):327–337. [PMC free article] [PubMed] [Google Scholar]
- Cathcart E. S., Skinner M., Cohen A. S. Immunogenicity of amyloid. Immunology. 1971 Jun;20(6):945–954. [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., VAZQUEZ J. J. Immunohistochemical analysis of amyloid by the fluorescence technique. J Exp Med. 1956 Nov 1;104(5):727–736. doi: 10.1084/jem.104.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin E. C., Pras M. Immunologic studies of water-soluble human amyloid fibrils. Comparative studies of eight amyloid preparations. J Exp Med. 1969 Oct 1;130(4):797–808. doi: 10.1084/jem.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Cuatrecasas P., Isersky C., Bladen H. A., Eanes E. D. Physical and chemical properties of amyloid fibers. II. Isolation of a unique protein constituting the major component from human splenic amyloid fibril concentrates. J Histochem Cytochem. 1969 Dec;17(12):769–780. doi: 10.1177/17.12.769. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Keiser H. R., Bladen H. A., Cuatrecasas P., Eanes E. D., Ram J. S., Kanfer J. N., DeLellis R. A. Amyloid. VI. A comparison of two morphologic components of human amyloid deposits. J Histochem Cytochem. 1968 Oct;16(10):633–644. doi: 10.1177/16.10.633. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Glenner G., Harada M., Isersky C., Cuatrecassas P., Page D., Keiser H. Human amyloid protein: diversity and uniformity. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1013–1019. doi: 10.1016/0006-291x(70)90186-5. [DOI] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. Individual antigenic specificity and cross-reactions among amyloid preparations from different individuals. Clin Exp Immunol. 1972 Apr;10(4):635–647. [PMC free article] [PubMed] [Google Scholar]
- LACHMANN P. J., MULLER-EBERHARD H. J., KUNKEL H. G., PARONETTO F. The localization of in vivo bound complement in tissue section. J Exp Med. 1962 Jan 1;115:63–82. doi: 10.1084/jem.115.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LETTERER E., CAESAR R., VOGT A. [Studies on electronoptic and immunomorphological structure of amyloid]. Dtsch Med Wochenschr. 1960 Oct 28;85:1909–1910. doi: 10.1055/s-0028-1112672. [DOI] [PubMed] [Google Scholar]
- Munthe E., Natvig J. B. Characterization of IgG complexes in eluates from rheumatoid tissue. Clin Exp Immunol. 1971 Feb;8(2):249–262. [PMC free article] [PubMed] [Google Scholar]
- Munthe E., Natvig J. B. Detection of genetic markers of human immunoglobulins by immunofluorescence technique. Ann N Y Acad Sci. 1971 Jun 21;177:326–334. doi: 10.1111/j.1749-6632.1971.tb35061.x. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Zucker-Franklin D., Rimon A., Franklin E. C. Physical, chemical, and ultrastructural studies of water-soluble human amyloid fibrils. Comparative analyses of nine amyloid preparations. J Exp Med. 1969 Oct 1;130(4):777–796. doi: 10.1084/jem.130.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri Ram J., DeLellis R. A., Glenner G. G. Amyloid. IV. Is human amyloid immunogenic? Int Arch Allergy Appl Immunol. 1968;34(3):269–282. [PubMed] [Google Scholar]
- The T. H., Feltkamp T. E. Conjugation of fluorescein isothiocyanate to antibodies. II. A reproducible method. Immunology. 1970 Jun;18(6):875–881. [PMC free article] [PubMed] [Google Scholar]
- VOGT A., KOCHEM H. G. [Histo-serological studies with fluorescein-labeled anticomplement. Demonstration of complement-binding substances in amyloid]. Z Zellforsch Mikrosk Anat. 1960;52:640–652. [PubMed] [Google Scholar]