Abstract
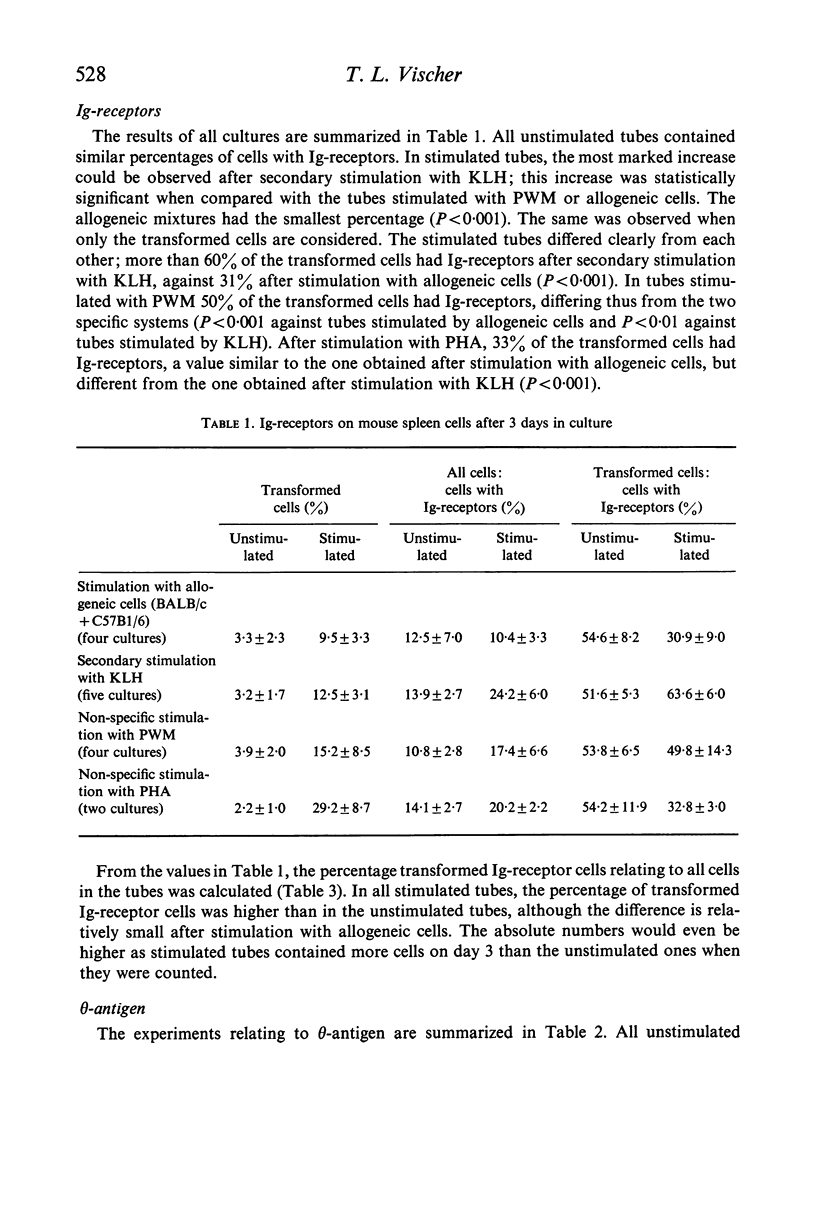
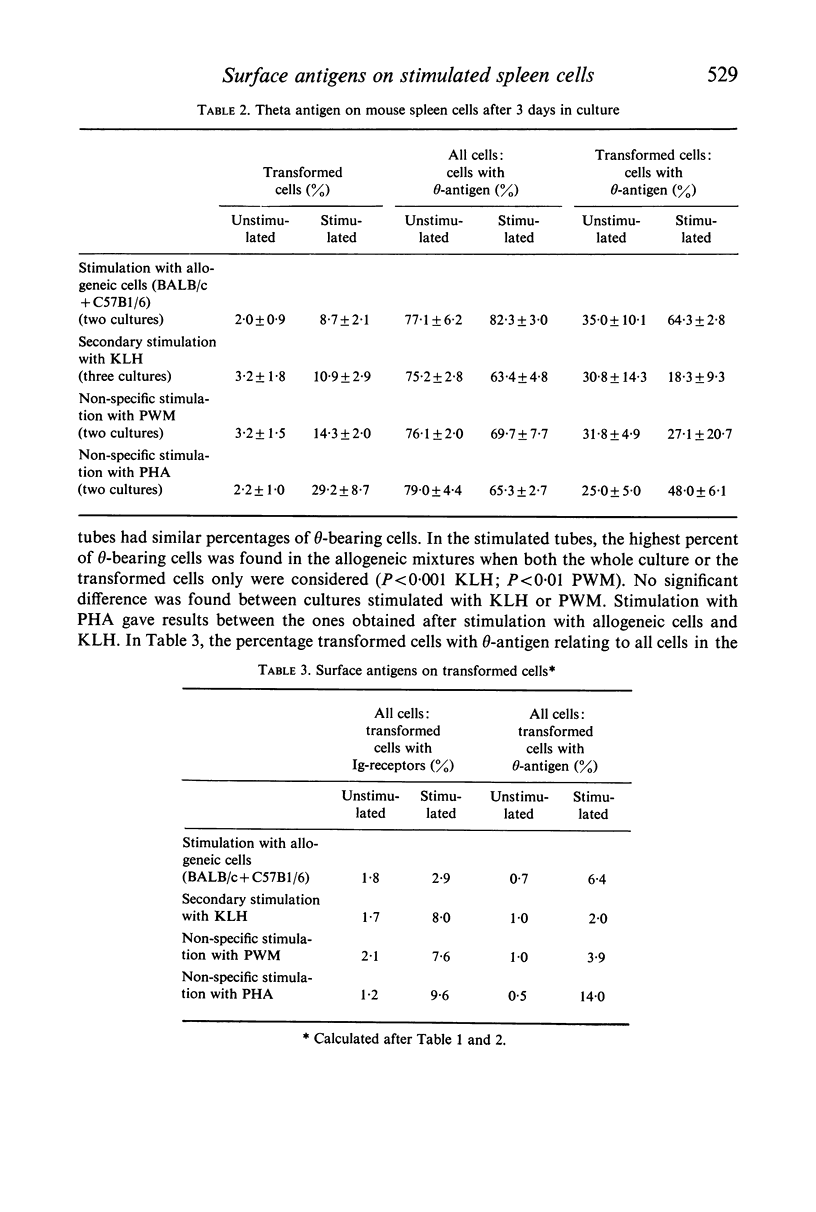
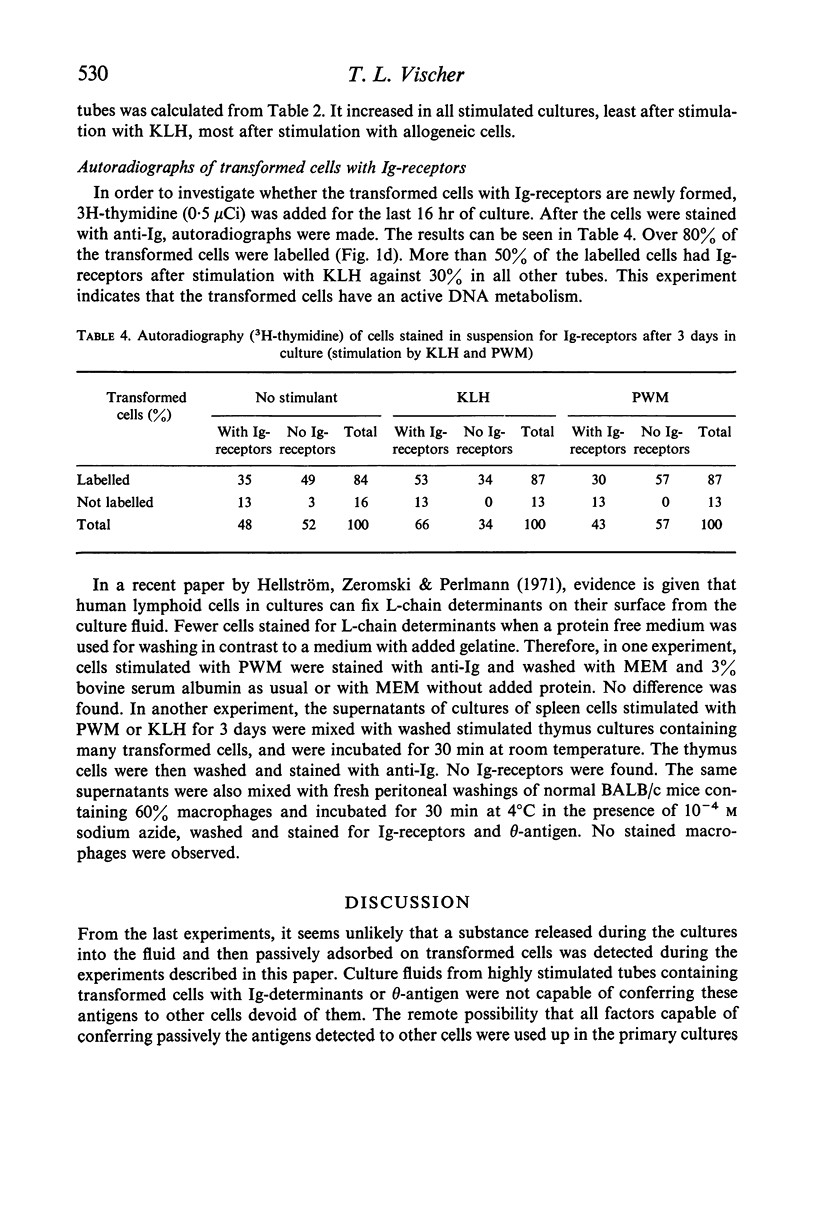
BALB/c spleen cells were stimulated with C57B1/6 cells, keyhole limpet haemocyanin (KLH), pokeweed mitogen (PWM) or phytohaemagglutinin (PHA). By the immunofluorescence method the development of transformed cells with immunoglobulin-like receptor molecules or theta antigen was investigated. Transformed Ig-receptor cells were predominant after stimulation with KLH, followed by PWM, PHA and allogeneic cells in decreasing order. Transformed cells with theta-antigen were clearly increased after stimulation with allogeneic cells. With all systems, more transformed cells with Ig-receptors were found in the stimulated cultures than in the control cultures, indicating that all stimulants have an effect on both cell types. The implications of the results on the immunologic interpretation of results obtained by in vitro stimulation of lymphocytes with the different stimulants are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankhurst A. D., Warner N. L. Surface immunoglobulins on mouse lymphoid cells. J Immunol. 1971 Aug;107(2):368–373. [PubMed] [Google Scholar]
- Benezra D., Gery I., Davies A. M. The relationship between lymphocyte transformation and immune responses. II. Correlations between transformation and humoral and cellular immune responses. Clin Exp Immunol. 1969 Jul;5(1):155–161. [PMC free article] [PubMed] [Google Scholar]
- Bradley J., Oppenheim J. J. The in vitro proliferation of lymphocytes from patients with hypogammaglobulinaemia. Clin Exp Immunol. 1967 Sep;2(5):549–557. [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Bockman D. E. Agammaglobulinaemia with B lymphocytes. Specific defect of plasma-cell differentiation. Lancet. 1971 Oct 9;2(7728):791–794. doi: 10.1016/s0140-6736(71)92742-5. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Goldberg L. S., Fudenberg H. H. Clinical, serologic and leukocyte function studies on patients with idiopathic "acquired" agammaglobulinemia and their families. Am J Med. 1970 Jan;48(1):48–53. doi: 10.1016/0002-9343(70)90097-5. [DOI] [PubMed] [Google Scholar]
- Gallily R., Garvey J. S. Primary stimulation of rats and mice with hemocyanin in soluton and adsorbed on bentonite. J Immunol. 1968 Nov;101(5):924–929. [PubMed] [Google Scholar]
- Gordon J., MacLean L. D. A lymphocyte-stimulating factor produced in vitro. Nature. 1965 Nov 20;208(5012):795–796. doi: 10.1038/208795a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Hellström U., Zeromski J., Perlmann P. Immunoglobulin light chain determinants on unstimulated and stimulated human blood lymphocytes, assayed by indirect immunofluorescence. Immunology. 1971 Jun;20(6):1099–1111. [PMC free article] [PubMed] [Google Scholar]
- Hosking C. S., Fitzgerald M. G., Simons M. J. Quantified deficiency of lymphocyte response to phytohaemagglutinin in immune deficiency diseases. Clin Exp Immunol. 1971 Oct;9(4):467–476. [PMC free article] [PubMed] [Google Scholar]
- Jones G., Torrigiani G., Roitt I. M. Immunoglobulin determinants on mouse lymphocytes. J Immunol. 1971 Jun;106(6):1425–1430. [PubMed] [Google Scholar]
- Lesley J. F., Kettman J. R., Dutton R. W. Immunoglobulins on the surface of thymus-derived cells engaged in the initiation of a humoral immune response. J Exp Med. 1971 Sep 1;134(3 Pt 1):618–629. doi: 10.1084/jem.134.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewi G., Temple A., Vischer T. L. The immunological significance in the guinea-pig of in vitro transformation of lymph node, spleen and peripheral blood lymphocytes. Immunology. 1968 Feb;14(2):257–264. [PMC free article] [PubMed] [Google Scholar]
- MUNOZ J. Production in mice of large volumes of ascites fluid containing antibodies. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):757–759. doi: 10.3181/00379727-95-23355. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Mills J. A. The immunologic significance of antigen induced lymphocyte transformation in vitro. J Immunol. 1966 Aug;97(2):239–247. [PubMed] [Google Scholar]
- Oppenheim J. J., Wolstencroft R. A., Gell P. G. Delayed hypersensitivity in the guinea-pig to a protein-hapten conjugate and its relationship to in vitro transformation of lymph node, spleen, thymus and peripheral blood lymphocytes. Immunology. 1967 Jan;12(1):89–102. [PMC free article] [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H. Cell cooperation in the immune response. Clin Exp Immunol. 1971 Jun;8(6):839–856. [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON M., DUTTON R. W. ANTIBODY SYNTHESIZING CELLS: APPEARANCE AFTER SECONDARY ANTIGENIC STIMULATION IN VITRO. Science. 1964 Oct 30;146(3644):655–656. doi: 10.1126/science.146.3644.655. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- Rieke W. O. Lymphocytes from thymectomized rats: immunologic, proliferative, and metabolic properties. Science. 1966 Apr 22;152(3721):535–538. doi: 10.1126/science.152.3721.535. [DOI] [PubMed] [Google Scholar]
- Spitler L. E., Lawrence H. S. Studies of lymphocyte culture: products of sensitive lymphocyte-antigen interaction. J Immunol. 1969 Nov;103(5):1072–1077. [PubMed] [Google Scholar]
- Stockman G. D., Gallagher M. T., Heim L. R., South M. A., Trentin J. J. Differential stimulation of mouse lymphoid cells by phytohemagglutinin and pokeweed mitogen. Proc Soc Exp Biol Med. 1971 Mar;136(3):980–982. doi: 10.3181/00379727-136-35410. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Carswell E. A., Thorbecke G. J. Surface antigens of immunocompetent cells. I. Effect of theta and PC.1 alloantisera on the ability of spleen cells to transfer immune responses. J Exp Med. 1970 Dec 1;132(6):1181–1190. doi: 10.1084/jem.132.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi T., Adler W. H., Smith R. T. Cellular recognition in vitro by mouse lymphocytes. Effects of neonatal thymectomy and thymus graft restoration on alloantigen and PHA stimulation of whole and gradient-separated subpopulations of spleen cells. J Exp Med. 1971 Jan 1;133(1):63–80. doi: 10.1084/jem.133.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L. Culture of mouse lymphoid cells in serum-free medium. J Immunol Methods. 1972 Jan;1(2):199–202. doi: 10.1016/0022-1759(72)90046-4. [DOI] [PubMed] [Google Scholar]
- Vischer T. L., Jaquet C. Effect of antibodies against immunoglobulins and the theta antigen on the specific and non-specific stimulation of mouse spleen cells in vitro. Immunology. 1972 Feb;22(2):259–266. [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Mäkelä O. Separation of normal and immune lymphoid cells by antigen-coated coated columns. Antigen-binding characteristics of membrane antibodies as analyzed by hapten-protein antigens. J Exp Med. 1970 Jul 1;132(1):110–126. doi: 10.1084/jem.132.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]


