Abstract
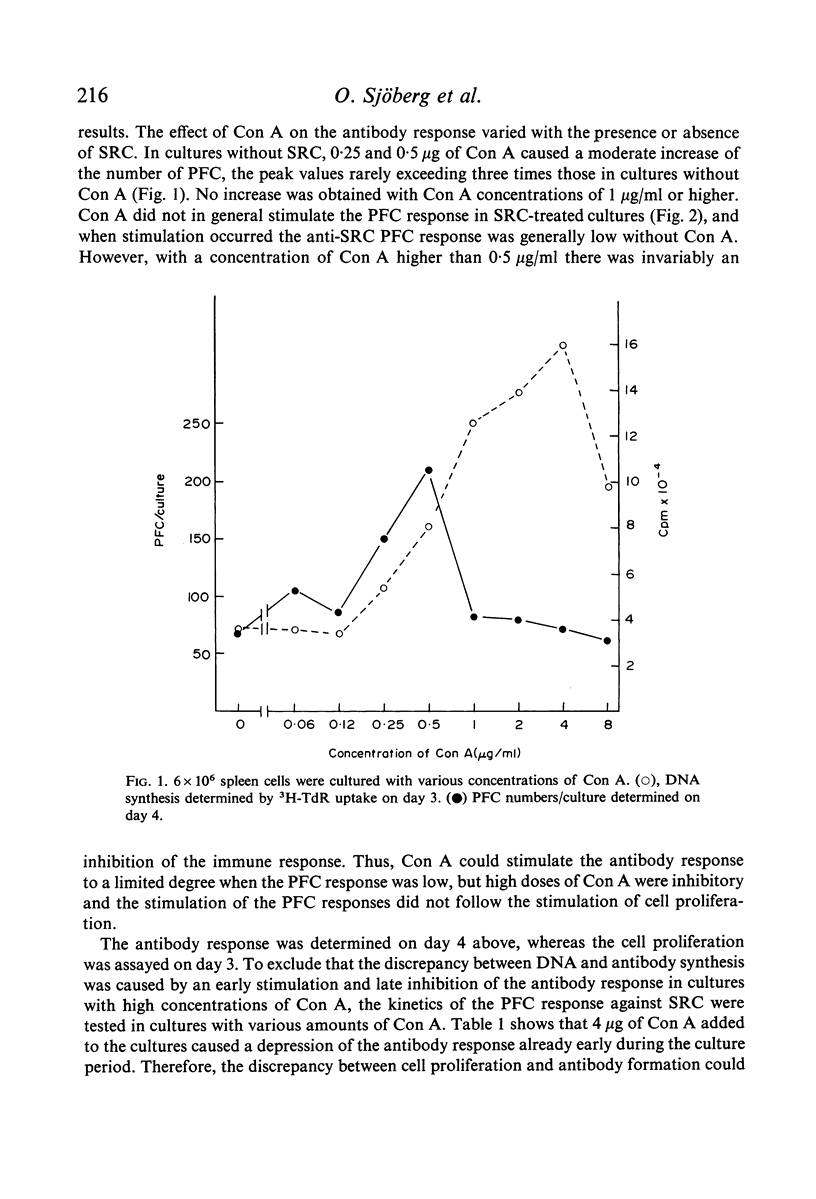
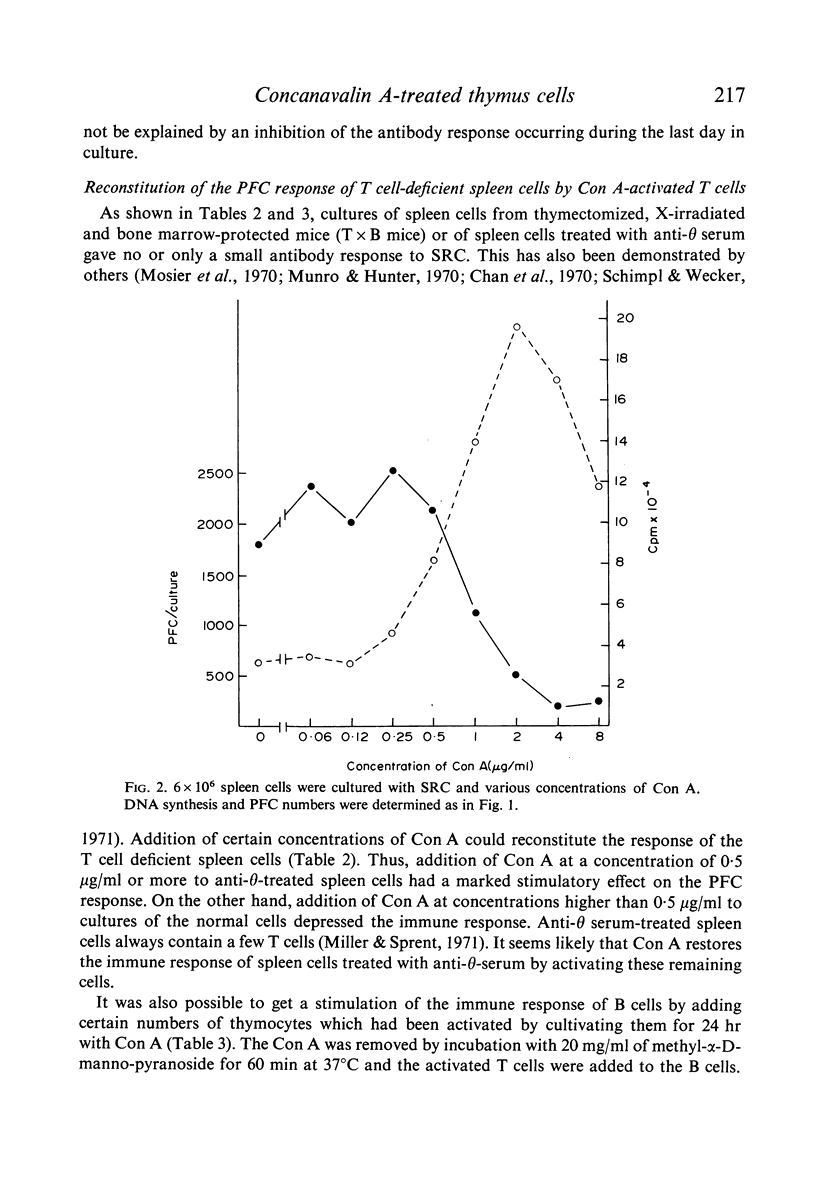
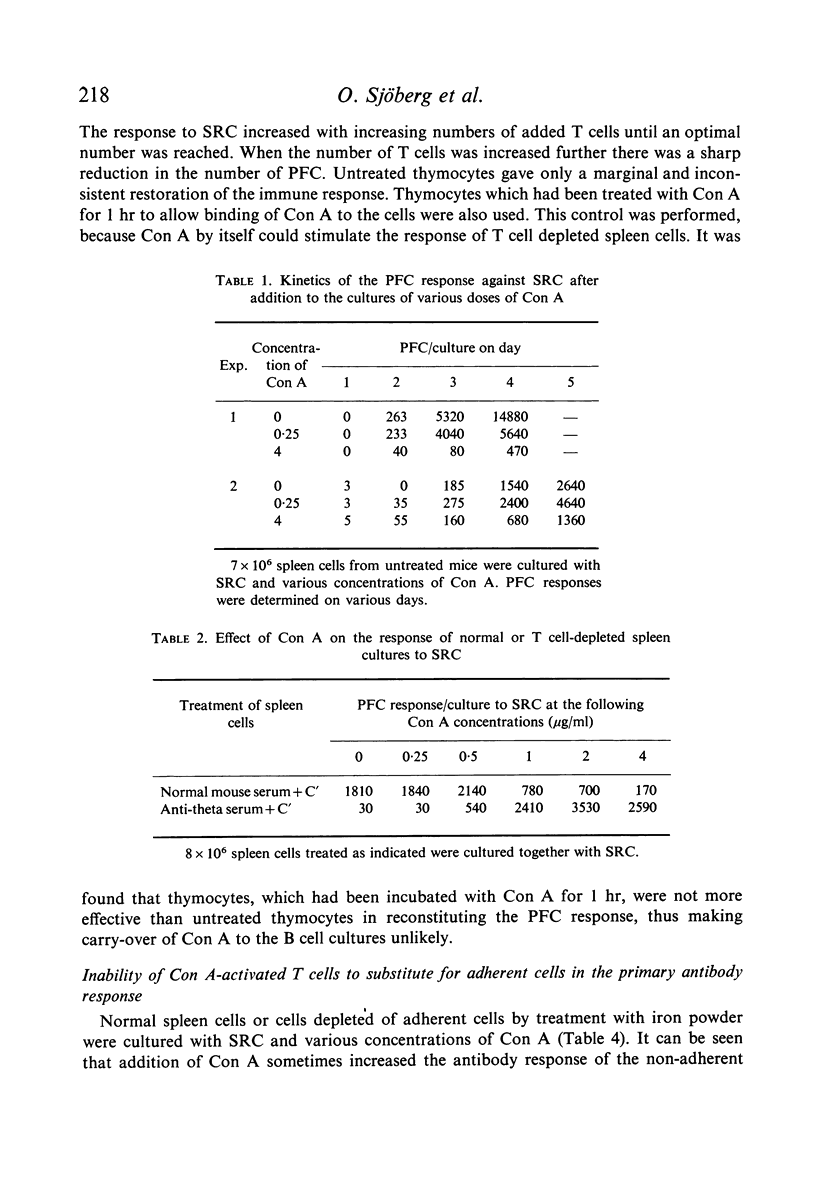
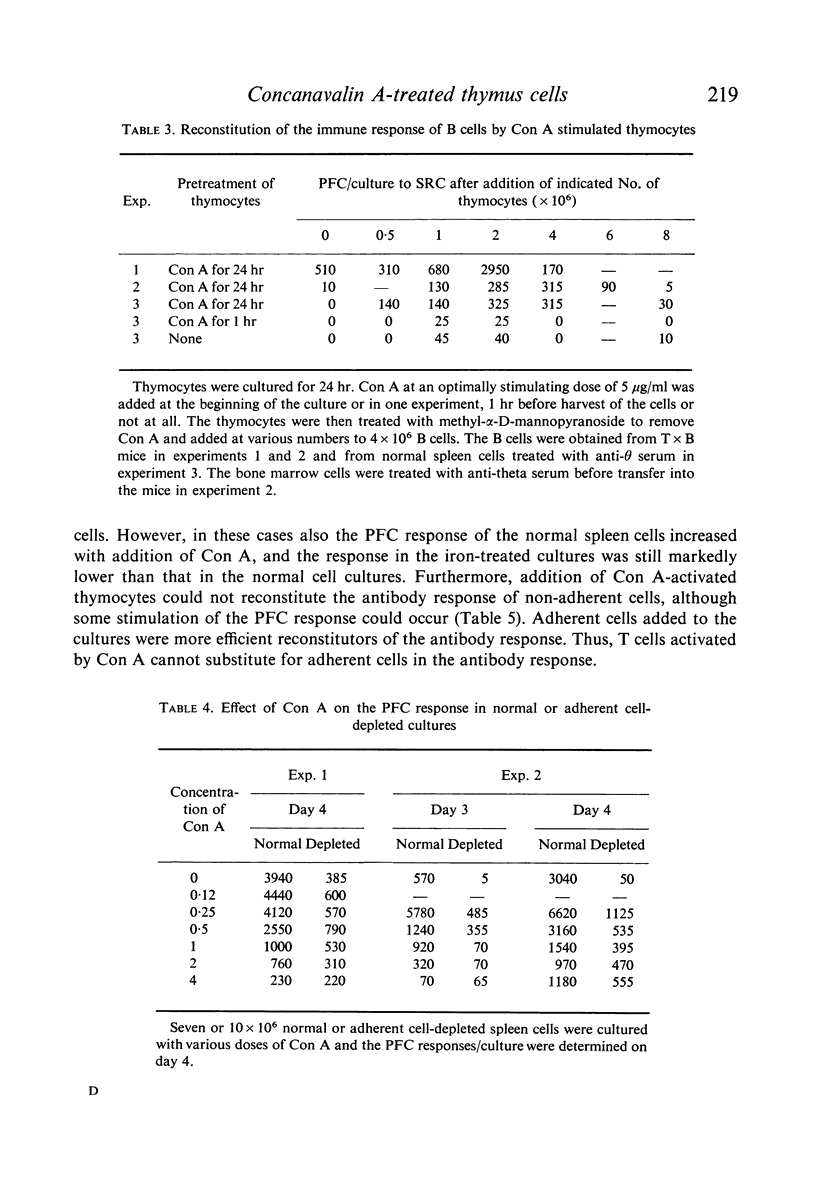
The effect of soluble Concanavalin A (Con A) on the primary antibody response in vitro against sheep red cells (SRC) by mouse spleen cells was studied. Stimulation of normal spleen cells with Con A caused a slight increase of the background number of plaque-forming cells (PFC). Cultures stimulated with SRC did not show an increased PFC response after Con A treatment, except in experiments where the PFC response against SRC was rather low in the absence of Con A. Concentrations of Con A higher than 0·5 μg/ml were inhibitory to the immune response of SRC-treated cultures also early during the culture period. In contrast, T cell depleted spleen cultures, incapable by themselves to respond to SRC, were reconstituted by addition of Con A at concentrations of 0·5 μg/ml or more. Presumably, residual T cells were activated by Con A. Con A activated T cells could reconstitute the PFC response in T cell-deficient cultures. In contrast, it was not possible to obtain more than a marginal stimulation of the antibody response in cultures of spleen cells depleted of adherent cells by addition of soluble Con A or Con A-activated thymocytes.
The results suggest that Con A may stimulate the antibody response by activation of T cells, and support the concept that activated T cells can non-specifically stimulate the antibody response of B cells. Large numbers of activated T cells were inhibitory to the immune response and it is suggested that this phenomenon is analagous to antigenic competition. Furthermore, activated T cells do not seem capable of substituting for adherent cells in the primary immune response in vitro, suggesting that adherent cells are important for the functions of B cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Britton S. When allogeneic mouse spleen cells are mixed in vitro the T-cells secrete a product which guides the maturation of B-cells. Scand J Immunol. 1972;1(1):89–98. doi: 10.1111/j.1365-3083.1972.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Chan E. L., Mishell R. I., Mitchell G. F. Cell interaction in an immune response in vitro: requirement for theta-carrying cells. Science. 1970 Dec 11;170(3963):1215–1217. doi: 10.1126/science.170.3963.1215. [DOI] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Basten A. Specific collaboration between T and B lymphocytes across a cell impermeable membrane in vitro. Nat New Biol. 1972 May 3;237(70):13–15. doi: 10.1038/newbio237013a0. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Easten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med. 1971 Jul 1;134(1):103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten antibody responses. 3. Stimulation of antibody synthesis and facilitation of hapten-specific secondary antibody responses by graft-versus-host reactions. J Exp Med. 1971 Feb 1;133(2):169–186. doi: 10.1084/jem.133.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth H. W., Williamson A. R. Cell surveillance model for lymphocyte cooperation. Nature. 1971 Dec 24;234(5330):454–456. doi: 10.1038/234454a0. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J. Lymphocyte cooperation. Proc R Soc Lond B Biol Sci. 1971 Jan 12;176(1045):425–426. doi: 10.1098/rspb.1971.0005. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Sprent J. Thymus-derived cells in mouse thoracic duct lymph. Nat New Biol. 1971 Apr 28;230(17):267–270. doi: 10.1038/newbio230267a0. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Fitch F. W., Rowley D. A., Davies A. J. Cellular deficit in thymectomized mice. Nature. 1970 Jan 17;225(5229):276–277. doi: 10.1038/225276a0. [DOI] [PubMed] [Google Scholar]
- Munro A., Hunter P. In vitro reconstitution of the immune response of thymus-deprived mice to sheep red blood cells. Nature. 1970 Jan 17;225(5229):277–278. doi: 10.1038/225277a0. [DOI] [PubMed] [Google Scholar]
- Möller G. Immunocyte triggering. Cell Immunol. 1970 Dec;1(6):573–582. doi: 10.1016/0008-8749(70)90023-7. [DOI] [PubMed] [Google Scholar]
- Möller G. Induction of antigenic competition with thymus-dependent antigens: effect on DNA synthesis in spleen cells. J Immunol. 1971 Jun;106(6):1566–1571. [PubMed] [Google Scholar]
- Möller G. Suppressive effect of graft versus host reactions on the immune response to heterologous red cells. Immunology. 1971 Apr;20(4):597–609. [PMC free article] [PubMed] [Google Scholar]
- Nisbet N. W., Simonsen M., Zaleski M. The frequency of antigen-sensitive cells in tissue transplantation. A commentary on clonal selection. J Exp Med. 1969 Mar 1;129(3):459–467. doi: 10.1084/jem.129.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Reconstitution of a thymus cell-deprived immune system by syngeneic and allogeneic thymocytes in vitro. Eur J Immunol. 1971 Aug;1(4):304–306. doi: 10.1002/eji.1830010419. [DOI] [PubMed] [Google Scholar]
- Sjöberg O., Andersson J., Möller G. Requirement for adherent cells in the primary and secondary immune response in vitro. Eur J Immunol. 1972 Apr;2(2):123–126. doi: 10.1002/eji.1830020206. [DOI] [PubMed] [Google Scholar]
- Sjöberg O. Antigenic competition in vitro of spleen cells subjected to a graft-versus-host reaction. Immunology. 1971 Aug;21(2):351–361. [PMC free article] [PubMed] [Google Scholar]
- Sjöberg O., Britton S. Antigenic competition in vitro between heterologous erythrocytes. Eur J Immunol. 1972 Jun;2(3):282–288. doi: 10.1002/eji.1830020318. [DOI] [PubMed] [Google Scholar]
- Sjöberg O. Effect of allogeneic cell interaction on the primary immune response in vitro. Cell types involved in suppression and stimulation of antibody synthesis. Clin Exp Immunol. 1972 Nov;12(3):365–375. [PMC free article] [PubMed] [Google Scholar]
- Taylor R. B., Iverson G. M. Hapten competition and the nature of cell-cooperation in the antibody response. Proc R Soc Lond B Biol Sci. 1971 Jan 12;176(1045):393–418. doi: 10.1098/rspb.1971.0003. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Blyth JL NOWELL P. C. Quantitative studies on the mixed lymphocyte interaction in rats. 3. Kinetics of the response. J Exp Med. 1968 Nov 1;128(5):1157–1181. doi: 10.1084/jem.128.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]


