Abstract
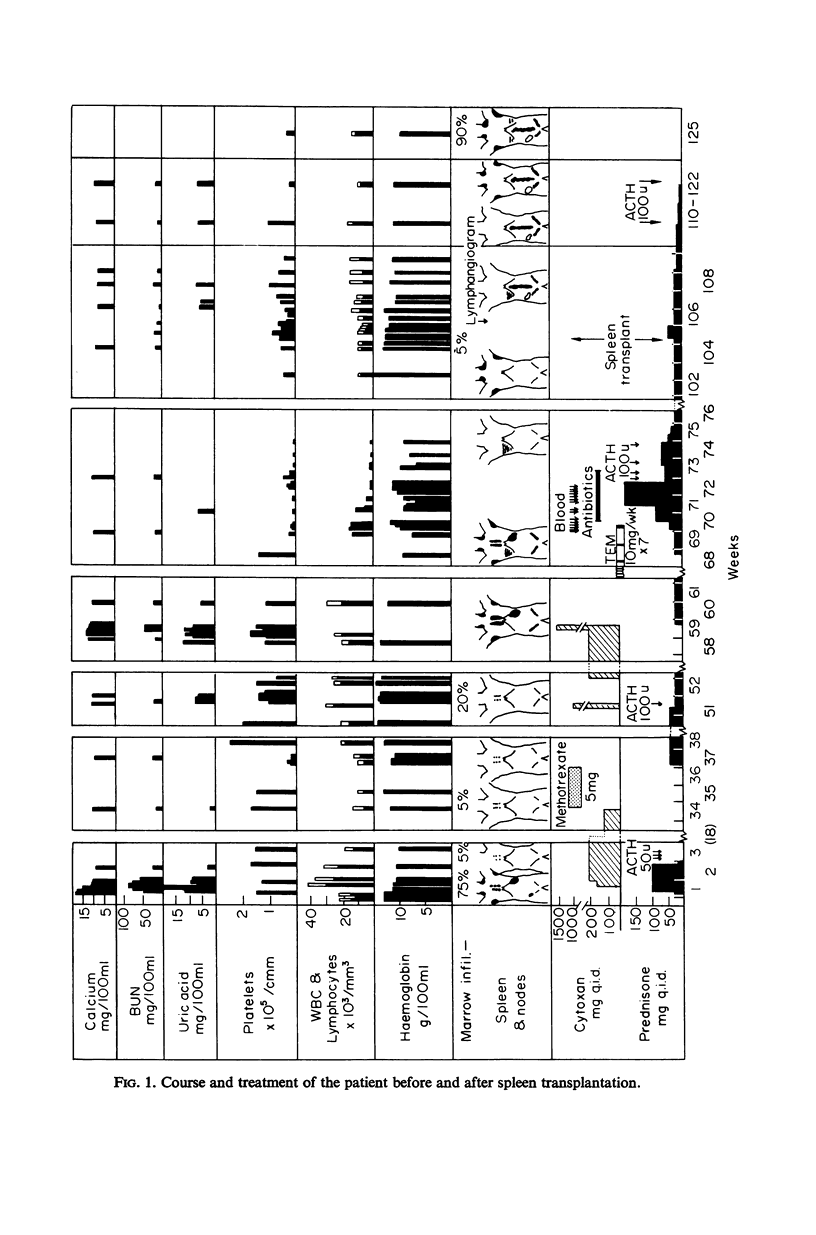
The results of spleen transplantation from a healthy individual to his leukaemic identical twin are reported. The rationale for the procedure and its significance in relation to present hypotheses of tumour growth and spread are reviewed. Although lymphocytes of both the donor and recipient showed specific immune capacity to destroy tumour cells in vitro, there was evidence for partial inhibition of the immunological effector process that destroys the tumour cells in the diminished lymphocyte phytoagglutinin stimulation, hypoglobulinaemia, and marked deficiency of IgA in the leukaemic twin. These abnormalities and the final relapse in spite of transplantation of the spleen as a source of immunocompetent cells could also be considered the result of growth potential in the tumour, capable of overriding any immunological control.
Full text
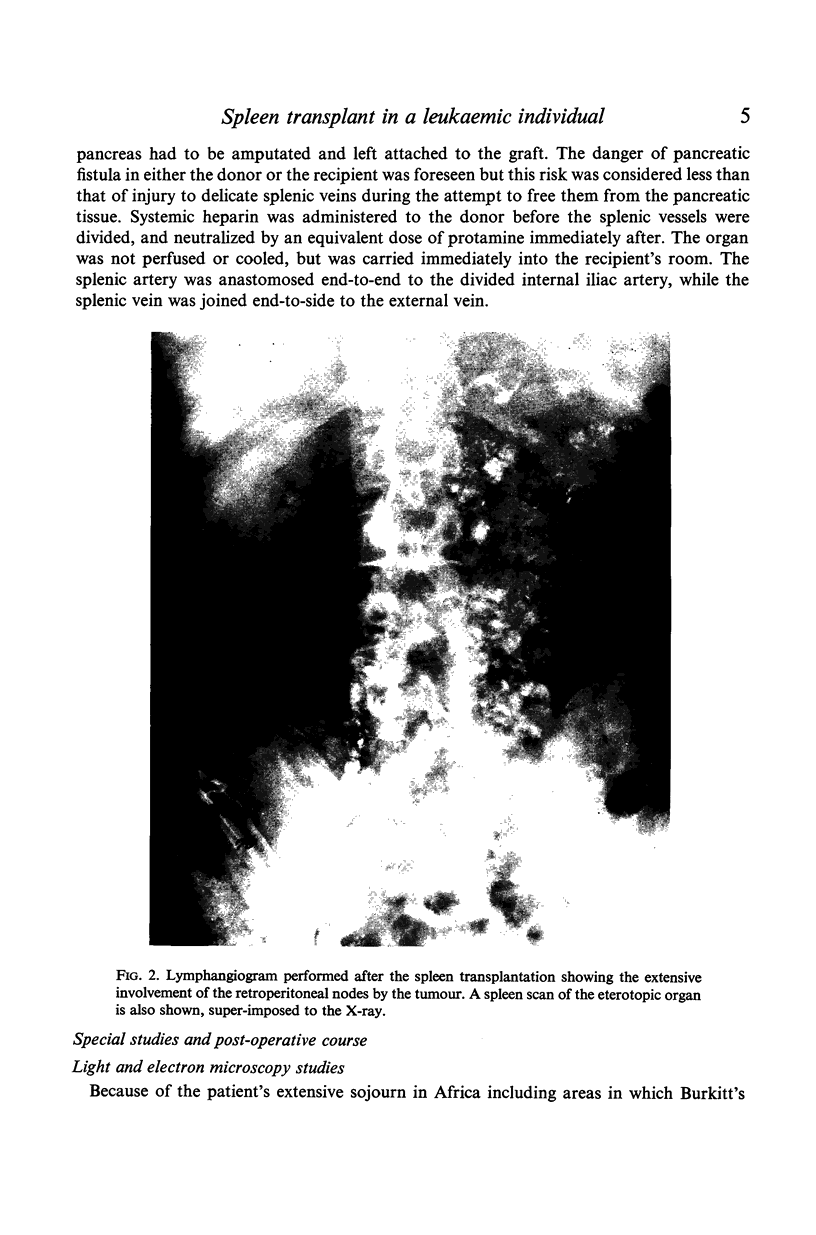
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON R. C., HERMANN H. W. Leukemia in twin children. J Am Med Assoc. 1955 Jun 25;158(8):652–654. doi: 10.1001/jama.1955.02960080028006d. [DOI] [PubMed] [Google Scholar]
- BLACK M. M., KERPE S., SPEER F. D. Lymph node structure in patients with cancer of the breast. Am J Pathol. 1953 May-Jun;29(3):505–521. [PMC free article] [PubMed] [Google Scholar]
- BURNET M. IMMUNOLOGICAL FACTORS IN THE PROCESS OF CARCINOGENESIS. Br Med Bull. 1964 May;20:154–158. doi: 10.1093/oxfordjournals.bmb.a070310. [DOI] [PubMed] [Google Scholar]
- Burkitt D. Long-term remissions following one and two-dose chemotherapy for African lymphoma. Cancer. 1967 May;20(5):756–759. doi: 10.1002/1097-0142(1967)20:5<756::aid-cncr2820200530>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- DUNPHY J. E. Some observations on the natural behavior of cancer in man. N Engl J Med. 1950 Feb 2;242(5):167-72, illust. doi: 10.1056/NEJM195002022420502. [DOI] [PubMed] [Google Scholar]
- Doak P. B., Montgomerie J. Z., North J. D., Smith F. Reticulum cell sarcoma after renal homotransplantation and azathioprine and prednisone therapy. Br Med J. 1968 Dec 21;4(5633):746–748. doi: 10.1136/bmj.4.5633.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLEY E. J. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953 Dec;13(12):835–837. [PubMed] [Google Scholar]
- GROSS L. IMMUNOLOGICAL DEFECT IN AGED POPULATION AND ITS RELATIONSHIP TO CANCER. Cancer. 1965 Feb;18:201–204. doi: 10.1002/1097-0142(196502)18:2<201::aid-cncr2820180211>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- HUNGERFORD D. A., DONNELLY A. J., NOWELL P. C., BECK S. The chromosome constitution of a human phenotypic intersex. Am J Hum Genet. 1959 Sep;11:215–236. [PMC free article] [PubMed] [Google Scholar]
- Hellström I., Hellström K. E., Evans C. A., Heppner G. H., Pierce G. E., Yang J. P. Serum-mediated protection of neoplastic cells from inhibition by lymphocytes immune to their tumor-specific antigens. Proc Natl Acad Sci U S A. 1969 Feb;62(2):362–368. doi: 10.1073/pnas.62.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON L. O., ROBSON M. J., MARKS E. K. The effect of x radiation on antibody formation. Proc Soc Exp Biol Med. 1950 Oct;75(1):145–152. doi: 10.3181/00379727-75-18127. [DOI] [PubMed] [Google Scholar]
- Jerusalem C. Relationship between malaria infection (Plasmodium berghei) and malignant lymphoma in mice. Z Tropenmed Parasitol. 1968 Mar;19(1):94–108. [PubMed] [Google Scholar]
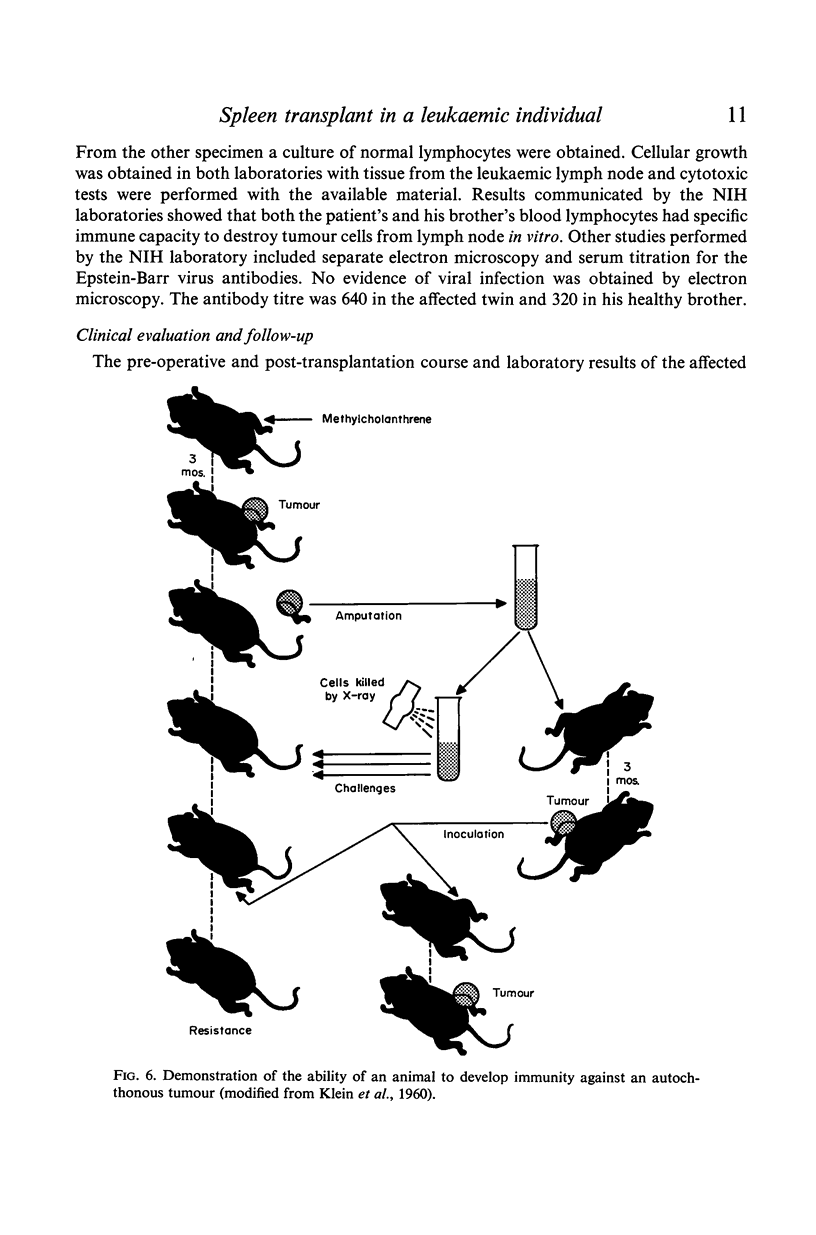
- KLEIN G., SJOGREN H. O., KLEIN E., HELLSTROM K. E. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960 Dec;20:1561–1572. [PubMed] [Google Scholar]
- Kafuko G. W., Baingana N., Knight E. M., Tibemanya J. Association of Burkitt's tumour and holoendemic malaria in West Nile District, Uganda: malaria as a possible aetiologic factor. East Afr Med J. 1969 Jul;46(7):414–436. [PubMed] [Google Scholar]
- Klein G., Oettgen H. F. Immunologic factors involved in the growth of primary tumors in human or animal hosts. Cancer Res. 1969 Sep;29(9):1741–1746. [PubMed] [Google Scholar]
- Lukes R. J., Butler J. J. The pathology and nomenclature of Hodgkin's disease. Cancer Res. 1966 Jun;26(6):1063–1083. [PubMed] [Google Scholar]
- MARCHIORO T. L., ROWLANDS D. T., Jr, RIFKIND D., WADDELL W. R., STARZL T. E., FUDENBERG H. SPLENIC HOMOTRANSPLANTATION. Ann N Y Acad Sci. 1964 Nov 30;120:626–651. doi: 10.1111/j.1749-6632.1964.tb34757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHE G., AMIEL J. L., SCHWARZENBERG L., CATTAN A., SCHNEIDER M. HAEMATOPOIETIC CHIMERA IN MAN AFTER ALLOGENIC (HOMOLOGOUS) BONE-MARROW TRANSPLANTATION. (CONTROL OF THE SECONDARY SYNDROME. SPECIFIC TOLERANCE DUE TO THE CHIMERISM). Br Med J. 1963 Dec 28;2(5373):1633–1635. doi: 10.1136/bmj.2.5373.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHISON N. A. Passive transfer of transplantation immunity. Proc R Soc Lond B Biol Sci. 1954 Feb 18;142(906):72–87. doi: 10.1098/rspb.1954.0007. [DOI] [PubMed] [Google Scholar]
- PAGE A. R., HANSEN A. E., GOOD R. A. Occurrence of leukemia and lymphoma in patients with agammaglobulinemia. Blood. 1963 Feb;21:197–206. [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- ROSENAU W., MOON H. D. Lysis of homologous cells by sensitized lymphocytes in tissue culture. J Natl Cancer Inst. 1961 Aug;27:471–483. [PubMed] [Google Scholar]
- Riesco A. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer. 1970 Jan;25(1):135–140. doi: 10.1002/1097-0142(197001)25:1<135::aid-cncr2820250120>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- WALKER R. I., FOWLER I. GRANULOCYTE INHIBITION OF HUMAN PERIPHERAL BLOOD LYMPHOCYTE GROWTH IN VITRO. Exp Cell Res. 1965 May;38:379–385. doi: 10.1016/0014-4827(65)90411-8. [DOI] [PubMed] [Google Scholar]
- WINN H. J. Immune mechanisms in homotransplantation. II. Quantitative assay of the immunologic activity of lymphoid cells stimulated by tumor homografts. J Immunol. 1961 Feb;86:228–239. [PubMed] [Google Scholar]
- WOODRUFF M. F., NOLAN B. PRELIMINARY OBSERVATIONS ON TREATMENT OF ADVANCED CANCER BY INJECTION OF ALLOGENEIC SPLEEN CELLS. Lancet. 1963 Aug 31;2(7305):426–429. doi: 10.1016/s0140-6736(63)92171-8. [DOI] [PubMed] [Google Scholar]
- Wilson R. E., Hager E. B., Hampers C. L., Corson J. M., Merrill J. P., Murray J. E. Immunologic rejection of human cancer transplanted with a renal allograft. N Engl J Med. 1968 Feb 29;278(9):479–483. doi: 10.1056/NEJM196802292780904. [DOI] [PubMed] [Google Scholar]