Abstract
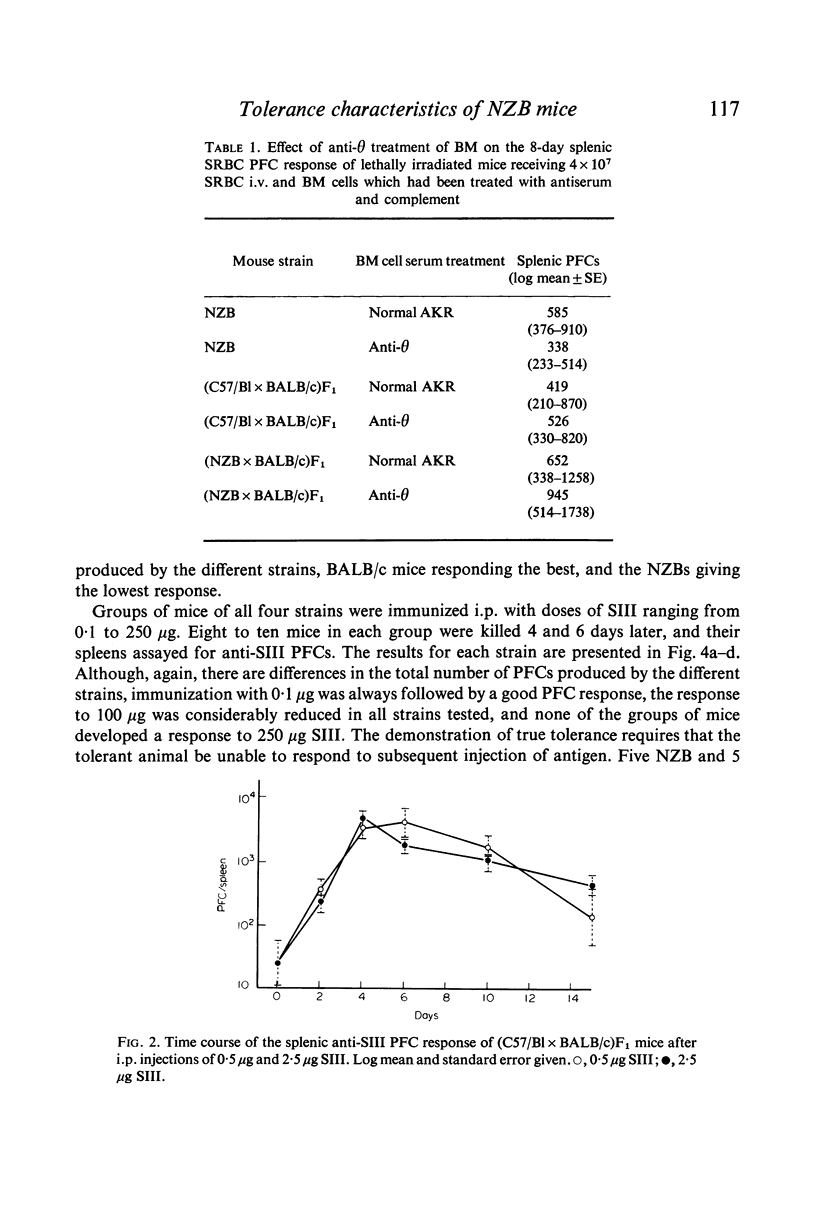
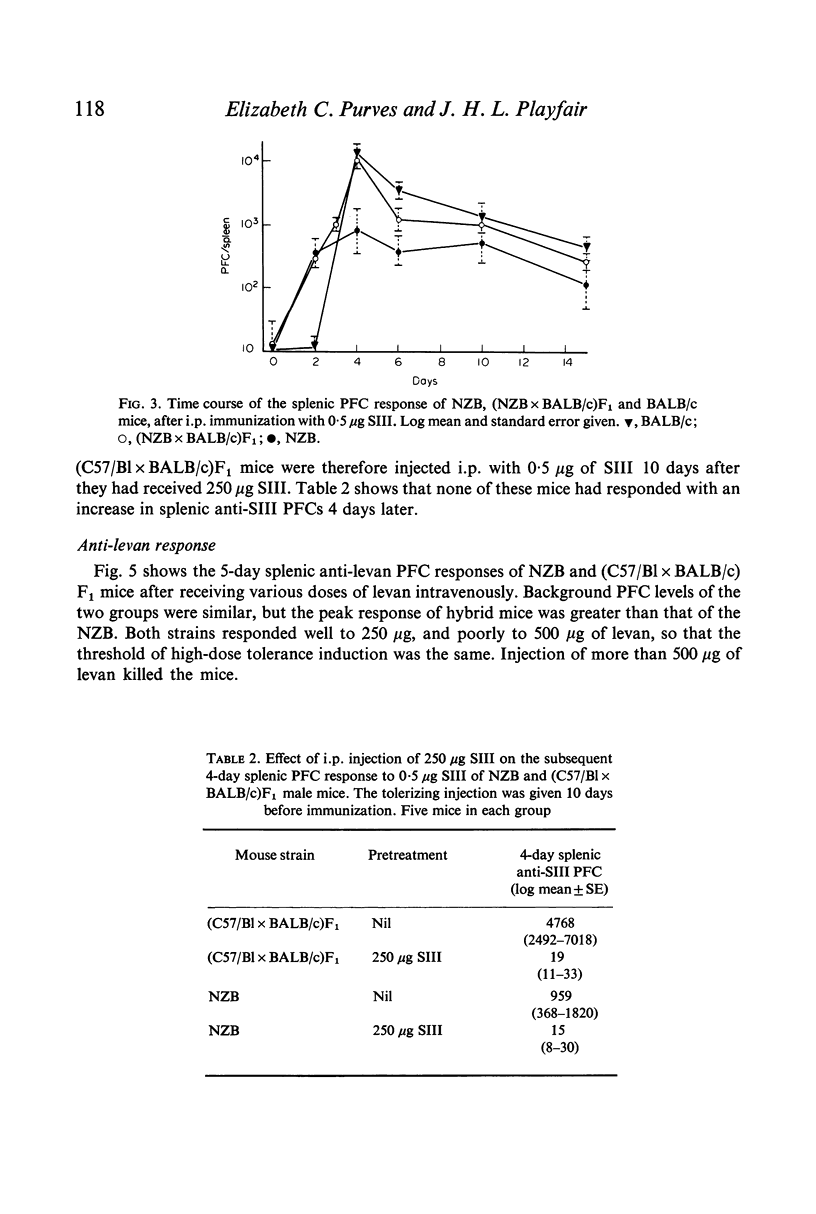
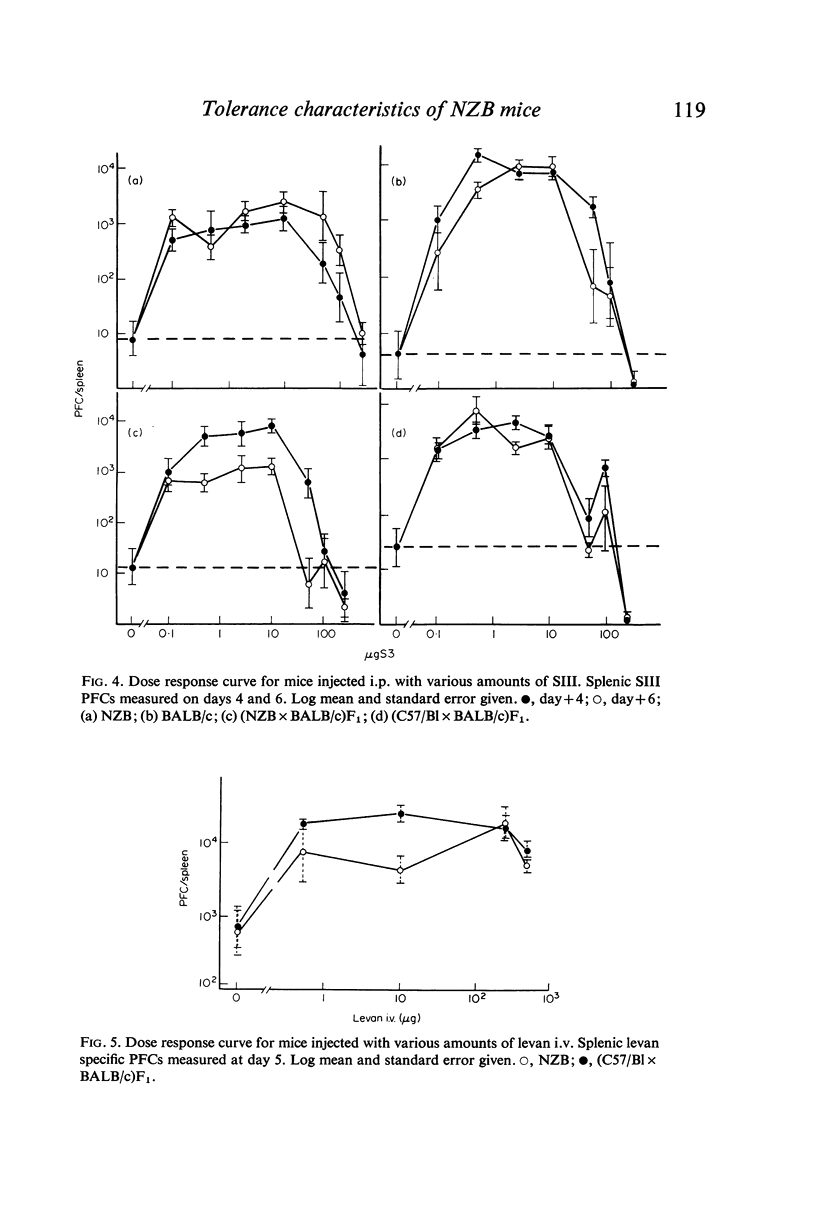
Splenic PFC dose-response curves were measured in normal mice and in mice of the autoimmune NZB strain for the thymus-independent antigens pneumococcal polysaccharide type 3 (SIII) and bacterial levan. There were differences between the strains in the maximal response achieved to each antigen, but the level at which high dose tolerance occurred was the same in all the strains.
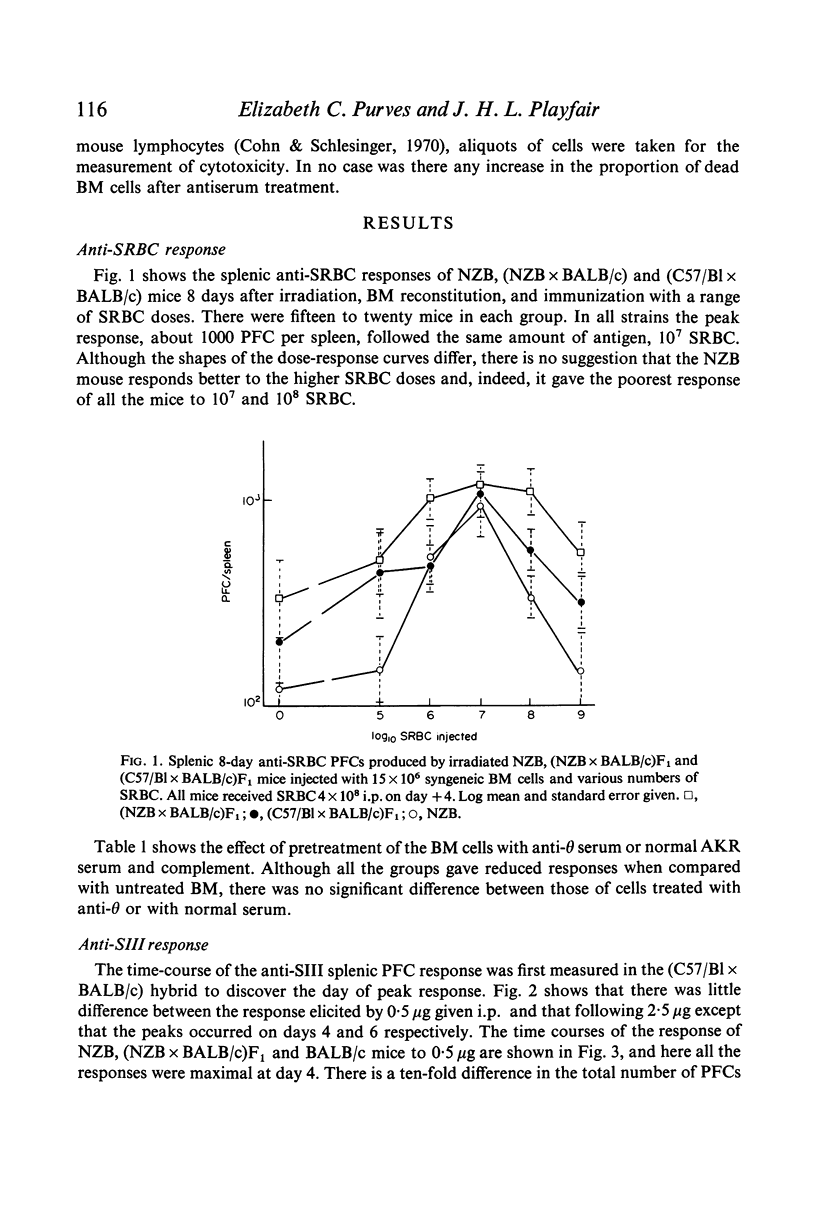
The splenic anti-sheep erythrocyte plaque-forming cell dose-response curve of lethally-irradiated, bone marrow restored NZB mice was compared with that of (C57/B1 × BALB/c)F1 hybrid and (NZB × BALB/c)F1 hybrid mice. Although the dose-response curves of the two hybrid stains were less sharp than that of the NZB, the peak response occurred at the same dose of SRBC (108, i.v.), and reached the same level, tailing off at higher doses.
These results indicate that the B cells of NZB mice display normal high-dose tolerance characteristics, at least to these three antigens.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J. Increased 7S antibody response to sheep erythrocytes in the 2-month-old NZB mouse. Clin Exp Immunol. 1969 Sep;5(3):251–263. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Lambert P. H., Dixon F. J. Comparison of the immune responsiveness of NZB and NZB X NZW F1 hybrid mice with that of other strains of mice. J Exp Med. 1969 Nov 1;130(5):1093–1105. doi: 10.1084/jem.130.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar A., Playfair J. H. The genetic basis of autoimmunity in NZB mice studied by progeny-testing. Clin Exp Immunol. 1971 Mar;8(3):479–490. [PMC free article] [PubMed] [Google Scholar]
- HELYER B. J., HOWIE J. B. Spontaneous auto-immune disease in NZB/BL mice. Br J Haematol. 1963 Apr;9:119–131. doi: 10.1111/j.1365-2141.1963.tb05450.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G. Cellular events in the induction and loss of tolerance to pneumococcal polysaccharides. Transplant Rev. 1972;8:50–75. doi: 10.1111/j.1600-065x.1972.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Westphal O. Synthesis and use of O-stearoyl polysaccharides in passive hemagglutination and hemolysis. Eur J Biochem. 1967 Mar;1(1):46–50. doi: 10.1007/978-3-662-25813-2_9. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Jacobs M. E., Gordon J. K., Talal N. Inability of the NZB-NZW F 1 thymus to transfer cyclophosphamide-induced tolerance to sheep erythrocytes. J Immunol. 1971 Aug;107(2):359–364. [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORINS L. C., HOLMES M. C. ANTINUCLEAR FACTOR IN MICE. J Immunol. 1964 Jul;93:148–154. [PubMed] [Google Scholar]
- Pantelouris E. M., Flisch P. A. Responses of athymic ("nude") mice to sheep red blood cells. Eur J Immunol. 1972 Jun;2(3):236–239. doi: 10.1002/eji.1830020308. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., Purves E. C. Antibody formation by bone marrow cells in irradiated mice. I. Thymus-dependent and thymus-independent responses to sheep erythrocytes. Immunology. 1971 Jul;21(1):113–121. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Purves E. C. Separate thymus dependent and thymus independent antibody forming cell precursors. Nat New Biol. 1971 Jun 2;231(22):149–151. doi: 10.1038/newbio231149a0. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Specific tolerance to sheep erythrocytes in mouse bone marrow cells. Nature. 1969 May 31;222(5196):882–883. doi: 10.1038/222882a0. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Strain differences in the immune responses of mice. 3. A raised tolerance threshold in NZB thymus cells. Immunology. 1971 Dec;21(6):1037–1043. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Staples P. J., Steinberg A. D., Talal N. Induction of immunologic tolerance in older New Zealand mice repopulated with young spleen, bone marrow, or thymus. J Exp Med. 1970 Jun 1;131(6):1223–1238. doi: 10.1084/jem.131.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples P. J., Talal N. Relative inability to induce tolerance in adult NZB and NZB-NZW F1 mice. J Exp Med. 1969 Jan 1;129(1):123–139. doi: 10.1084/jem.129.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyan M. L., Herzenberg L. A., Gibbs P. R. Lymphoid precursors: thymus independent antibody production. J Immunol. 1969 Dec;103(6):1283–1287. [PubMed] [Google Scholar]
- Weir D. M., McBride W., Naysmith J. D. Immune response to a soluble protein antigen in NZB mice. Nature. 1968 Sep 21;219(5160):1276–1277. doi: 10.1038/2191276a0. [DOI] [PubMed] [Google Scholar]


