Abstract
The frequencies of nonselected mutations among adaptive Lac+ revertants of Escherichia coli strains with and without the error-prone DNA polymerase IV (Pol IV) were compared. This frequency was more than sevenfold lower in the Pol IV-defective strain than in the wild-type strain. Thus, the mutations that occur during hypermutation are due to Pol IV.
When populations of microorganisms are exposed to nonlethal selections, mutations that relieve the selective pressure arise (7), a phenomenon called adaptive mutation (6). Although it originally seemed that only useful mutations appeared (7), it is now clear that selected mutations are accompanied by nonselected mutations, i.e., the process is not directed to useful genes (12).
Most research on adaptive mutation has focused on a strain of Escherichia coli, called FC40, that cannot utilize lactose (Lac−) but that readily reverts to lactose utilization (Lac+) when lactose is its only carbon source (6). The process that produces adaptive Lac+ mutations is not the same as that which produces Lac+ mutations during normal growth. Unlike growth-dependent mutations, almost all adaptive Lac+ mutations are dependent on recombination functions (6, 10, 24). While several different types of sequence changes revert the Lac− allele during growth, adaptive Lac+ mutations are almost all −1-bp frameshifts (17, 39). In addition, the high rate of adaptive mutation requires that the lac allele be on the F′ episome and that conjugal functions be expressed (18, 19, 20, 35). Recent evidence suggests that production of DNA nicks is the crucial conjugal function required for adaptive mutation (37).
One to five percent of the Lac+ colonies that appear during lactose selection consist not of Lac+ revertants but of cells that have amplified the unreverted Lac− allele (11, 16). This proportion increases if the experiment continues past the normal 5 days (25, 34) and is higher in Salmonella enterica serovar Typhimurium than in E. coli (1). One hypothesis to explain adaptive mutation in FC40 is that all Lac+ revertants start out as amplifiers; true Lac+ mutations arise within the amplified arrays and then these arrays disappear (14, 26). An alternative hypothesis is that amplification affects only a minority of the cells and that most Lac+ mutations are the result of error-prone DNA synthesis primed by recombination intermediates (reviewed in reference 13).
During selection, a subpopulation of cells experiences a state of greatly heightened mutation (12, 21, 38, 45). This phenomenon, called hypermutation, was predicted by Hall (23) and modeled by Ninio (32), Boe (3), and Cairns (5; see appendix in reference 38). During lactose selection, about 0.1% of the FC40 cells are hypermutators and have a mutation rate that is 200-fold higher than normal. Because the hypermutator population is small, only about 10% of the adaptive Lac+ mutations arise in hypermutators; the rest of the Lac+ mutations arise in normal cells via the recombination-dependent mechanisms discussed above (38). But because hypermutators accumulate multiple mutations, the population of Lac+ revertants is enriched for second, nonselected mutations. The hypermutator state must be transient because, when assayed, cells with multiple mutations have a normal mutation rate (21, 38, 45).
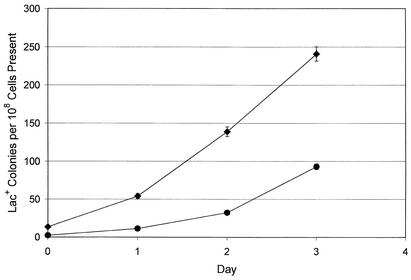
Recently a family of error-prone, DNA-dependent DNA polymerases has been discovered (reviewed in reference 22). These polymerases are found in all three domains of life, and their cellular functions are the subject of much current research. In E. coli, two of these, DNA polymerases IV and V (Pol IV and V), are induced as part of the SOS response to DNA damage. Pol V and its homologues are responsible for replicating past DNA lesions, but Pol IV and its homologues have a rather restricted ability to perform this function (28, 31, 43). When replicating undamaged DNA, both Pol IV and Pol V are highly error prone (43), and overproduction of Pol IV is a powerful mutator (28, 47). But the loss of either polymerase has only modest effects on normal, growth-dependent mutation rates (2, 40, 42). Pol V plays no role in adaptive mutation in FC40 (15), although it is implicated in other types of mutation in starving cells (44). In contrast, if Pol IV is defective, the rate at which adaptive Lac+ revertants accumulate in FC40 falls three- to fivefold (13, 30) (see Fig. 1). Thus, most Lac+ adaptive mutations are due to Pol IV; in its absence, the remainder are due to another polymerase, probably Pol III (13, 30).
FIG. 1.
Accumulation of Lac+ mutations in Lac− cells incubated on lactose minimal medium. Diamonds, FC1383 (dinB+); circles, FC1394 (dinB mutant). Because it takes 2 days for a Lac+ revertant to produce a visible colony, the curves have been displaced on the graph to a position corresponding to 2 days earlier on the x axis. Data are expressed as the Lac+ colonies appearing each day divided by the number of Lac− cells present on the plates 2 days earlier, cumulated. Each point is the mean of results for 20 plates for Lac+ and for 10 plates for Lac− cells ± 95% confidence intervals (some of which are smaller than the symbols). Frequencies and confidence intervals were calculated according to the formulae for ratios described previously (36).
It has been hypothesized that Pol IV is also responsible for the multiple mutations that occur during transient hypermutation (13, 21, 26). We tested this hypothesis by comparing the frequencies of second, nonselected mutations among Lac+ adaptive revertants of a wild-type and a Pol IV-defective strain. If Pol IV is responsible for the mutations that occur during the hypermutable state, then the frequency of Lac+ revertants carrying second, nonselected mutations should be lower in a strain missing Pol IV than in the wild-type strain. If Pol IV is not responsible for these mutations, then the opposite should be true—the Lac+ revertants of a Pol IV− strain should be enhanced for hypermutators and thus should carry second mutations at a frequency higher than that of the Lac+ revertants of the wild-type strain.
Pol IV is encoded by the dinB gene (sometimes called dinP) (46), which is the first gene of an operon (27). To avoid any effects due to the other genes in the operon, we used an allele of dinB, ΔdinB::Zeo, that is not polar (4). FC40 carries two copies of dinB, one on the chromosome and one on the episome, and both of these were replaced by ΔdinB::Zeo. The absence of Pol IV was confirmed by immunoblot analysis using polyclonal antibody to Pol IV (obtained from H. Omori). FC40 and its doubly ΔdinB::Zeo derivative were made Xyl− (unable to metabolize xylose) and Camr (resistant to chloramphenicol), yielding FC1383 and FC1394, respectively. Thus, the doubly ΔdinB::Zeo strain is FC1394 and its isogenic doubly dinB+ control is FC1383, which is equivalent to FC40. As previously described (38), we used loss of motility (Mot−) as a second phenotype. Since mutations in about 50 genes affect motility (29), this one phenotype screens for mutations in about 1% of the genome. Both Lac+ and Lac− clones, obtained as previously described (38), were isolated during the course of the experiment whose results are shown in Fig. 1. These clones were tested for loss of motility. Mot− clones were further tested for normal mutation rates (39).
Table 1 summarizes the frequency of Lac+ and Lac− clones that were also Mot−. Of the Lac+ revertants of the dinB+ strain, FC1383, 0.6% were Mot−, whereas less than 0.07% of the nonreverted Lac−cells collected over the same period were Mot−. Thus, Mot− defects were enriched more than sevenfold in the Lac+ population. In contrast, in the dinB mutant strain, FC1394, less than 0.08% of the Lac+ clones were Mot−, a frequency that was not different from that among FC1394 Lac− cells (Table 1). The >7-fold difference in the frequencies of Mot− defects among Lac+ revertants of the dinB+ strain and the dinB mutant strain is significant (χ2 ∼ 5.5; P ∼ 0.02). Thus, Pol IV is required for the mutations that appear during transient hypermutation.
TABLE 1.
Frequencies of clones with Mot− defectsa
| Clone | No. of Lac+ clones screened | No. of Lac+ Mot− clones observed | No. of Lac+ Mot− clones/total no. of Lac+ clones (%) | No. of Lac− clones screened | No. of Lac− Mot− clones observed | No. of Lac− Mot− clones/total no. of Lac− clones (%) | Ratio of Lac+ Mot− clones/Lac− Mot− clones |
|---|---|---|---|---|---|---|---|
| FC1383 (dinB+) | 1,536 | 9 | 0.58 | 1,428 | 0 | <0.07 | >8b |
| FC1394 (dinB mutant) | 1,217 | 0 | <0.08 (>7d) | 1,248 | 0 | <0.08 (NAe) | NAc (>8) |
The Yates correction for small numbers was used (48). Values in parentheses are ratios of the value for FC1383 to the value for FC1394. NA, not applicable.
The difference in the frequencies of Mot− mutant among the Lac+ and Lac− clones of FC1383 is significant (χ2 = 6.5, P = 0.01).
To make this ratio significant at the 5% level, 6 of the 1,217 Lac+ clones screened would have had to be Mot−.
The difference in the frequencies of Mot− mutants among the Lac+ clones of FC1383 and FC1394 is significant (χ2 = 5.5, P = 0.02).
To make this ratio significant at the 5% level, 7 of the 1,428 Lac− clones of FC1383 screened would have had to be Mot−.
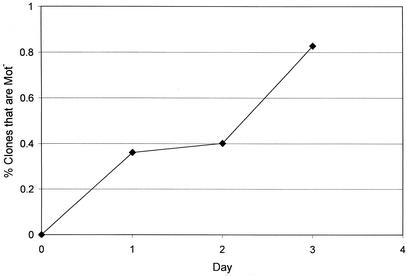
As was previously found (38), the proportion of Lac+ clones that were Mot− in the dinB+ strain increased over time (Fig. 2). This result suggests that the majority of hypermutators do not die or pass out of the hypermutator state during the course of the experiment (21, 38).
FIG. 2.
Appearance of Lac+ clones that were also Mot− during incubation on lactose minimal medium. Because it takes 2 days for a Lac+ revertant to produce a visible colony, the curve has been displaced on the graph to a position corresponding to 2 days earlier on the x axis. Data are expressed as the daily percentages of the Lac+ clones of the dinB+ strain, FC1383, that were composed of Lac+ Mot− cells.
The results presented here include only pure clones; we excluded Lac+ or Lac− clones that contained both mutant and nonmutant cells. As was observed previously (38), some of the Lac+ clones of the Pol IV-proficient strain contained such mixtures, which could mean that the state of hypermutation persisted for several cell divisions after Lac+ revertants began to grow.
Our results show that Lac+ revertants of a strain that is missing Pol IV do not accumulate nonselected mutations among their Lac+ revertants at the same frequency that a Pol IV-proficient strain does. Because the Lac+ cells that carry second mutations have in all probability passed through a state of hypermutation, this result is evidence that the mutations that occur during this state are due to the errors made by Pol IV. Slechta et al. (41) recently reported that a strain with a noninducible lexA allele likewise does not accumulate nonselected mutations among its Lac+ revertants at the frequency that a lexA+ strain does. Since LexA is the repressor of dinB (8, 33), these results, in combination with ours, suggest that the hypermutable state requires the induction of Pol IV.
While our results show that Pol IV is necessary for hypermutation, they do not show that it is sufficient. Methyl-directed mismatch repair (MMR), which corrects replication errors, is active in lactose-starved cells (14), and hypermutators are not heritably defective in MMR (21, 38, 45). Loss of MMR raises the frequency of Mot− mutants in Lac− cells, but it does not affect the frequency of Mot− mutants in Lac+ revertants (38). These results strongly suggest that the hypermutators are transiently defective in MMR. One possibility is that MMR is saturated by the errors produced by Pol IV. But, levels of two MMR enzymes decline in stationary-phase cells (9). Thus, stationary-phase cells may be particularly susceptible to becoming temporarily MMR− because of occasional defects in or loss of these proteins, as was predicted by Ninio (3, 32). We suggest that the extraordinary mutation rate of the hypermutators is due to two factors—the induction of Pol IV and the transient loss of MMR. The rest of the population (which accounts for the majority of the Lac+ mutations) may have various mutation rates, depending on the levels and activities of Pol IV and MMR.
Hypermutators are a minority (about 0.1% of the population), and they produce only a minority of the adaptive mutations (about 10% of the Lac+ mutants), but they account for nearly all of the cells bearing two or more mutations (38). Thus, hypermutators may become important when more than one mutation is required to meet selective conditions (5, 32, 38). The cellular role of Pol IV in E. coli and other organisms is under intense investigation. The fact that Pol IV in FC40 is responsible for most of the adaptive mutations and for all the multiple mutations suggests that Pol IV is active in stationary cells. Under adverse conditions, cells may employ this error-prone polymerase to produce variants that allow their descendents to survive.
Acknowledgments
We thank Jeffrey H. Miller, Roger Woodgate, Antonio Fernández de Henestrosa, and Haruo Ohmori for strains and reagents. We are grateful to John Cairns for reading a previous version of this manuscript.
This work was supported by NSF grant MCB-9996308 and USPHS grants NIH-NIGMS GM54084 and G651575.
REFERENCES
- 1.Andersson, D. I., E. S. Slechta, and J. R. Roth. 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282:1133-1135. [DOI] [PubMed] [Google Scholar]
- 2.Bhamre, S., B. B. Gadea, C. A. Koyama, S. J. White, and R. G. Fowler. 2001. An aerobic recA-, umuC-dependent pathway of spontaneous base-pair substitution mutagenesis in Escherichia coli. Mutat. Res. 473:229-247. [DOI] [PubMed] [Google Scholar]
- 3.Boe, L. 1992. Translational errors as the cause of mutations in Escherichia coli. Mol. Gen. Genet. 231:469-471. [DOI] [PubMed] [Google Scholar]
- 4.Borden, A., P. I. O'Grady, D. Vandewiele, A. R. Fernández de Henestrosa, C. W. Lawrence, and R. Woodgate. 2002. Escherichia coli DNA polymerase III can replicate efficiently past a T-T cis-syn cyclobutane dimer if DNA polymerase V and the 3′ to 5′ exonuclease proofreading function encoded by dnaQ are inactivated. J. Bacteriol. 184:2674-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns, J. 1998. Mutation and cancer: the antecedents to our studies of adaptive mutation. Genetics 148:1433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns, J., J. Overbaugh, and S. Miller. 1988. The origin of mutants. Nature 335:142-145. [DOI] [PubMed] [Google Scholar]
- 8.Courcelle, J., A. Khodursky, B. Peter, P. O. Brown, and P. C. Hanawalt. 2001. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158:41-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, G., H.-C. T. Tsui, and M. E. Winkler. 1996. Depletion of the cellular amounts of the MutS and MutH methyl-directed mismatch repair proteins in stationary-phase Escherichia coli K-12 cells. J. Bacteriol. 178:2388-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, P. L. 1993. Adaptive mutation: the uses of adversity. Annu. Rev. Microbiol. 47:467-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster, P. L. 1994. Population dynamics of a Lac− strain of Escherichia coli during selection for lactose utilization. Genetics 138:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster, P. L. 1997. Nonadaptive mutations occur on the F′ episome during adaptive mutation conditions in Escherichia coli. J. Bacteriol. 179:1550-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster, P. L. 2000. Adaptive mutation in Escherichia coli. Cold Spring Harbor Symp. Quant. Biol. 65:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster, P. L., and J. Cairns. 1992. Mechanisms of directed mutation. Genetics 131:783-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster, P. L., and J. Cairns. 1998. Adaptive mutation of a lacZ amber allele. Genetics 150:1329-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster, P. L., G. Gudmundsson, J. M. Trimarchi, H. Cai, and M. F. Goodman. 1995. Proofreading-defective DNA polymerase II increases adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:7951-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster, P. L., and J. M. Trimarchi. 1994. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science 265:407-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, P. L., and J. M. Trimarchi. 1995. Adaptive reversion of an episomal frameshift mutation in Escherichia coli requires conjugal functions but not actual conjugation. Proc. Natl. Acad. Sci. USA 92:5487-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster, P. L., and J. M. Trimarchi. 1995. Conjugation is not required for adaptive reversion of an episomal frameshift mutation in Escherichia coli. J. Bacteriol. 177:6670-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galitski, T., and J. R. Roth. 1995. Evidence that F plasmid transfer replication underlies apparent adaptive mutation. Science 268:421-423. [DOI] [PubMed] [Google Scholar]
- 21.Godoy, V. G., F. S. Gizatullin, and M. S. Fox. 2000. Some features of the mutability of bacteria during nonlethal selection. Genetics 154:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman, M. F., and B. Tippin. 2000. The expanding polymerase universe. Nat. Rev. Mol. Cell Biol. 1:101-109. [DOI] [PubMed] [Google Scholar]
- 23.Hall, B. G. 1990. Spontaneous point mutations that occur more often when they are advantageous than when they are neutral. Genetics 126:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris, R. S., S. Longerich, and S. M. Rosenberg. 1994. Recombination in adaptive mutation. Science 264:258-260. [DOI] [PubMed] [Google Scholar]
- 25.Hastings, P. J., H. J. Bull, and S. M. Rosenberg. 2000. Adaptive amplification: an inducible chromosomal instability mechanism. Cell 103:723-731. [DOI] [PubMed] [Google Scholar]
- 26.Hendrickson, H., E. S. Slechta, U. Bergthorsson, D. I. Andersson, and J. R. Roth. 2002. Amplification-mutagenesis: evidence that “directed” adaptive mutation and general hypermutability result from growth with a selected gene amplification. Proc. Natl. Acad. Sci. USA 99:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, S.-R., G. Maenhaut-Michel, M. Yamada, Y. Yamamoto, K. Matsui, T. Sofuni, T. Nohmi, and H. Ohmori. 1997. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc. Natl. Acad. Sci. USA 94:13792-13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, S. R., K. Matsui, M. Yamada, P. Gruz, and T. Nohmi. 2001. Roles of chromosomal and episomal dinB genes encoding DNA pol IV in targeted and untargeted mutagenesis in Escherichia coli. Mol. Genet. Gen. 266:207-215. [DOI] [PubMed] [Google Scholar]
- 29.MacNab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 30.McKenzie, G. J., P. L. Lee, M.-J. Lombardo, P. J. Hastings, and S. M. Rosenberg. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571-579. [DOI] [PubMed] [Google Scholar]
- 31.Napolitano, R., R. Janel-Bintz, J. Wagner, and R. P. P. Fuchs. 2000. All three SOS-inducible DNA polymerases (Pol II, Pol IV and Pol V) are involved in induced mutagenesis. EMBO J. 19:6259-6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ninio, J. 1991. Transient mutators: a semiquantitative analysis of the influence of translation and transcription errors on mutation rates. Genetics 129:957-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohmori, H., E. Hatada, Y. Qiao, M. Tsuji, and R. Fukuda. 1995. dinP, a new gene in Escherichia coli, whose product shows similarities to UmuC and its homologues. Mutat. Res. 347:1-7. [DOI] [PubMed] [Google Scholar]
- 34.Powell, S. C., and R. M. Wartell. 2001. Different characteristics distinguish early versus late arising adaptive mutations in Escherichia coli FC40. Mutat. Res. 473:219-228. [DOI] [PubMed] [Google Scholar]
- 35.Radicella, J. P., P. U. Park, and M. S. Fox. 1995. Adaptive mutation in Escherichia coli: a role for conjugation. Science 268:418-420. [DOI] [PubMed] [Google Scholar]
- 36.Rice, J. A. 1995. Mathematical statistics and data analysis, p. 1-602. Wadsworth Publishing Company, Belmont, Calif.
- 37.Rodriguez, C., J. Tompkin, J. Hazel, and P. L. Foster. 2002. Induction of a DNA nickase in the presence of its target site stimulates adaptive mutation in Escherichia coli. J. Bacteriol. 184:5599-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosche, W. A., P. L. Foster, and J. Cairns. 1999. The role of transient hypermutators in adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA 96:6862-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg, S. M., S. Longerich, P. Gee, and R. S. Harris. 1994. Adaptive mutation by deletions in small mononucleotide repeats. Science 265:405-407. [DOI] [PubMed] [Google Scholar]
- 40.Sargentini, N. J., and K. C. Smith. 1981. Much of spontaneous mutagenesis in Escherichia coli is due to error-prone DNA repair: implications for spontaneous carcinogenesis. Carcinogenesis 2:863-872. [DOI] [PubMed] [Google Scholar]
- 41.Slechta, E. S., J. Liu, D. I. Andersson, and J. R. Roth. 2002. Evidence that selected amplification of a bacterial lac frameshift allele stimulates Lac(+) reversion (adaptive mutation) with or without general hypermutability. Genetics 161:945-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strauss, B. S., R. Roberts, L. Francis, and P. Pouryazdanparast. 2000. Role of the dinB gene product in spontaneous mutation in Escherichia coli with an impaired replicative polymerase. J. Bacteriol. 182:6742-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang, M., P. Pham, X. Shen, J.-S. Taylor, M. O'Donnell, R. Woodgate, and M. F. Goodman. 2000. Roles of E. coli DNA polymerase IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature 404:1014-1018. [DOI] [PubMed] [Google Scholar]
- 44.Timms, A. R., W. Muriel, and B. A. Bridges. 1999. A UmuD,C-dependent pathway for spontaneous G:C to C:G transversions in stationary phase Escherichia coli mut Y. Mutat. Res. 435:77-80. [DOI] [PubMed] [Google Scholar]
- 45.Torkelson, J., R. S. Harris, M.-J. Lombardo, J. Nagendran, C. Thulin, and S. M. Rosenberg. 1997. Genome-wide hypermutation in a subpopulation of stationary-phase cells underlies recombination-dependent adaptive mutation. EMBO J. 16:3303-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner, J., Gruz, P., S. R. Kim, Yamada, M., K. Matsui, R. P. Fuchs, and T. Nohmi. 1999. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol. Cell 4:281-286. [DOI] [PubMed] [Google Scholar]
- 47.Wagner, J., and T. Nohmi. 2000. Escherichia coli DNA polymerase IV mutator activity: genetic requirements and mutational specificity. J. Bacteriol. 182:4587-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zar, J. H. 1984. Biostatistical analysis, p. 1-718. Prentice Hall, Englewood Cliffs, N.J.