Abstract
Rabbits were immunized with peripheral cells, thoracic duct cells and various lymphoblast cell lines.
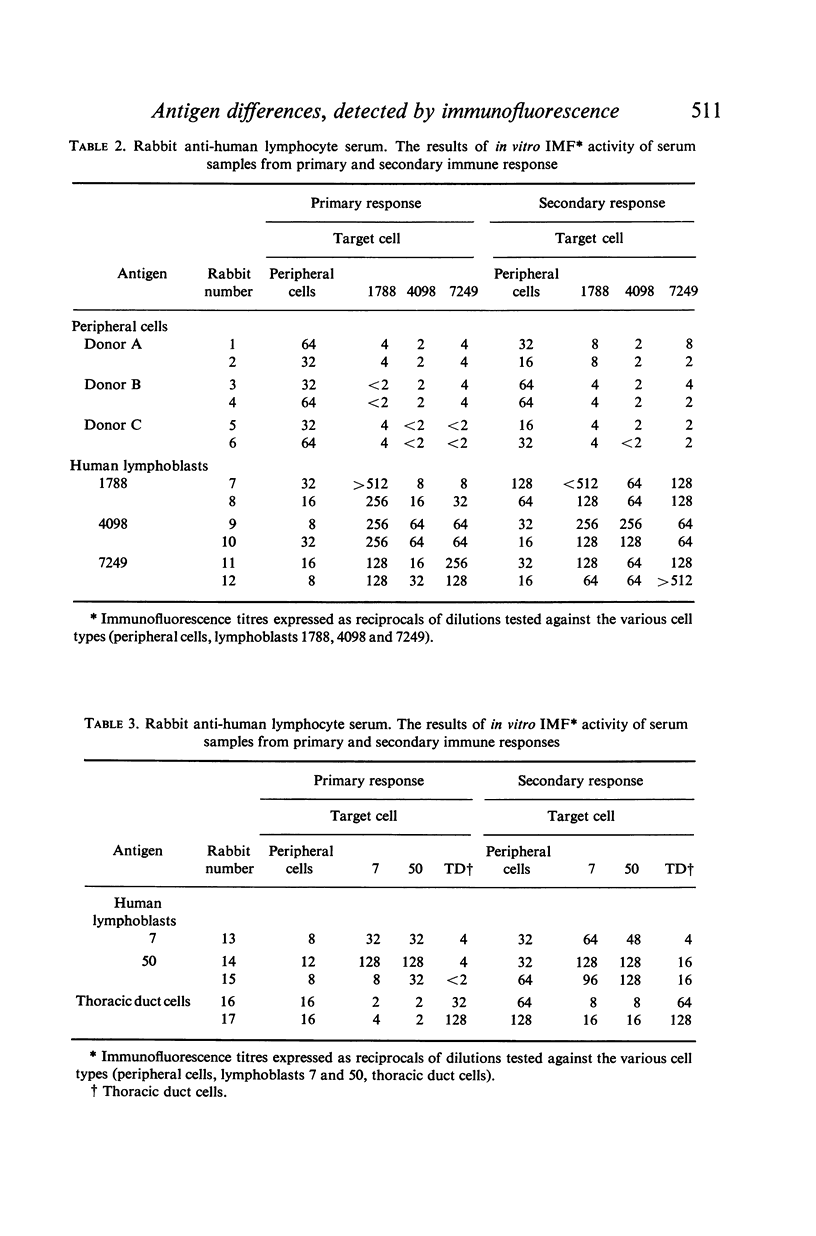
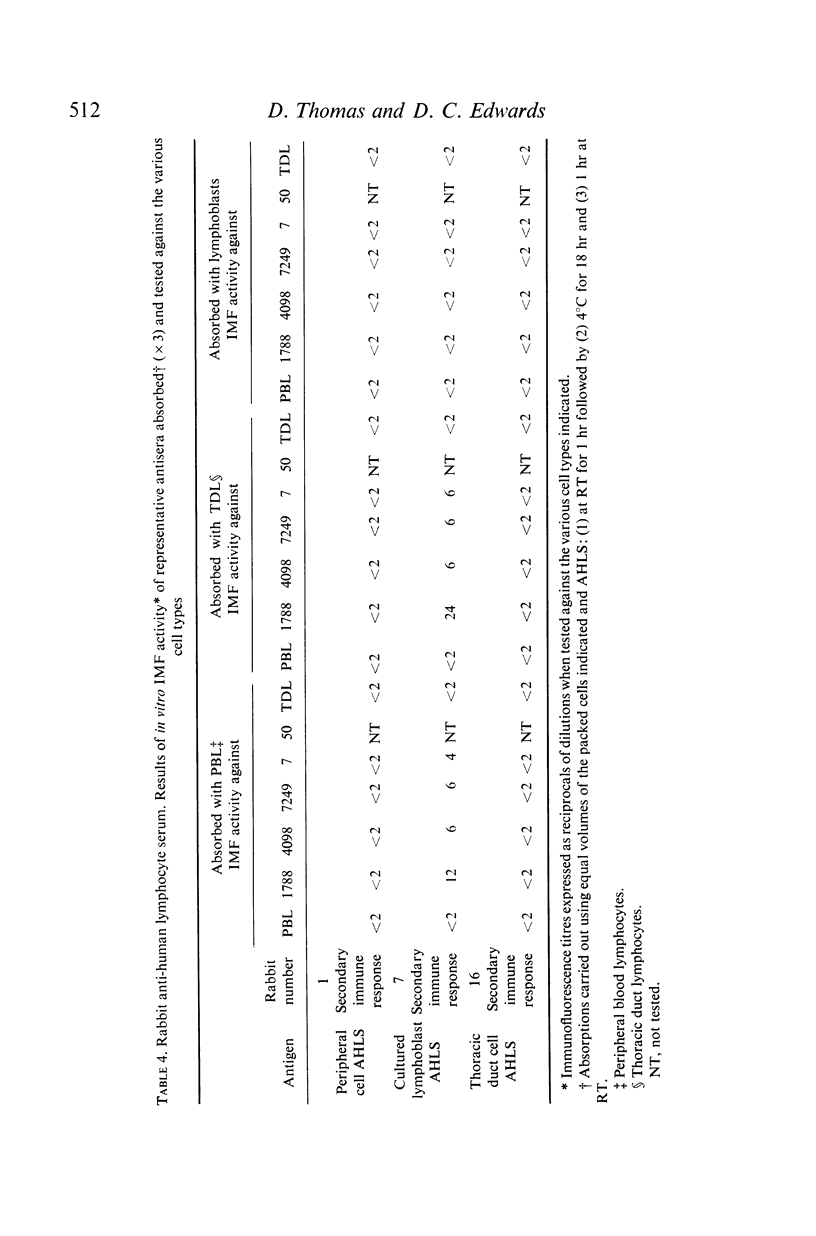
Bleedings were taken approximately 4 weeks after each injection corresponding to the primary and secondary immune response. The immunofluorescence (IMF) titre of each serum was estimated using the specific antigen cell and the cells used to raise the other sera. In general the titres were higher when the specific antigen cells were used. In absorption experiments it was found that anti-peripheral cell sera and anti-thoracic duct cell sera were readily absorbed by the specific or non-specific cells, but the anti-lymphoblast sera, although readily absorbed by the specific or other lymphoblast cells, could not be completely absorbed with peripheral and thoracic duct cells.
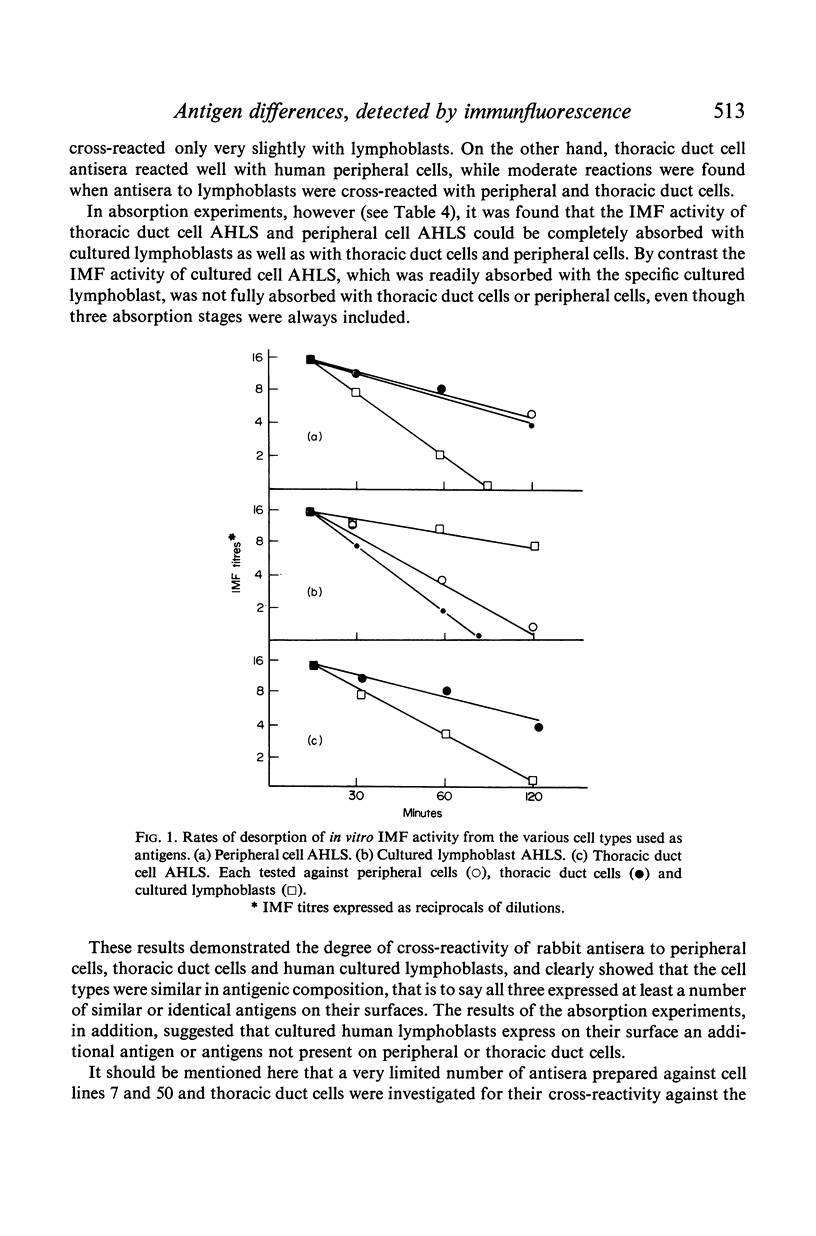
The IMF test was, by minor modifications, adapted to allow preliminary studies to be made of the desorption of anti-human lymphocyte globulin from the lymphocytes and it is concluded from these investigations that, while peripheral cells, thoracic duct cells and cultured human lymphoblasts may contain similar and/or identical antigens, they also express similar but not identical antigens, while cultured lymphoblasts in addition possess antigens absent from peripheral and thoracic duct lymphocytes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. W. Tumour specific antigens associated with chemically induced tumours. Rev Eur Etud Clin Biol. 1970 Jun-Jul;15(6):593–598. [PubMed] [Google Scholar]
- Dumonde D. C. Tissue-specific antigens. Adv Immunol. 1966;5:245–412. doi: 10.1016/s0065-2776(08)60275-8. [DOI] [PubMed] [Google Scholar]
- HAMBURGER R. N., PIOUS D. A., MILLS S. E. ANTIGENIC SPECIFICITIES ACQUIRED FROM THE GROWTH MEDIUM BY CELLS IN TISSUE CULTURE. Immunology. 1963 Sep;6:439–449. [PMC free article] [PubMed] [Google Scholar]
- Lance E. M., Ford P. J., Ruszkiewicz M. The use of subcellular fractions to raise anti-lymphocytic serum. Immunology. 1968 Oct;15(4):571–580. [PMC free article] [PubMed] [Google Scholar]
- Lance E. M. The selective action of antilymphocyte serum on recirculating lymphocytes: a review of the evidence and alternatives. Clin Exp Immunol. 1970 Jun;6(6):789–802. [PMC free article] [PubMed] [Google Scholar]
- Najarian J. S., Simmons R. L., Gewurz H., Moberg A., Merkel F., Moore G. E. Anti-serum to cultured human lymphoblasts: preparation, purification and immunosuppressive properties in man. Ann Surg. 1969 Oct;170(4):617–632. doi: 10.1097/00000658-196910000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perper R. J., Yu T. Z., Kooistra J. B. The in vitro specificity of antilymphocyte sera produced by either cultured or noncultured lymphocytes. Int Arch Allergy Appl Immunol. 1970;37(4):418–431. doi: 10.1159/000230804. [DOI] [PubMed] [Google Scholar]
- Prehn R. T. Cancer antigens in tumors induced by chemicals. Fed Proc. 1965 Sep-Oct;24(5):1018–1022. [PubMed] [Google Scholar]
- Steel C. M., Hardy D. A., Ling N. R., Dick H. M., Mackintosh P., Crichton W. B. The interaction of normal lymphocytes nd cells from lymphoid cell lines. 3. Studies on activation in an autochthonous system. Immunology. 1973 Jan;24(1):177–189. [PMC free article] [PubMed] [Google Scholar]
- Symes M. O., Riddell A. G. The viability of human spleen cells after cooling in vitro. Br J Surg. 1966 Sep;53(9):794–798. doi: 10.1002/bjs.1800530916. [DOI] [PubMed] [Google Scholar]
- Thomas D., Mosedale B., Zola H. The use of the indirect fluorescent antibody technique in assessing the activity of antilymphocytic sera and antilymphocytic globulins. Clin Exp Immunol. 1971 Jun;8(6):987–991. [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Woodrooffe J. G., Mosedale B., Ward P. D., Zola H., Edwards D. C., Balner H. Antibody titres of antilymphocytic sera and globulins determined by immunofluorescence and their correlation with immunosuppressive activity. Clin Exp Immunol. 1972 Aug;11(4):569–578. [PMC free article] [PubMed] [Google Scholar]
- Thomson A. E., Bull J. M., Robinson M. A. A procedure for separating viable lymphocytes from human blood and some studies on their susceptibility to hypotonic shocks. Br J Haematol. 1966 Jul;12(4):433–446. doi: 10.1111/j.1365-2141.1966.tb05652.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Grimes A. J. Defibrination of normal human blood for in vitro cell studies. Nature. 1968 Apr 13;218(5137):178–180. doi: 10.1038/218178a0. [DOI] [PubMed] [Google Scholar]
- Woiwod A. J., Courtenay J. S., Edwards D. C., Epps H. B., Knight R. R., Mosedale B., Phillips A. W., Rahr L., Thomas D., Woodrooffe J. G. The preparation and properties of horse antihuman lymphocyte serum and globulin. Transplantation. 1970 Aug;10(2):173–186. doi: 10.1097/00007890-197008000-00004. [DOI] [PubMed] [Google Scholar]
- Zola H., Mosedale B., Thomas D. The preparation and properties of antisera to subcellular fractions from lymphocytes. Transplantation. 1970 Mar;9(3):259–272. doi: 10.1097/00007890-197003000-00010. [DOI] [PubMed] [Google Scholar]
- Zola H., Thomas D., Mosedale B., Courtenay J. S. Immunosuppressive antisera to soluble extracts from mouse and human lymphocytes. Transplantation. 1971 Jul;12(1):49–53. doi: 10.1097/00007890-197107000-00008. [DOI] [PubMed] [Google Scholar]


