Abstract
The ability of mouse lymphoid cells to show non-specific cytotoxicity to 51Cr-labelled mastocytoma cells in vitro was assessed by the release of 51Cr.
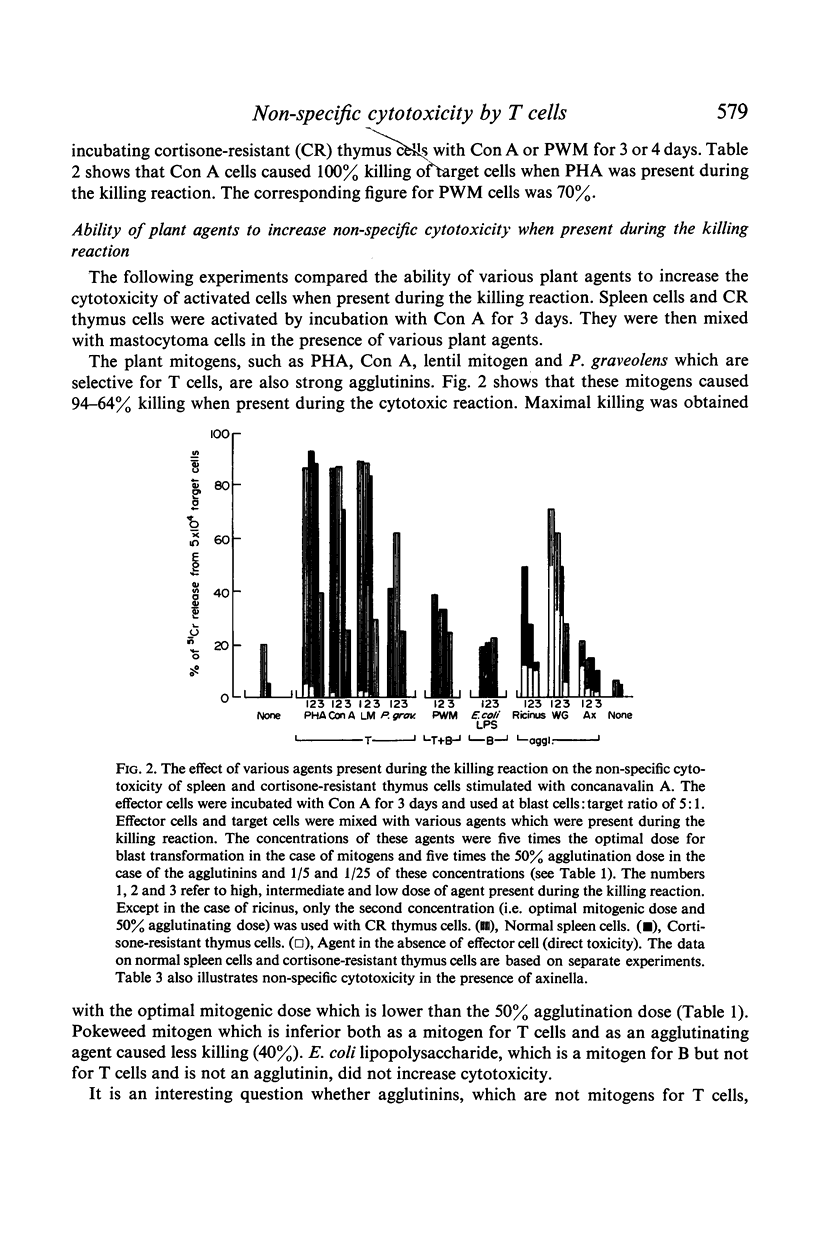
Little cytotoxicity was shown by normal lymphoid cells. However, incubation for several days with mitogens for T cells, such as PHA, Con A, P. graveolens and lentil mitogen, activated spleen cells for cytotoxic killing providing PHA was present during the killing reaction. Pokeweed mitogen, which is an inferior mitogen for T cells, was less active. Three agglutinins, which did not transform T cells, were ineffective. Con A and PWM also activated cortisone-resistant thymus cells.
Activated T cells only killed target cells when a plant agent was present during the killing reaction. The T cell mitogens, which are also strong agglutinins, were effective. PWM was less effective and agents which were not T cell mitogens, including leucoagglutinins, had a variable and usually small effect.
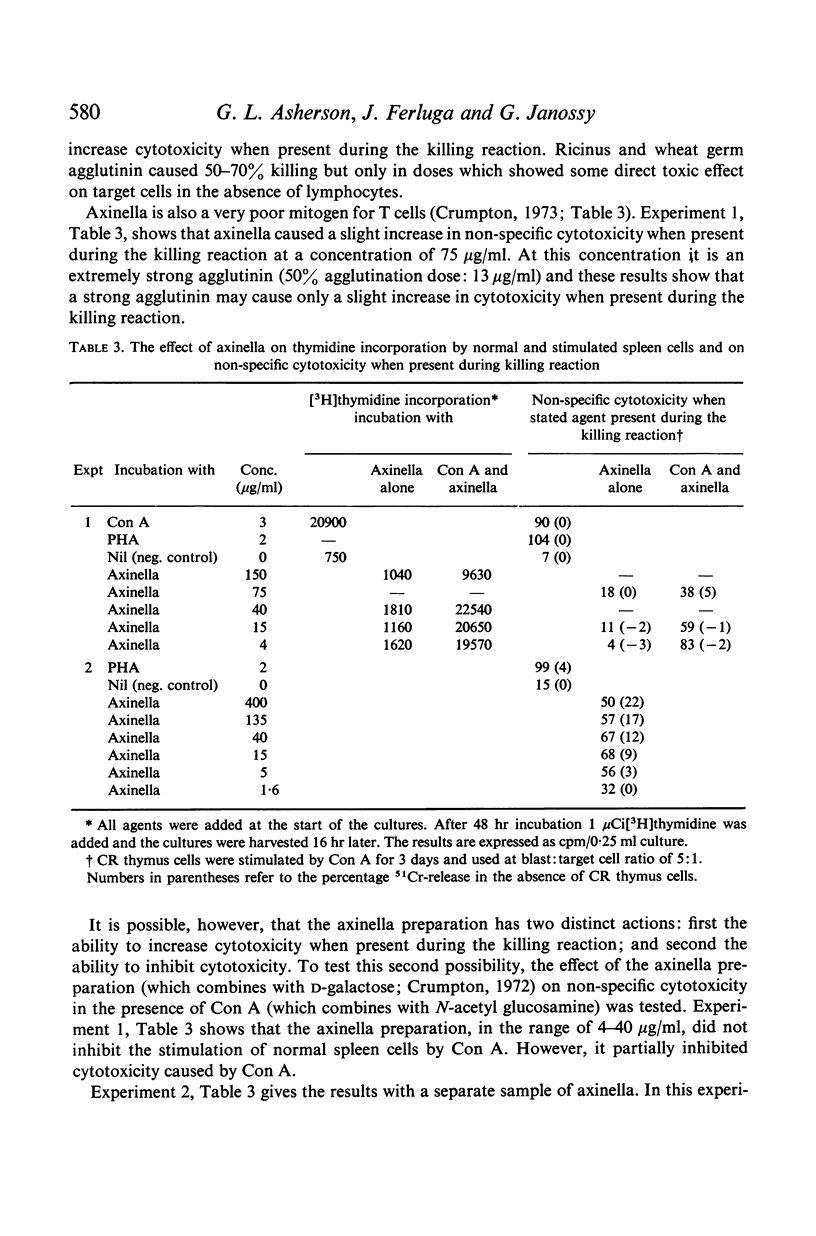
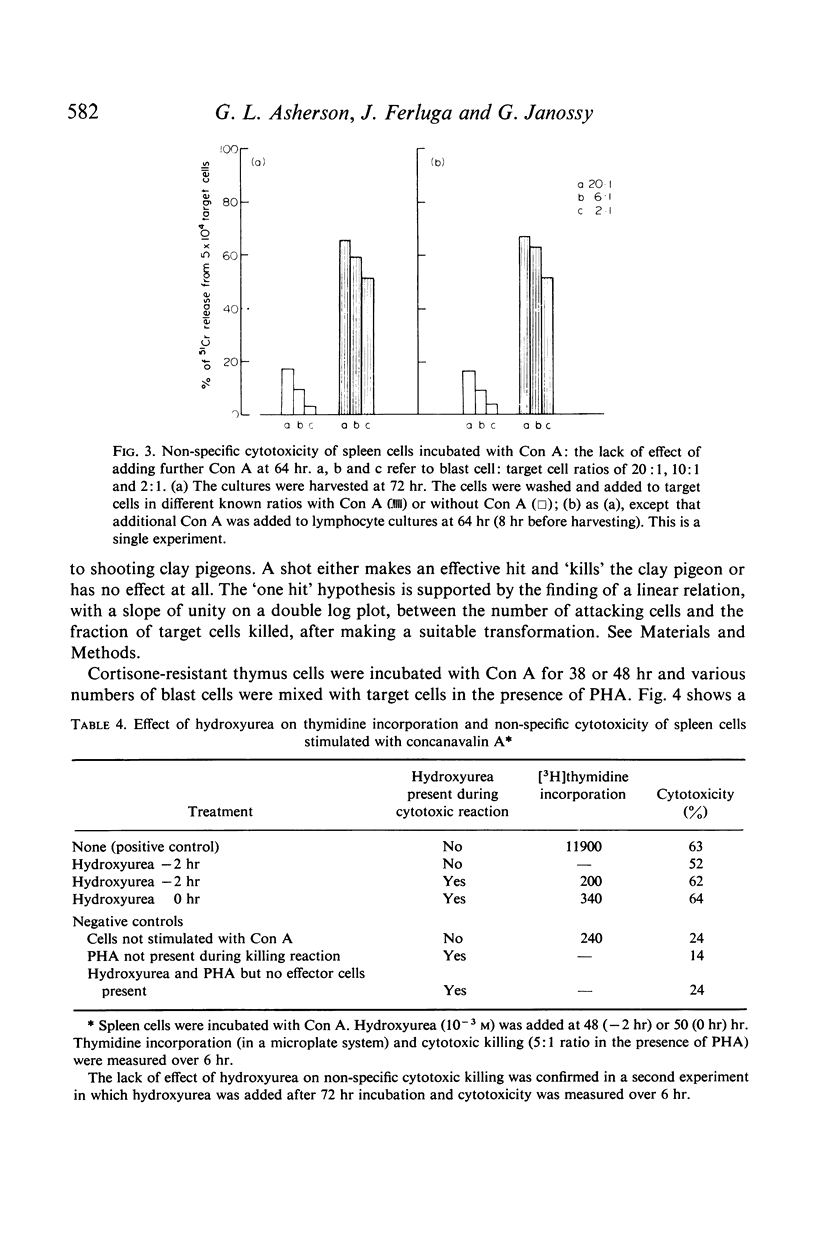
Cell division was not essential for the activation of cytotoxic cells as colcemid diminished but did not abolish activation. DNA synthesis was not required during the killing reaction as judged by the failure of hydroxyurea, an inhibitor of DNA synthesis, to block cytotoxicity when added 2 hr before the killing reaction.
The relation between the number of lymphocytes added and the number of target cells killed was compatible with the `one hit' hypothesis that an effective interaction between a single attacking cell and a target cell led to target cell death.
Non-specific cytotoxicity does not require a strong antigenic difference between attacking and target cells as syngeneic lymphocytes will kill mastocytoma cells. Killing of human cell lines also occurs. The data suggest that the present type of non-specific cytotoxicity is due to activated T cells (presumably blasts) and that plant mitogens are required both for the conversion of the resting T cells to activated cells and to approximate the activated T cells to the target cells and/or initiate a special terminal cytotoxic activation.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Rudolf H., Chapuis B. Studies of allograft immunity in mice. I. Induction, development and in vitro assay of cellular immunity. Immunology. 1970 Apr;18(4):501–515. [PMC free article] [PubMed] [Google Scholar]
- Börjeson J., Reisfeld R., Chessin L. N., Welsh P. D., Douglas S. D. Studies on human peripheral blood lymphocytes in vitro. I. Biological and physicochemical properties of the pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):859–872. doi: 10.1084/jem.124.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. In vitro cytotoxic activity of thymus cells sensitized to alloantigens. Nature. 1970 Jul 4;227(5253):72–73. doi: 10.1038/227072a0. [DOI] [PubMed] [Google Scholar]
- Curtis A. S., Greaves M. F. The inhibition of cell aggregation by a pure serum protein. J Embryol Exp Morphol. 1965 Jun;13(3):309–326. [PubMed] [Google Scholar]
- Evans R., Alexander P. Rendering macrophages specifically cytotoxic by a factor released from immune lymphoid cells. Transplantation. 1971 Sep;12(3):227–229. doi: 10.1097/00007890-197109000-00015. [DOI] [PubMed] [Google Scholar]
- Ferluga J., Asherson G. L., Becker E. L. The effect of organophosphorus inhibitors, p-nitrophenol and cytochalasin B on cytotoxic killing of tumour cells by immune spleen cells, and the effect of shaking. Immunology. 1972 Oct;23(4):577–590. [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Hollander N., Feldman M. The development of hypersensitive lymphocytes in cell culture. J Exp Med. 1971 Oct 1;134(4):1062–1082. doi: 10.1084/jem.134.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Wigzell H., Blomgren H., Svedmyr E. A. Cells mediating specific in vitro cytotoxicity. II. Probable autonomy of thymus-processed lymphocytes (T cells) for the killing of allogeneic target cells. J Exp Med. 1972 Apr 1;135(4):890–906. doi: 10.1084/jem.135.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Henney C. S. Quantitation of the cell-mediated immune response. I. The number of cytolytically active mouse lymphoid cells induced by immunization with allogeneic mastocytoma cells. J Immunol. 1971 Dec;107(6):1558–1566. [PubMed] [Google Scholar]
- Holm G., Perlmann P. Cytotoxic potential of stimulated human lymphocytes. J Exp Med. 1967 Apr 1;125(4):721–736. doi: 10.1084/jem.125.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. II. discriminating stimulation of lymphocyte subpopulations by phytomitogens and heterologous antilymphocyte sera. Clin Exp Immunol. 1972 Mar;10(3):525–536. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Shohat M., Greaves M. F., Dourmashkin R. R. Lymphocyte activation. IV. The ultrastructural pattern of the response of mouse T and B cells to mitogenic stimulation in vitro. Immunology. 1973 Feb;24(2):211–227. [PMC free article] [PubMed] [Google Scholar]
- Lonai P., Feldman M. Cooperation of lymphoid cells in an in vitro graft reaction system. II. The "bone marrow-derived" cell. Transplantation. 1971 May;11(5):446–456. doi: 10.1097/00007890-197105000-00003. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Waksman B. H. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. 3. Analysis of mechanism. J Exp Med. 1968 Dec 1;128(6):1267–1279. doi: 10.1084/jem.128.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavy L., Treves A. J., Feldman M. Capacity of thymic cells to effect target cell lysis following treatment with concanavalin A. Cell Immunol. 1972 Apr;3(4):623–628. doi: 10.1016/0008-8749(72)90124-4. [DOI] [PubMed] [Google Scholar]
- Wagner H., Feldmann M., Boyle W., Schrader J. W. Cell-mediated immune response in vitro. 3. The requirement for macrophages in cytotoxic reactions against cell-bound and subcellular alloantigens. J Exp Med. 1972 Aug 1;136(2):331–343. doi: 10.1084/jem.136.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Harris A. W., Feldmann M. Cell-mediated immune response in vitro. II. The role of thymus and thymus-derived lymphocytes. Cell Immunol. 1972 May;4(1):39–50. doi: 10.1016/0008-8749(72)90004-4. [DOI] [PubMed] [Google Scholar]


