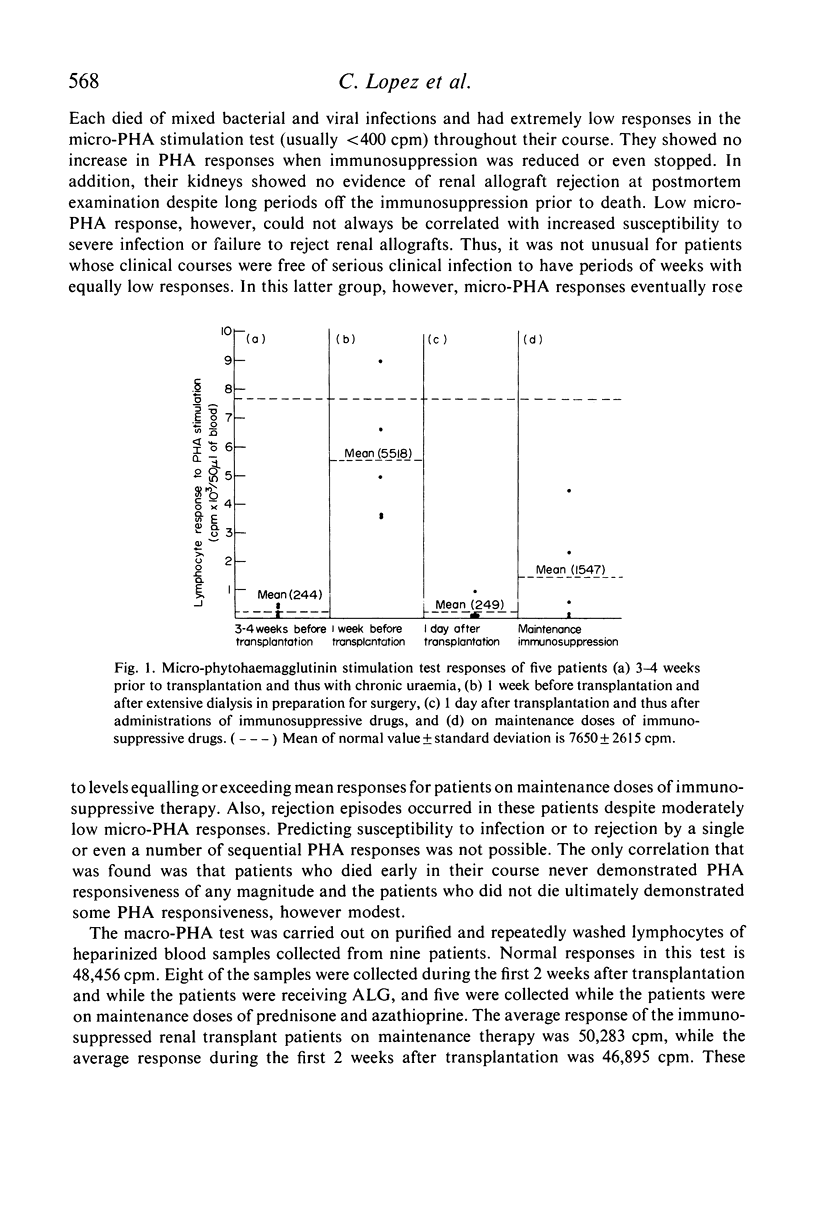
Abstract
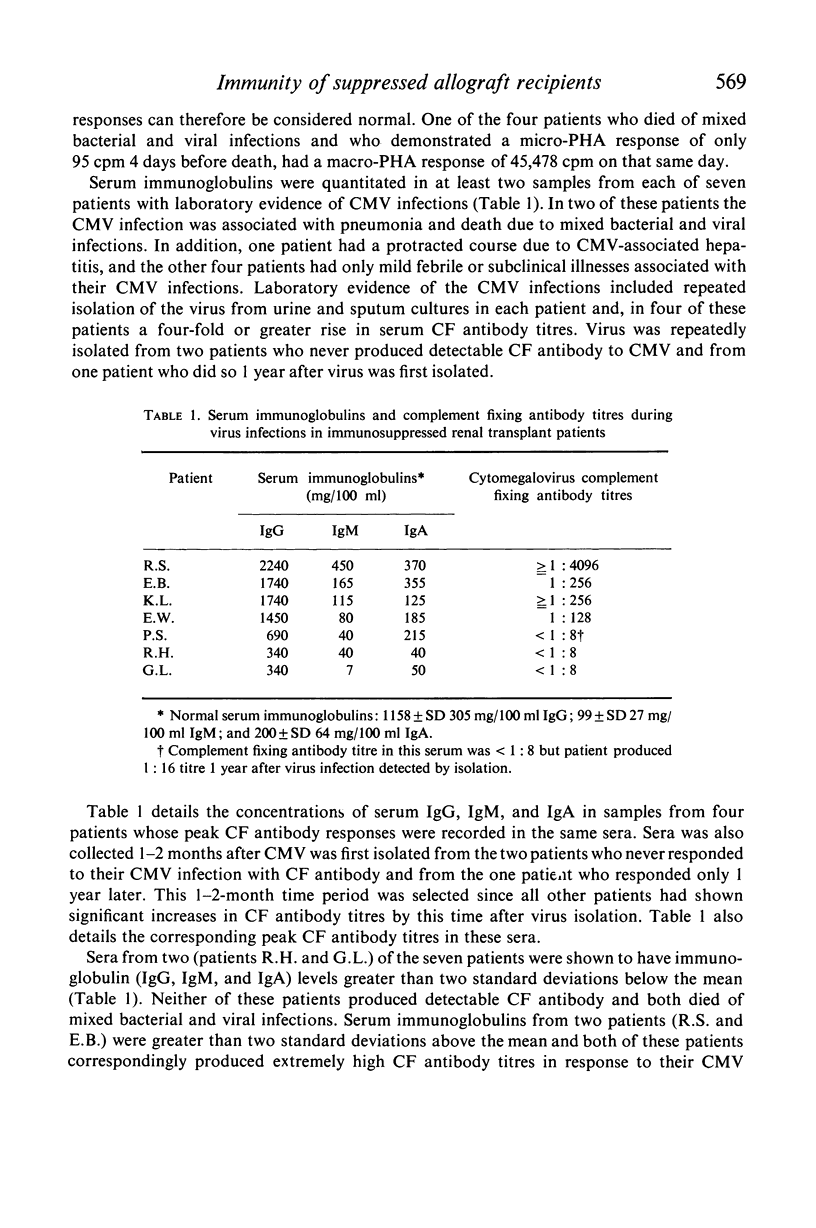
Cell-mediated and humoral immune responses were determined in immunosuppressed renal transplant recipients. A micro-phytohaemagglutinin (PHA) stimulation test utilizing whole heparinized blood and a macro-PHA test utilizing separated, washed lymphocytes were used to study cell-mediated immunity. Humoral immune status was estimated by determining quantitative immunoglobulins and complement fixing (CF) antibody titres to cytomegalovirus (CMV) infection. Micro-PHA responses were found to be markedly depressed in patients undergoing immunosuppressive therapy and in patients with chronic uraemia. Macro-PHA responses were normal, indicating that serum factors were responsible for the depressed micro-PHA responses. Antibody responses to CMV infections were found to be ten to a hundred times higher than in normal persons. An inverse relationship was demonstrated between micro-PHA responses and peak CF antibody titres to CMV infections. Humoral immune responses appeared to compensate for depressed cell-mediated immunity as measured by the micro-PHA test. Four patients had very low micro-PHA responses, did not respond to their CMV infections with CF antibody, and died of mixed bacterial and viral infections. Serum immunoglobulins of two were studied and were shown to be greater than two standard deviations below the normal mean. These patients appeared more suppressed than other patients receiving similar therapy and thus probably retained higher concentrations of suppressive drugs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENT L., BROWN J., MEDAWAR P. B. Skin transplantation immunity in relation to hypersensitivity. Lancet. 1958 Sep 13;2(7046):561–564. doi: 10.1016/s0140-6736(58)90202-2. [DOI] [PubMed] [Google Scholar]
- Bach M. C., Sahyoun A., Adler J. L., Schlesinger R. M., Breman J., Madras P., P'eng F., Monaco A. P. High incidence of fungus infections in renal transplantation patients treated with antilymphocyte and conventional immunosuppression. Transplant Proc. 1973 Mar;5(1):549–553. [PubMed] [Google Scholar]
- Craighead J. E., Hanshaw J. B., Carpenter C. B. Cytomegalovirus infection after renal allotransplantation. JAMA. 1967 Sep 4;201(10):725–728. [PubMed] [Google Scholar]
- Craighead J. E. Immunologic response to cytomegalovirus infection in renal allograft recipients. Am J Epidemiol. 1969 Dec;90(6):506–513. doi: 10.1093/oxfordjournals.aje.a121096. [DOI] [PubMed] [Google Scholar]
- Daniels J. C., Sakai H., Remmers A. R., Jr, Sarles H. E., Fish J. C., Cobb E. K., Levin W. C., Ritzmann S. E. In vitro reactivity of human lymphocytes in chronic uraemia: analysis and interpretation. Clin Exp Immunol. 1971 Feb;8(2):213–227. [PMC free article] [PubMed] [Google Scholar]
- Fine R. N., Grushkin C. M., Malekzadeh M., Wright H. T., Jr Cytomegalovirus syndrome following renal transplantation. Arch Surg. 1972 Oct;105(4):564–570. doi: 10.1001/archsurg.1972.04180100015006. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huraux J. M., Bricout F., Idatte J. M., Leroux-Robert C., Regnard J., Lagrange P., Kourilsky O., Acar J. Renal transplantation and viral infections. I. A retrospective serological study of renal transplantation recipients. Rev Eur Etud Clin Biol. 1972 Aug-Sep;17(7):670–679. [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Enhanced immune responsiveness to a thymus-independent antigen early after adult thymectomy: evidence for short-lived inhibitory thymus-derived cells. Eur J Immunol. 1972 Apr;2(2):114–118. doi: 10.1002/eji.1830020204. [DOI] [PubMed] [Google Scholar]
- LEVIN R. H., LANDY M., FREI E., 3rd THE EFFECT OF 6-MERCAPTOPURINE ON IMMUNE RESPONSE IN MAN. N Engl J Med. 1964 Jul 2;271:16–22. doi: 10.1056/NEJM196407022710103. [DOI] [PubMed] [Google Scholar]
- Lopez C., Simmons R. L., Mauer M., Park B., Najarian J. S., Good R. A. Role of virus infections in immunosuppressed renal transplant patients. Transplant Proc. 1973 Mar;5(1):803–808. [PubMed] [Google Scholar]
- MERRILL J. P., FRIEDMAN E. A., WILSON R. E., MARSHALL D. C. The production of "delayed type" cutaneous hypersensitivity to human donor leukocytes as a result of the rejection of skin homografts. J Clin Invest. 1961 Apr;40:631–635. doi: 10.1172/JCI104294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Wood M. L., Russell P. S. Some effects of purified heterologous antihuman lymphocyte serum in man. Transplantation. 1967 Jul;5(4 Suppl):1106–1114. doi: 10.1097/00007890-196707001-00046. [DOI] [PubMed] [Google Scholar]
- Park B. H., Good R. A. A new micromethod for evaluating lymphocyte responses to phytohemagglutinin: quantitative analysis of the function of thymus-dependent cells. Proc Natl Acad Sci U S A. 1972 Feb;69(2):371–373. doi: 10.1073/pnas.69.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. M., Szmuness W., Millian S. J., David D. S. A serologic study of cytomegalovirus infections associated with blood transfusions. N Engl J Med. 1971 May 20;284(20):1125–1131. doi: 10.1056/NEJM197105202842004. [DOI] [PubMed] [Google Scholar]
- Rifkind D., Goodman N., Hill R. B., Jr The clinical significance of cytomegalovirus infection in renal transplant recipients. Ann Intern Med. 1967 Jun;66(6):1116–1128. doi: 10.7326/0003-4819-66-6-1116. [DOI] [PubMed] [Google Scholar]
- STERN H., ELEK S. D. THE INCIDENCE OF INFECTION WITH CYTOMEGALOVIRUS IN A NORMAL POPULATION. A SEROLOGICAL STUDY IN GREATER LONDON. J Hyg (Lond) 1965 Mar;63:79–87. doi: 10.1017/s0022172400044983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLLMER H. The local effect of cortisone on the tuberculin reaction. J Pediatr. 1951 Jul;39(1):22–32. doi: 10.1016/s0022-3476(51)80277-4. [DOI] [PubMed] [Google Scholar]
- WAKSMAN B. H., ARBOUYS S., ARNASON B. G. The use of specific "lymphocyte" antisera to inhibit hypersensitive reactions of the "delayed" type. J Exp Med. 1961 Dec 1;114:997–1022. doi: 10.1084/jem.114.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]


