Abstract
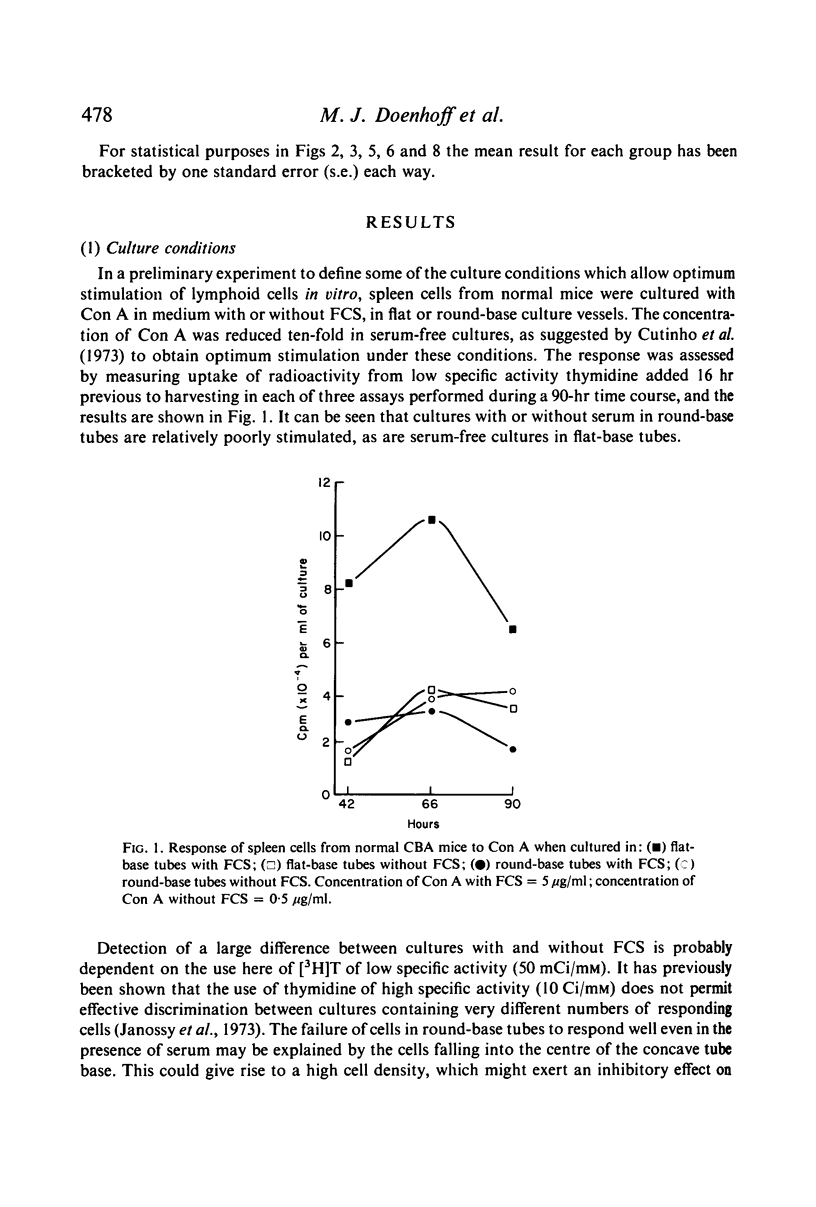
In the culture and assay systems described here the majority of mouse spleen cells stimulated by soluble concanavalin A and phytohaemagglutinin have been shown to be T cells, whereas bacterial endotoxin (LPS) stimulates B cells almost exclusively. These observations appear to be independent of the tissue source, the length of the culture period, and the presence of supernatants from other cultured T cells. Pokeweed mitogen (PWM) is capable of stimulating both T and B cells, the relative numbers of each probably being affected by the length of the culture period and variability between different PWM samples. Preliminary evidence is presented for mature LPS-responsive cells being able to persist for long periods in the mouse peripheral lymphoid system. The importance of using culture conditions that allow selective stimulation of T and B cells is discussed, especially in relation to their accurate quantitation.
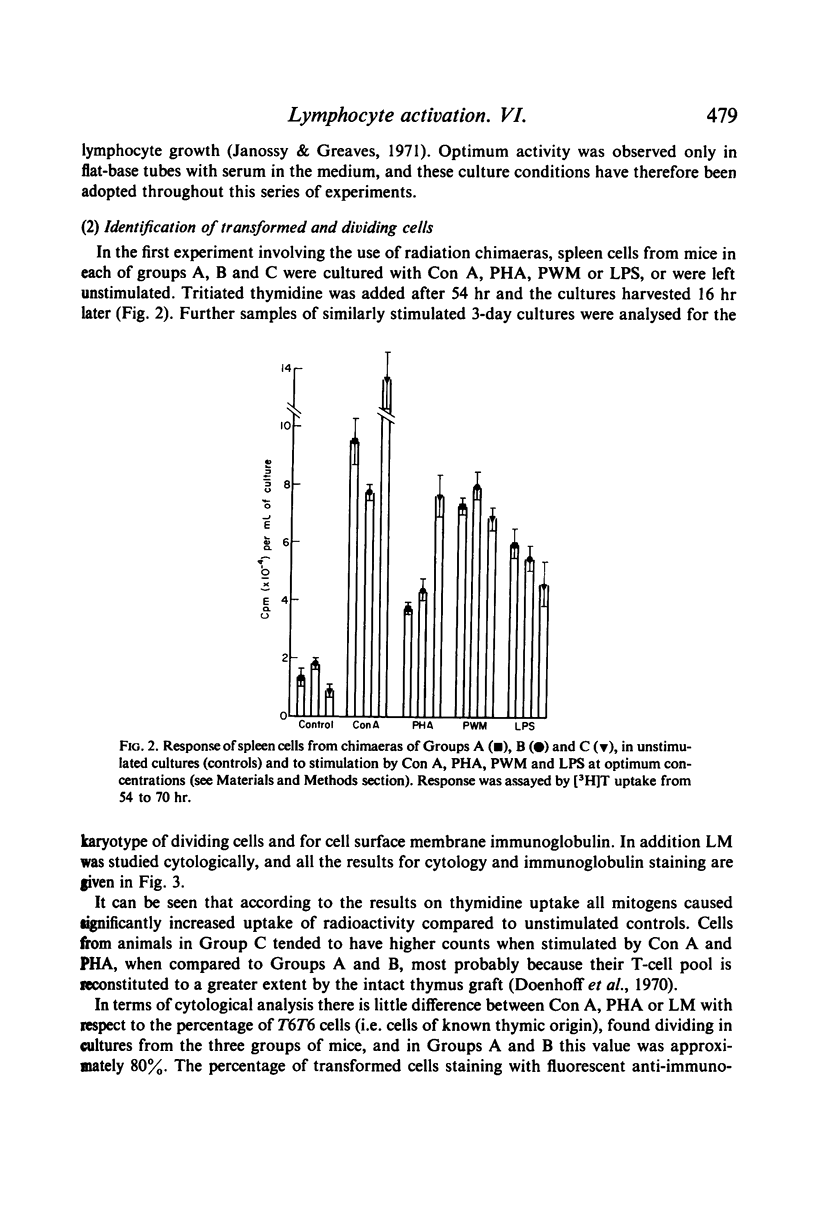
Full text
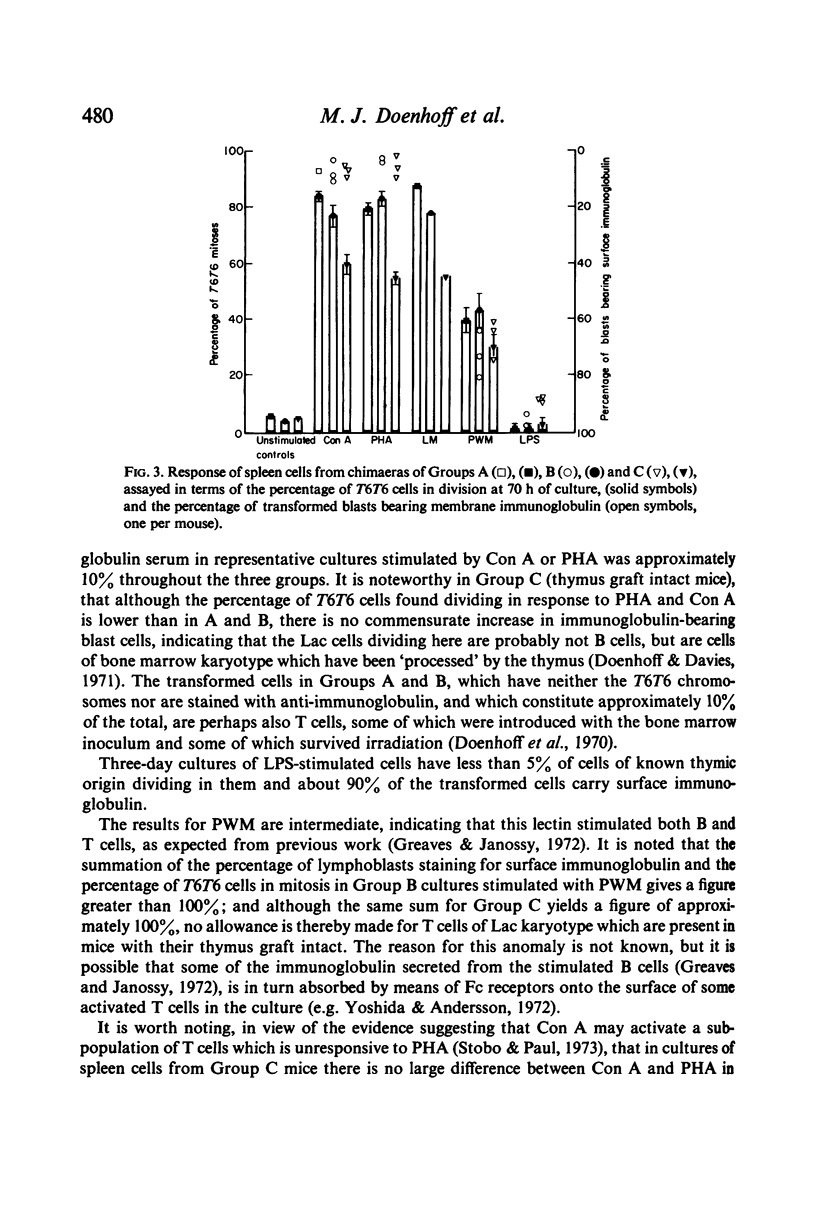
PDF


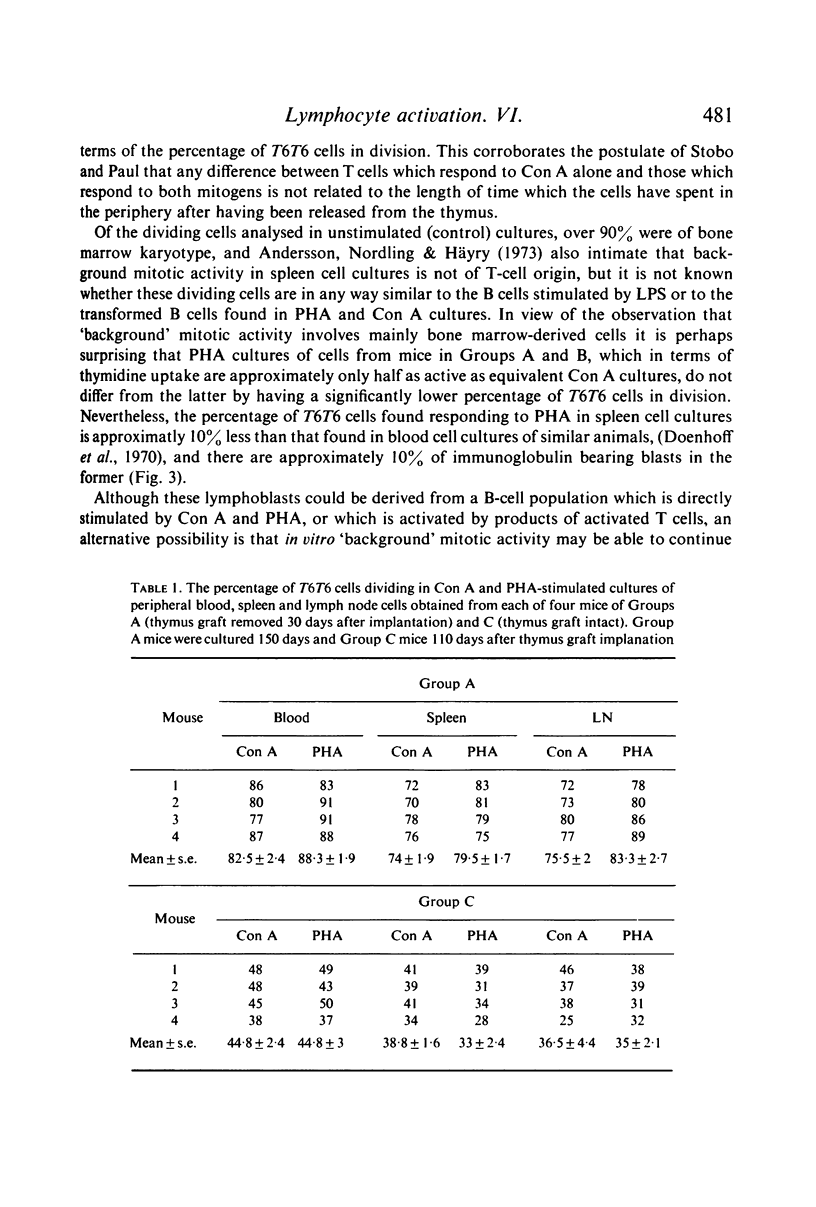




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Edelman G. M., Möller G., Sjöberg O. Activation of B lymphocytes by locally concentrated concanavalin A. Eur J Immunol. 1972 Jun;2(3):233–235. doi: 10.1002/eji.1830020307. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. B lymphocytes can be stimulated by concanavalin A in the presence of humoral factors released by T cells. Eur J Immunol. 1972 Feb;2(1):99–101. doi: 10.1002/eji.1830020119. [DOI] [PubMed] [Google Scholar]
- Andersson L. C., Nordling S., Häyry P. Proliferation of B and T cells in mixed lymphocyte cultures. J Exp Med. 1973 Jul 1;138(1):324–329. doi: 10.1084/jem.138.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börjeson J., Reisfeld R., Chessin L. N., Welsh P. D., Douglas S. D. Studies on human peripheral blood lymphocytes in vitro. I. Biological and physicochemical properties of the pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):859–872. doi: 10.1084/jem.124.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Möller G., Anderson J., Bullock W. W. In vitro activation of mouse lymphocytes in serum-free medium: effect of T and B cell mitogens on proliferation and antibody synthesis. Eur J Immunol. 1973 May;3(5):299–306. doi: 10.1002/eji.1830030509. [DOI] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J. Reconstitution of the T-cell pool after irradiation of mice. Cell Immunol. 1971 Feb;2(1):82–90. doi: 10.1016/0008-8749(71)90027-x. [DOI] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S., Janossy G. Lymphocyte activation. 3. Binding sites for phytomitogens on lymphocyte subpopulations. Clin Exp Immunol. 1972 Mar;10(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J. Isolation and characterization of a phytohemagglutinin from the lentil. Biochemistry. 1969 Jun;8(6):2436–2441. doi: 10.1021/bi00834a028. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F., Doenhoff M. J., Snajdr J. Lymphocyte activation. V. Quantitation of the proliferative responses to mitogens using defined T and B cell populations. Clin Exp Immunol. 1973 Aug;14(4):581–596. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. II. discriminating stimulation of lymphocyte subpopulations by phytomitogens and heterologous antilymphocyte sera. Clin Exp Immunol. 1972 Mar;10(3):525–536. [PMC free article] [PubMed] [Google Scholar]
- Phillips B., Roitt I. M. Evidence for transformation of human B lymphocytes by PHA. Nat New Biol. 1973 Feb 21;241(112):254–256. doi: 10.1038/newbio241254a0. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Vassalli P. Study of the thymic-derived or -independent nature of mouse spleen cells induced to proliferate in culture by various mitogens and antigens. Eur J Immunol. 1973 Aug;3(8):477–483. doi: 10.1002/eji.1830030805. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Vassalli P. Thymus-independent (B) cell proliferation in spleen cell cultures of mouse radiation chimeras stimulated by phytohemagglutinin or allogeneic cells. J Exp Med. 1972 Oct 1;136(4):962–967. doi: 10.1084/jem.136.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Basten A. Circulating T and B lymphocytes of the mouse. II. Lifespan. Cell Immunol. 1973 Apr;7(1):40–59. doi: 10.1016/0008-8749(73)90181-0. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]


